†These authors contributed equally.
Focal cerebral ischemia-reperfusion injury is closely related to hyperglycemia and gut microbiota imbalance, while gut microbiota contributes to the regulation of brain function through the gut-brain axis. Previous studies in patients with diabetes have found that 'yam gruel' is a classic medicated diet made from Dioscorea polystachya, increases the content of Bifidobacterium, regulates oxidative stress, and reduces fasting blood glucose levels. The research reported here investigated the effects of 'yam gruel' on the cognitive function of streptozotocin-induced diabetic rats with focal cerebral ischemia-reperfusion injury and explored the mechanism underlying the role of the gut-brain axis in this process. 'Yam gruel' was shown to improve cognitive function as indicated by increased relative content of probiotic bacteria, and short-chain fatty acids in the intestinal tract and cerebral cortex reduced oxidative stress and inflammatory response and promotion of the expression of neurotransmitters and brain-derived neurotrophic factor. Thus, it is concluded that 'yam gruel' has a protective effect on cognitive function via a mechanism related to the gut-brain axis.
Diabetes and stroke are common and frequently-occurring diseases that are closely related and often occur together. Studies have reported that stroke is more likely to occur in the presence of diabetes than in its absence and that patients with diabetes complicated by stroke have a poor prognosis (O'Donnell et al., 2016). Cerebral ischemia caused by a stroke can lead to reperfusion injury, which eventually aggravates brain tissue damage and dysfunction (Lin et al., 2016; Yang et al., 2019). Diabetes is a metabolic disease characterized by hyperglycemia that aggravates brain damage caused by acute stroke via a mechanism possibly related to hyperglycemia-induced oxidative stress and inflammatory response (Li et al., 2010). Studies have reported that long-term hyperglycemia generates a considerable number of oxygen free radicals in the brain. Brain tissue and nerve cells become peroxidized, thereby leading to their necrosis and cognitive impairment (Sun et al., 2016). Excessive oxidation reaction increases vascular oxygen consumption and pressure, and the production of oxygen free radicals. Hyperglycaemia also increases blood viscosity and leads to atherosclerosis and thrombosis, which further aggravates cerebral ischemia-reperfusion injury (Chehaib et al., 2016; Chorepsima et al., 2017).
Further, the inflammatory response is closely related to progressive neurological injury in ischemic stroke (Fu et al., 2015; Nakata et al., 2017). Pro-inflammatory cytokines aggravate both brain injury after stroke and cognitive dysfunction by down-regulating nerve growth factor (Winek et al., 2016). Additionally, studies have shown that increased pro-inflammatory factors associated with diabetes affect nerve signal transduction and are an important reason for a decline in cognitive function (Liu et al., 2018; Monguió-Tortajada et al., 2018). Currently, the clinical treatment of diabetes in conjunction with focal cerebral ischemia-reperfusion injury remains a significant problem as drug therapy often has side effects. Recently investigators have started to explore other effective treatment methods.
A porridge 'yam gruel,' consisting of raw Dioscorea polystachya common name Chinese yam or 'Huai Qing Fu' yam (Zhang, 2018), is a traditional medicated diet derived from the book 'Records of Traditional Chinese and Western Medicine in Combination.' Previous studies have found that 'yam gruel' can increase the content of Bifidobacterium in the intestinal tract, regulate oxidative stress, relieve inflammatory response and reduce fasting blood glucose (FBG) levels in patients with diabetes (Pang et al., 2017). Studies in animal models of cognitive dysfunction have further revealed that the active components of yam regulate the composition of gut microbiota and improve memory dysfunction (Dong et al., 2018; Tohda et al., 2017). A considerable number of studies have shown that gut probiotics affect the regulation of cognitive function due to two-way communication channels between the gut and the brain, referred to as the 'gut-brain axis' (Hamidi et al., 2019; Martin et al., 2018).
In diabetes with focal cerebral ischemia-reperfusion injury, brain function is impaired, and any gut microbiota imbalance is aggravated, as manifested by decreased probiotics, such as Bifidobacterium and Lactobacillus, and an increase in harmful bacteria (Winek et al., 2016). Oxidative stress and inflammatory response are involved in the regulation of post-stroke cognitive dysfunction (Xu et al., 2017), which may bridge between gut microbiota and the occurrence of post-stroke cognitive dysfunction. The current research investigates the effects of 'yam gruel' on the cognitive function of diabetic rats with focal cerebral ischemia-reperfusion injury via the gut-brain axis. The objective is to provide a new method and target for the treatment of diabetes when associated with such injury.
'Yam gruel' was prepared according to a previously described method (Pang et al., 2017; Zhang, 2018). A raw Dioscorea polystachya was peeled and sliced and 125 g of yam slices mixed with 50 mL of water. The mixture was homogenized to make a paste. The paste was added to 250 mL of cold water and boiled over medium and low heat for 30 seconds, respectively. The boiling procedure was repeated three times, with intervals of 30 seconds. During the boiling process, the paste was stirred gently. It contained approximately 250 mL of 'yam gruel' at a concentration of about 0.5 g/mL. The 'yam gruel' was then cooled to 37 °C for use.
Eight-week-old male Wistar rats weighing approximately 170-190 g were purchased from Shanghai Slack Laboratory Animal Co., Ltd (Shanghai, P. R. China). Rats were randomly housed in cages maintained at 25 ± 2 °C and 60% relative humidity under a 12-hour light/dark inverted cycle with free access to water and food. All animal experiments were approved by the Animal Experiment Committee of the Fujian University of Traditional Chinese Medicine (2019-023), and the entire experiment was carried out in accordance with the guidelines for the care and protection of laboratory animals of the National Institute of Health.
2.2.1 Induction of diabetes
After adaptive feeding for one week, all rats were fed for six weeks with a high-sugar/high-fat diet (10.0% lard, 15.0% sucrose, 4.0% cholesterol, 10.0% yolk powder, 0.3% chocolate and 60.7% standard diet) provided by Minhou Wushi Experimental Animal Trading Co., Ltd. (Fuzhou, P. R. China). Subsequently, rats were fasted overnight and intraperitoneally injected twice with a 1% streptozotocin (STZ) (Sigma, USA) solution at a dosage of 25 mg/kg (two days). Blood glucose levels were observed via the tail vein after 72 hours of STZ injection, and rats with an FBG level greater than 11.1 mmol/L for two consecutive days were confirmed as type 2 diabetes models (T2DM). After four weeks, these rats were used for the induction of focal cerebral ischemia-reperfusion.
2.2.2 Induction of focal cerebral ischemia-reperfusion
Focal cerebral ischemia-reperfusion surgery was performed, according to Longa et al. (1989). Rats were anesthetized and fixed on the operating table in a supine position. The left common carotid artery (CCA) and external carotid artery were ligated after the skin of the neck were incised. A small cut was made near the distal ligation of the CCA. A nylon monofilament (Watson Biotechnology Co., Ltd., Fuzhou, P. R. China) with a heparin-coated tip was inserted from the CCA into the internal carotid artery (ICA). The insertion depth was approximately 18-20 mm. The thread was gently moved until a sense of resistance was felt, that is, the threaded plug reached the beginning of the middle cerebral artery (MCA), thereby blocking the blood flow in the MCA and causing focal cerebral ischemia. The nylon monofilament and ICA were fixed with a surgical thread, and the surgical opening was sutured. After two hours of ischemia, the thread was extracted carefully, and reperfusion was performed. The operation was the same in the sham group except that the nylon monofilament was not inserted. Body temperature was maintained at 37 °C during the operation and animal recovery. After 24 hours of reperfusion, neurological deficits were observed, and rats with one to three points were selected for further study. These rats were then randomly divided into the model and 'yam gruel' groups.
2.2.3 Groups and administration
A total of 27 rats were randomly divided into three groups: (1) sham (normal saline, 15 mL/kg/day, n = 9), (2) model (normal saline, 15 mL/kg/day, n = 9) and (3) 'yam gruel' ('yam gruel', 15 mL/kg/day, n = 9) groups. All rats were fed once daily by gavage for four consecutive weeks.
Neurological deficits were evaluated 24 hours after reperfusion and at the end of a four-week intervention by an individual blind to the experiment. The degree of the neurological deficit was characterized as 0 points when no neurological deficits, 1 point when the right forepaw could not fully stretch when the tail was lifted, 2 points when turning around into a circle when crawling, 3 points when falling to the right when crawling and 4 points when unable to walk on their own and loss of consciousness.
Spatial learning and memory function of rats were evaluated during the last 5 days of the intervention by using the Morris water maze test as described by Li et al. (2015). The Morris water maze comprised a circular pool (diameter 120 cm, height 50 cm, water depth 30 cm maintained at 25 °C). The pool was divided into four equal quadrants identified by four entry points on the pool wall. A circular escape platform was located in the center of one quadrant and immobilized 2 cm beneath the water surface. Animals were tested every morning for five consecutive days. On days 1-4, a positioning navigation test was conducted, where a rat was gently placed in the water within each of the four quadrants in turn (facing the pool wall) and allowed 90 seconds to find the platform. If the rat reached the platform successfully and stayed for 5 seconds, then the escape latency was recorded by a computer (Top Scan Lite Animal Behavior Analysis System, Clever Sys Inc., USA). If the rat failed to find the platform within 90 seconds, it was guided onto the platform for 10 seconds, and the escape latency recorded as 90 seconds. A space exploration experiment was conducted on day 5, where the platform was removed, and the rats were individually placed into the water at a position opposite the quadrant of the original platform. The number of times crossing the position of the first platform within 90 seconds was recorded.
At the end of the intervention, rats were evaluated for neurological deficits and then anesthetized with 2% pentobarbital sodium (60 mg/kg, i.p.). Subsequently, the abdominal aortic blood of each rat was collected and divided into two parts; one was used to measure FBG, and the other was centrifuged at 12,000 × g for 10 minutes at 4 °C to collect serum for the detection of inflammatory and oxidative stress-related indicators. The left hippocampus and cerebral cortices and colon contents were obtained and stored at -80 °C for later use. Additionally, the appropriate amount of the left hippocampus was fixed in 4% paraformaldehyde for hematoxylin and eosin (HE) staining.
2.5.1 16S rRNA gene sequencing technology
16S rRNA gene sequencing technology was used to detect changes in gut microbiota. DNA integrity was checked by 1% agarose gel electrophoresis following total DNA extraction. Subsequently, the V3-V4 hypervariable region of the 16S rRNA gene was amplified from genomic DNA by using primers 341F (CCTACGGGNGGCWGCAG) and 805R (GACTACHVGGGTATCTAATCC). The polymerase chain reaction (PCR) conditions were: initial denaturation (94 °C, 3 minutes) followed by 18 cycles of denaturing (94 °C, 3 seconds), annealing (55 °C, 30 seconds), elongation (72 °C, 30 seconds) and a final extension (72 °C, 5 minutes). Amplification products were purified and quantified for sequencing with the Illumina MiSeq System (Illumina Inc., CA, USA). Raw reads were filtered to obtain accurate and reliable clean reads, which were then spliced based on overlaps to obtain clean tags. Operational taxonomic unit (OTU) clustering and species classification analysis was carried out based on valid data.
2.5.2 Gas chromatography-mass spectrometry
Following pretreatment of colon content and cerebral cortical samples, short-chain fatty acids (SCFAs) contents (acetic, propionic and butyric acids) in the colon and cerebral cortex and GABA and 5-hydroxytryptamine (5-HT) contents in the cerebral cortex were analyzed by gas chromatography-mass spectrometry (Agilent Co., USA) with helium (1.0 mL/min)used as the carrier gas. The initial column temperature was 100 °C and maintained for 30 seconds. The temperature was then raised to 180 °C at 8 °C/minute and maintained for 1 minute, then further raised to 200 °C at 20 °C/minute, then maintained for 5 minutes. Additionally, the temperature of the detector and the sample inlet was set to 240 °C and 200 °C, respectively. The inlet temperature was 250 °C when testing for GABA. The oven temperature was initialized at 100 °C, increased to 295 °C at a rate of 8 °C/minute, and maintained at 295 °C for 5 minutes. The conditions for 5-HT detection were: carrier gas helium; column temperature 110 °C for 3 minutes, raised to 225 °C at 8 °C/minute and further raised to 300 °C at 20 °C/minute; injection, interface and ion source temperatures were 280, 260 and 110 °C, respectively.
2.5.3 Superoxide dismutase (SOD) and malondialdehyde (MDA) measurements
SOD and MDA serum levels were measured following the manufacturers' instructions using commercially available kits, purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, P. R. China).
2.5.4 Tumour necrosis factor α (TNF-α), interleukin 1β (IL-1β) and lipopolysaccharide (LPS) measurements
The serum concentrations of TNF-α (Thermo Fisher Scientific, Vienna, Austria), IL-1β (Multi Sciences (Lianke) Biotech, Co., Ltd., Hangzhou, P. R. China) and LPS (Nanjing Jiancheng Bioengineering Institute, Nanjing, P. R. China) were detected by commercial assay kits following the manufacturers' instructions.
2.5.5 RNA extraction and real-time quantitative PCR (RT-qPCR)
RT-qPCR was used to detect the gene expression of brain-derived neurotrophic factor (BDNF). Total RNA was extracted according to the manufacturers' instructions from the frozen hippocampus tissues (Servicebio Technology Co., Ltd., Wuhan, P. R. China) then reverse transcribed into cDNA using a RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific Inc.). Primers for RT-qPCR were: BDNF forward, 5′-GTGTGACAGTATTAGCGAGTGGG-3′ and reverse, 5′-ACGATTGGGTAGTTCGGCATT-3′; GAPDH (as an internal standard) forward, 5′-TTCCTACCCCCAATGTATCCG-3′ and reverse, 5′-CATGAGGTCCACCACCCTGTT-3′. Amplification and detection of quantitative PCR were performed using a 7500 real-time fluorescence quantitative PCR system (Corbett Company, Australia). PCR amplification conditions were: initial denaturation (95 °C, 10 minutes); 40 cycles (95 °C, 15 seconds); 60 °C for 60 seconds; melting curve (60 °C to 95 °C at 0.3 °C/15 seconds). Data were calculated using the 2-ΔΔCt method.
2.5.6 Western blotting
Western blot was performed to measure the protein expression of BDNF. The hippocampus tissue was ground in liquid nitrogen, cleaved with RIPA lysis buffer (Boster Biological Technology Co., Ltd., Wuhan, P. R. China) and the homogenate centrifuged at 14,000 × g (4 °C, 15 minutes). A BCA protein quantification kit (Boster Biological Technology Co., Ltd., Wuhan, P. R. China) was used to quantify the protein concentration. Additionally, 50 μg of protein and 3 μL of the marker were loaded and separated by SDS-PAGE, transferred to PVDF membrane and blocked with 5% skimmed milk (room temperature, 2 hours). The membranes were then incubated with primary antibodies (BDNF: 1 : 200, Santa Cruz, USA) overnight (4 °C), washed with TBST (10 minutes × 3), incubated with horseradish peroxidase and labeled (1: 5000, Abcam, USA, room temperature, 2 hours). After washing with TBST three times, an automatic gel imaging system (Liuyi Instrument Factory, Beijing, P. R. China) was used for scanning and photography. The grey value of protein bands was measured, and the protein expression of BDNF analyzed.
2.5.7 FBG measurement
Blood glucose levels were measured with a reagent kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, P. R. China) according to the glucose oxidase method.
2.5.8 HE staining
HE staining was used for histopathology in the hippocampus CA1 region. Brain sections (4 mm) were sliced from the coronal plane. Dewaxed sections (xylene) were hydrated with alcohol from high to low concentration and then stained with HE. Pathological changes in the brain tissue were observed and photographed under a light microscope (DM4000B LED, Leica, Wetzlar, Germany).
Statistical analysis was performed with SPSS 20.0 software (IBM; Armonk, NY, USA). Values are expressed as mean ± SD. Neurological deficit scores were obtained using the Student's t-test, and escape latency were analyzed by two-way repeated-measures ANOVA. All other data between groups were analyzed with one-way ANOVA, followed by the least significant difference or Dunnett's T3 posthoc tests. Statistical significance was assumed for P < 0.05.
3.1.1 Characterisation of gut microbiota
The characterization of gut microbiota was analyzed using OTU numbers and the Chao1 and Shannon diversity indices. Fig. 1 shows that the OTU numbers and Chaol and Shannon indices were significantly lower in the model group when compared to the sham group (P < 0.05). 'Yam gruel' supplementation further reduced the above three indicators (P < 0.05).
 Figure 1.
Figure 1.Effect of 'yam gruel' on the characterization of gut microbiota. (A) OUT numbers of each group; (B) Chao1 index of each group; (C) Shannon diversity index of each group. The OTU numbers and Chao1 and Shannon indices were significantly lower in the model group when compared with the sham group. Compared with the model group, the above three indicators in the 'yam gruel' group were further reduced. Values are the means ± SD, n = 9. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
3.1.2 Relative abundances of different bacteria in gut microbiota
At the phylum level (Fig. 2A), Firmicutes and Bacteroidetes were the two major bacterial phyla in the gut microbiota of the three groups. An increase in the Firmicutes/Bacteroidetes (F: B) ratio was indicative of dysbiosis. The F: B ratio was higher in the model group than in the sham group. However, 'yam gruel' treatments decreased the F: B ratio compared with the model group (P < 0.05). At the genus level (Fig. 2B), the relative abundance of SCFAs-producing bacteria, such as Lactobacillus, Ruminococcus, and Clostridium, showed lower abundance in the model group than in the sham group. By contrast, 'yam gruel' treatment significantly increased the abundance of Lactobacillus, Ruminococcus, and Clostridium (P < 0.05).
 Figure 2.
Figure 2.Effect of 'yam gruel' on the relative abundances of gut microbiota at the phylum (A) and genus (B) taxonomic levels in each group. Species that could not be annotated at the genus level were classified into the 'unassigned' category. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group.
3.1.3 SCFAs contents
'Yam gruel' increased SCFAs content. As shown in Fig. 3, SCFAs content in the colon (Fig. 3A) and cerebral cortex (Fig. 3B) were decreased in the model group when compared with the sham group (P < 0.05). 'Yam gruel' treatment reversed this situation and significantly increased SCFAs content in the colon and cerebral cortex (P < 0.01).
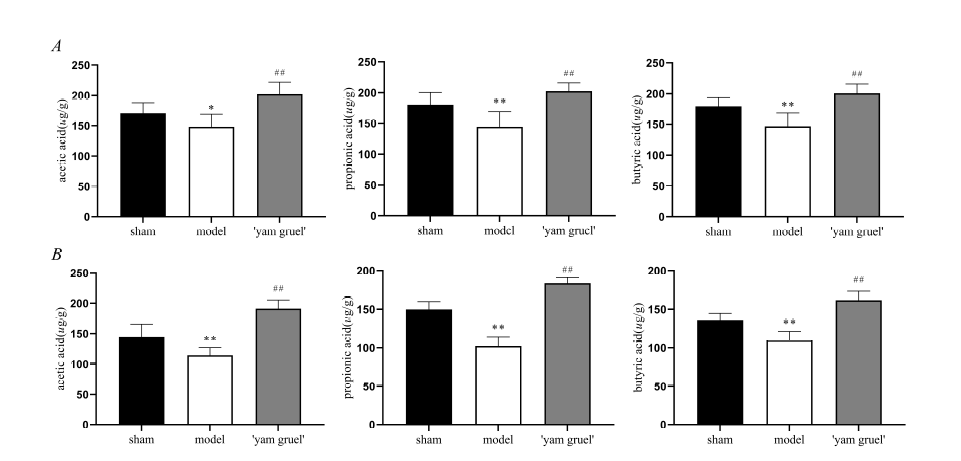 Figure 3.
Figure 3.Effect of 'yam gruel' on SCFAs. (A) The content of SCFAs in the colon content of each group; (B) The content of SCFAs in the cerebral cortical of each group. The content of SCFAs in the colon content and cerebral cortical in the model group were significantly reduced when compared with the sham group. However, compared with the model group, the content of SCFAs in the colon content and cerebral cortical in the 'yam gruel' group were significantly increased. Values are the means ± SD, n = 9. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
3.1.4 LPS contents
The LPS levels in serum were higher in the model group than in the sham group (P < 0.01). After four weeks of 'yam gruel' supplementation, LPS levels were significantly decreased (P < 0.05, Fig. 4).
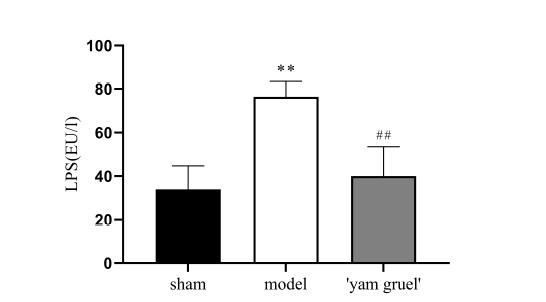 Figure 4.
Figure 4.Effect of 'yam gruel' on LPS. The levels of LPS in the serum in the model group were significantly increased when compared with the sham group. Compared with the model group, the levels of LPS in the serum in the 'yam gruel' group were significantly reduced. Values are the means ± SD, n = 9. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
3.2.1 Effect of 'yam gruel' on SOD and MDA
'Yam gruel' effectively improves the antioxidant capacity of rats. Compared with those in the sham group, SOD and MDA contents were either decreased or increased, respectively, in the model group (P < 0.05). Following treatment with 'yam gruel,' SOD levels increased, and MDA levels were decreased when compared with the model group (P < 0.05, Fig. 5).
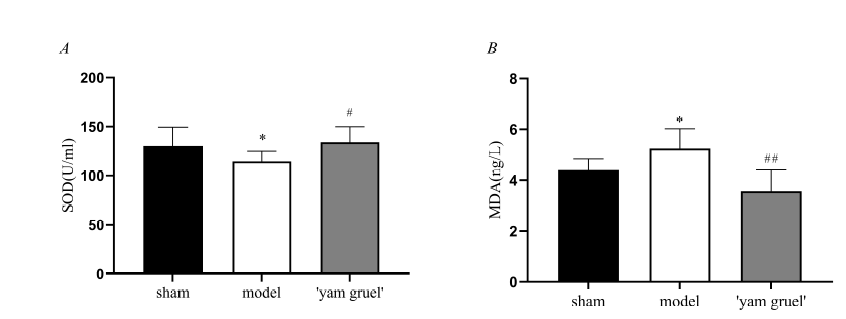 Figure 5.
Figure 5.Effect of 'yam gruel' on SOD and MDA. (A) SOD levels of each group; (B) MDA levels of each group. The content of SOD was decreased while the content of MDA was increased in the model group when compared with the sham group. Compared with the model group, the content of SOD increased, and the content of MDA decreased in the 'yam gruel' group. Values are the means ± SD, n = 9. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
3.2.2 Effect of 'yam gruel' on TNF-α and IL-1β
'Yam gruel' had an anti-inflammatory effect on diabetic rats with focal cerebral ischemia-reperfusion injury. TNF-α and IL-1β levels in serum were higher in the model group than in the sham group (P < 0.01). Following four weeks of 'yam gruel' treatment, TNF-α, and IL-1β levels were significantly decreased (P < 0.05, Fig. 6).
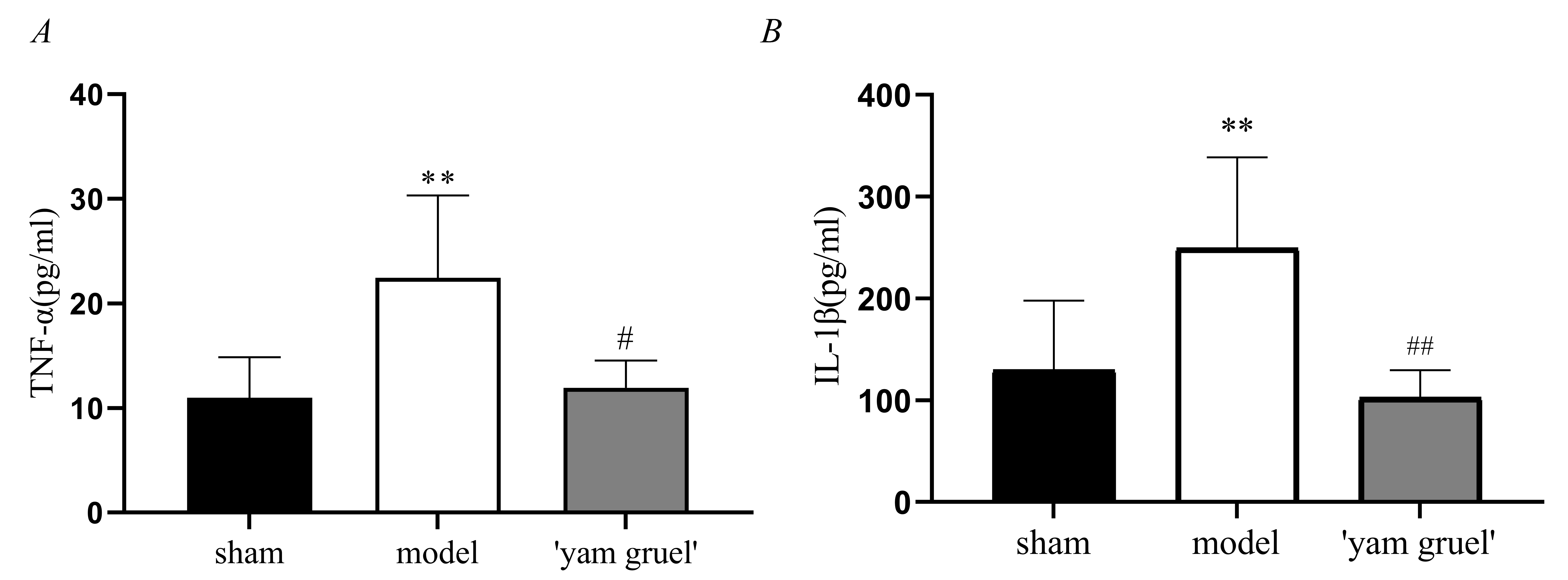 Figure 6.
Figure 6.Effect of 'yam gruel' on TNF-α and IL-1β. (A) TNF-α levels of each group; (B) IL-1β levels of each group. The levels of TNF-α and IL-1β in the serum in the model group were significantly increased when compared with the sham group. However, compared with the model group, the levels of TNF-α and IL-1β in the serum in the 'yam gruel' group were significantly reduced. Values are the means ± SD, n = 9. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
3.3.1 Effect of 'yam gruel' on GABA and 5-HT
GABA (Fig. 7A) and 5-HT (Fig. 7B) levels were significantly reduced in the model group when compared with the sham group (P < 0.01). However, 'yam gruel' treatment significantly increased GABA and 5-HT levels when compared with the model group (P < 0.01).
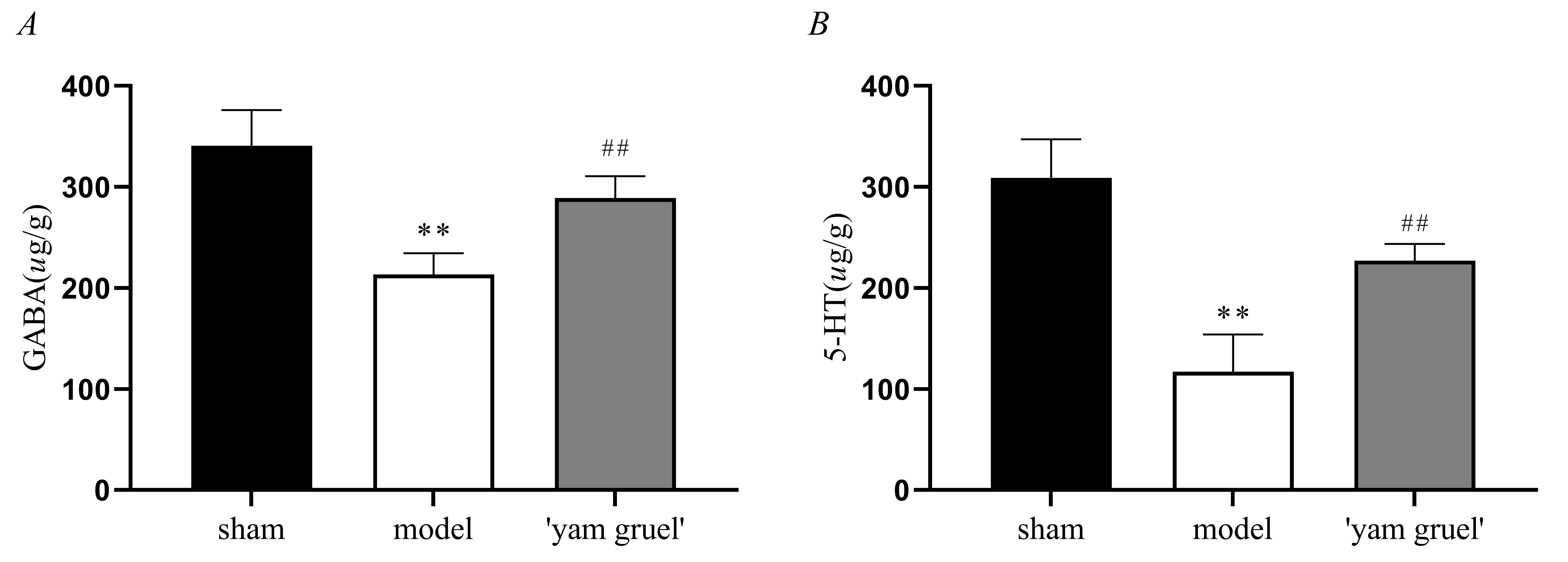 Figure 7.
Figure 7.Effect of 'yam gruel' on GABA and 5-HT. (A) GABA levels of each group; (B) 5-HT levels of each group. The levels of GABA and 5-HT in the cerebral cortex in the model group were significantly decreased when compared with the sham group. However, compared with the model group, the levels of GABA and 5-HT in the cerebral cortex in the 'yam gruel' group were significantly increased. Values are the means ± SD, n = 9. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
3.3.2 Effect of 'yam gruel' on BDNF expression
BDNF gene (Fig. 8A) and protein (Fig. 8B and Fig. 8C) expression levels were significantly decreased in the model group when compared with the sham group (P < 0.05). RT-qPCR and Western blot showed that both the BDNF gene and protein expression levels were increased in the 'yam gruel' group (P < 0.05).
 Figure 8.
Figure 8.Effect of 'yam gruel' on the expression of BDNF. (A) mRNA levels of BDNF of each group; (B) and (C) Protein levels of BDNF of each group. The expression levels of the BDNF gene and protein in the model group were decreased when compared with the sham group. However, compared with the model group, the expression levels of the BDNF gene and protein in the 'yam gruel' group were significantly increased. Values are the means ± SD. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
To study the effect of 'yam gruel' on the blood glucose levels of rats, FBG was measured after four weeks of 'yam gruel' administration. As shown in Fig. 9, hyperglycemia was found in both the sham and model groups, but no statistical difference was found between the two groups. However, treatment with 'yam gruel' significantly reduced the FBG levels in STZ-induced hyperglycaemic rats with focal cerebral ischemia-reperfusion injury (P < 0.01).
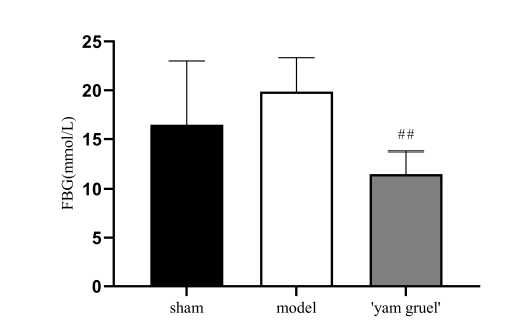 Figure 9.
Figure 9.Effect of 'yam gruel' on FBG. Hyperglycemia was found in both the sham group and the model group, and there was no statistical difference between the two groups. Compared with the model group, the FBG levels in the 'yam gruel' group were significantly reduced. Values are the means ± SD, n = 9. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
3.5.1 Effect of 'yam gruel' on histopathological changes
As shown in Fig. 10, the cells in the sham group were neatly arranged, with nuclei in the center and clear intercellular space. On the contrary, in the model group, the cells were disarranged, intercellular boundaries were blurred, and the nuclei were hyperchromatic and irregular. However, these histopathological changes were ameliorated by 'yam gruel.'
 Figure 10.
Figure 10.Effect of 'yam gruel' on histopathological (magnification, x400). In the model group, the cells were disarranged, intercellular boundaries were blurred, and the nuclei were hyperchromatic and irregular. However, these histopathological changes were ameliorated in the 'yam gruel' group. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group.
3.5.2 Effect of 'yam gruel' on neurological damage
The neuroprotective effect of 'yam gruel' was demonstrated by the neurological deficit scores. Fig. 11 shows that after 24 hours of reperfusion, no neurological deficit was observed in the sham group, whereas the ischemia-reperfusion-treated rats showed different degrees of the neurological defect (P < 0.01). However, the neurological deficit score was significantly reduced after the administration of 'yam gruel' when compared with the model group (P < 0.05).
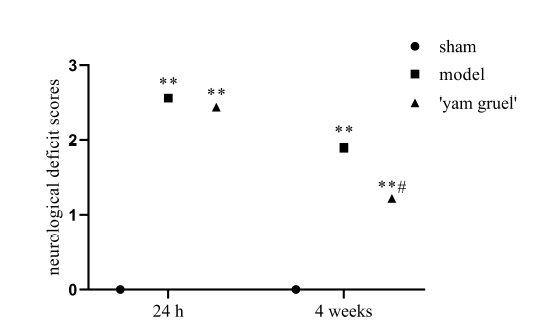 Figure 11.
Figure 11.Effect of 'yam gruel' on neurological damage. After 24 hours of reperfusion, there was no neurological deficit in the sham group, while the ischemia-reperfusion treated rats showed different degrees of neurological defects. However, following the administration of 'yam gruel,' the score of the neurological deficit rats was significantly reduced compared to the model group. Values are the means ± SD, n = 9. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
3.5.3 Effect of 'yam gruel' on spatial learning and memory ability
As shown in Fig. 12, when compared with the sham group, the escape latency of the model group was prolonged, and the number of times crossing the position of the original platform was decreased (P < 0.05). On the contrary, 'yam gruel' supplementation shortened escape latency and increased the number of platform crossings (P < 0.05).
 Figure 12.
Figure 12.Effect of 'yam gruel' on spatial learning and memory ability. (A) The escape latency of each group; (B) The number of times crossing the position of the original platform of each group. The escape latency was prolonged, and the number of times crossing the position of the original platform was decreased in the model group when compared with the sham group. However, compared with the model group, the escape latency was shortened, and the number of times crossing the position of the original platform was increased in the 'yam gruel' group. Values are the means ± SD, n = 9. sham = sham group; model = model group; and 'yam gruel' = 'yam gruel' group. Symbols show significant differences between the following groups: *P < 0.05 and **P < 0.01 vs. sham group; #P < 0.05 and ##P < 0.01 vs. model group.
The effects of 'yam gruel' on the cognitive function of diabetic rats with focal cerebral ischemia-reperfusion injury were studied, and the mechanism of the role of the gut-brain axis in this process explored. Results showed that the cognitive function of diabetic rats with focal cerebral ischemia-reperfusion injury was impaired. Gut microbiota and their metabolites, oxidative stress, inflammatory response, neurotransmitter, and BDNF and cognitive function-related indicators were significantly improved after the administration of 'yam gruel.' It is suggested that 'yam gruel' improves the cognitive function of diabetic rats with focal cerebral ischemia-reperfusion injury via the gut-brain axis.
'Yam gruel' was prepared from raw Dioscorea polystachya. Its effects may be related to the active components of Dioscorea polystachya in the 'yam gruel.' Dioscorea polystachya contains more than 20 active components, such as diosgenin, yam polysaccharide, allantoin, and mucus protein (Wu et al., 2016). The active components of yam have a variety of functions, including antioxidation, anti-inflammation, regulation of glycolipid metabolism, regulation of gut microbiota, and enhancement of body immunity (Gong et al., 2019; Go et al., 2015; Tohda et al., 2017). Studies of animal models of cognitive dysfunction have shown that 125 mg/kg of diosgenin gavage intervention for four weeks improves memory dysfunction and cognitive function in mice (Chiu et al., 2011). According to the diosgenin extraction (0.0918%) (Xue et al., 2019) and drying (26.5%) (Ning and Zhang, 2008) rates of raw Dioscorea polystachya, 136 g of dried yam, equivalent to 513 g of raw yam, is needed to extract 125 mg of diosgenin.
In the study reported here, every rat in the 'yam gruel' group was given 5 mL of 'yam gruel' made daily from 2.5 g raw Dioscorea polystachya, which was equivalent to 70.188 g raw Dioscorea polystachya. The dosage was then only 1/28th of that amount. In another study of cognitive dysfunction patients and healthy persons, taking 80 mg of diosgenin daily also improved cognitive function (Tohda et al., 2017). The above 80 mg diosgenin is equivalent to the extract of 87 g dry yam and 328 g raw Dioscorea polystachya. In a previous study, patients with T2DM took 'yam gruel' made from 125 g raw Dioscorea polystachya once a day for three consecutive months of intervention. This process increased the contents of Bifidobacterium in the intestinal tract of T2DM patients, improved oxidative stress, and reduced blood glucose levels (Pang et al., 2017a,b). 'Yam gruel' provides a variety of yam components, and a complementary superposition effect may exist between them. Hence, taking less 'yam gruel' made from raw Dioscorea polystachya every day may provide a better effect.
Gut probiotics help regulate brain function and behavior. Known as the gut-brain axis, a distinctive two-way communication channel that involves neural, endocrine, and inflammatory mechanisms are observed between the gut and the brain (Hamidi et al., 2019; Martin et al., 2018). This axis closely links the gut, gut microbiota, and brain function and can effectively improve cognitive function by participating in oxidative stress and inflammatory responses (Hoffman and Lumpkin, 2018; Xu et al., 2017). In the present research, 'yam gruel' adjusted the composition of gut microbiota in rats, enhanced the relative abundance of SCFAs-producing bacteria, such as Lactobacillus, Ruminococcus, and Clostridium (Koh et al., 2016) and increased SCFAs content. Thus, 'yam gruel' may function through the gut-brain axis based on regulating the composition of gut microbiota and its metabolites, alleviating oxidative stress and inflammatory response, further up-regulate the expression of neurotransmitter and BDNF and finally improve cognitive function in rats.
Oxidative stress is involved in the pathophysiological process of diabetic vascular complications and stroke (Zhang et al., 2017). Currently, clinical trial and animal studies have shown that after stroke, the body will consume antioxidant markers, such as SOD and glutathione peroxidase; generate related oxidation products, such as MDA and sequester oxygen free radicals in the brain, leading to an excessive occurrence of oxidative stress, thus aggravating brain injury (Fang et al., 2018). After diabetes associated with focal cerebral ischemia-reperfusion, oxidative stress affects brain function and aggravates cognitive dysfunction. Relevant studies have shown that probiotics, such as Lactobacillus, Clostridium butyricum, and SCFAs have antioxidant effects that reduce oxidative stress, alleviate brain damage and improve cognitive dysfunction (Davari et al., 2013; Kim et al., 2018; Liu et al., 2017; Swidsinski et al., 2012). In the research described here, treatment with 'yam gruel' regulated the composition of gut microbiota and reduced the FBG levels of diabetic rats with focal cerebral ischemia-reperfusion. In terms of oxidative stress, the levels of SOD increased, whereas levels of MDA decreased, and oxidative stress in rats was alleviated.
Moreover, HE staining showed cell necrosis in the model group that was significantly improved by a 'yam gruel' diet. This finding suggests that 'yam gruel' can both effectively reduce the blood glucose levels of diabetic rats with focal cerebral ischemia-reperfusion and participate in the oxidative stress response after apoplexy, thereby improving brain injury and beneficially affecting cognitive function. Such effects may be mediated via the gut-brain axis.
Stroke led to a decrease of probiotics and an increase of pathogenic bacteria in the gut and then induced increased LPS production and intestinal permeability. LPS stimulated the secretion of inflammatory factors IL-1, interleukin 6 (IL-6) and TNF-α through NF-κB, thus aggravating the inflammatory response (Chen et al., 2019). Inflammatory responses have been reported to aggravate progressive neurological injury in ischemic stroke (Fu et al., 2015; Nakata et al., 2017). The latter two studies have shown that some enteroendocrine cells directly form intestinal epithelial-neural synapses with vagus neurons, which transmit inflammatory signals to nerve cells and affect brain function (Hoffman and Lumpkin, 2018; Kaelberer et al., 2018). Gut microbiota may regulate brain function with the help of the gut-brain axis. Intestinal probiotics and SCFAs change the intestinal environment, inhibit the production of LPS (Kim et al., 2018; Tan et al., 2014), reduce the release of pro-inflammatory factors, such as IL-6 and TNF-α and protect BDNF. SCFAs reach the brain through the blood-brain barrier, play an anti-inflammatory role, participate in the regulation of relevant neurotransmitters (for example GABA, 5-HT) and consequently affect development, aging and necrosis or apoptosis of important cognitive functional areas, such as the hippocampus (Braniste et al., 2014). In the present research, 'yam gruel' increased the SCFAs content, decreased the levels of LPS, TNF-α, and IL-1β and up-regulated the expression of BDNF, GABA, and 5-HT by adjusting the composition of gut microbiota. These results suggest that through the gut-brain axis, supplementation by 'yam gruel' could improve the inflammatory response and protect cognitive function after stroke.
In the research described here, 'yam gruel' was used as an intervention in diabetic rats with focal cerebral ischemia-reperfusion injury. 'Yam gruel' mediates gut microbiota and its metabolites, regulates oxidative stress, and inflammatory response promotes the expression of neurotransmitter and BDNF and improves cognitive function. The production of 'yam gruel' is simple, its cost is low, it is easy to administer, and it was found to be effective in this experiment. Clinical trials should be conducted to explore the effectiveness of 'yam gruel' in diabetic patients with focal cerebral ischemia-reperfusion injury.
Shuqin Pang and Zongting Luo designed the present research. Carol Chunfeng Wang reviewed and revised the grammar of the manuscript. Xuepei Hong, Jian Zhou, Fang Chen, Li Ge, and Xia Li performed the experiments. Yanling Dai, Yilan Wu, and Jiahui Zhang analyzed the data. Shuqin Pang and Zongting Luo wrote the manuscript. All authors read and approved the final version of the manuscript.
The present research was approved by the Ethics Committee of Fujian University of Traditional Chinese Medicine (Fuzhou, P. R. China). Informed consent was obtained.
We acknowledge the support of fund 2015Y2001-51/Science and Technology Platform Construction Project of Fujian Science and Technology Department, 81904269/National Natural Science Foundation of P. R. China.
The authors declare no conflict of interest.
