Multiple sclerosis is a progressive autoimmune disorder of the myelin sheath and is the most common inflammatory disease of young adults. Up to 65% of multiple sclerosis patients have cognitive impairments such as memory loss and difficulty in understanding and maintaining attention and concentration. Many pharmacological interventions have been used to reverse motor impairments in multiple sclerosis patients; however, none of these drugs improve cognitive function. Melatonin can diffuse through the blood-brain barrier and has well-known antioxidant and anti-inflammatory properties with almost no side effects; it is, therefore, a promising neuroprotective supplement for many neurological diseases, such as multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, ischemic stroke, and fibromyalgia. However, only some researches have assessed the effect of melatonin on cognitive dysfunction in multiple sclerosis. Here, we evaluated the effects of melatonin supplementation on memory defects induced by cuprizone in a mouse model of multiple sclerosis. Cuprizone (400 mg/kg) and melatonin (80 mg/kg) were administered to SWR/J mice daily for 5 weeks. Open field, tail-flick, and novel object recognition behavioral tests were performed. Also, expression of cAMP-response element-binding protein, synaptophysin, and postsynaptic density protein 95 were measured in the prefrontal cortex. Melatonin significantly improved the memory defects induced by cuprizone toxicity by up-regulating cAMP-response element-binding protein and by increasing expression of the synapse-associated synaptophysin and postsynaptic density protein 95 genes in the prefrontal cortex. These results indicate that melatonin may provide protective effects against memory impairments associated with multiple sclerosis.
Multiple sclerosis (MS) is a progressive inflammatory autoimmune disease of the myelin sheath that affects around 2.3 million people worldwide (Browne et al., 2014). It is the most common demyelination disorder of the central nervous system (Kashani et al., 2014). Demyelination disrupts the central nervous system signals, leading to various functional abnormalities associated with the affected neurons (Goldenberg, 2012). Moreover, demyelination lesions affect cognitive function in 65% of MS patients (Rahn et al. 2012). However, episodic memory loss and slow information processing speed, which correlate with working memory defects, are the most common mental and cognitive changes seen in MS patients (Mousavi et al., 2018; Sumowski et al., 2018).
Cognition can be described as any form of mental function, intellectual activity, or information processing (Wessinger and Clapham, 2009) with memory forming the basis of these activities (Brod et al., 2013; Unsworth et al., 2009). Memory is a highly complicated process that requires multiple synapses and the coordinated action of many cytoplasmic and nuclear proteins (Takeuchi et al., 2013). There are two broad types of memory, short- and long-term memory, which are subdivided into sensory and working memory, and declarative and non-declarative memory, respectively (Barrett et al., 2010). Each type of memory has a particular mode of action involving various synaptic and cellular events (Takeuchi et al., 2013). Disruption to synaptic plasticity strongly correlates with impaired learning and memory (Peng et al., 2017). cAMP-response element-binding protein (Creb), synaptophysin (Syp), and postsynaptic density protein 95 (Psd95) are proteins that have a fundamental function in axonal transport, synaptic plasticity, and neurogenesis (Ortega-Martínez, 2015). Downregulation of such proteins and focal demyelination in specific brain regions, such as the hippocampus and prefrontal cortex (PFC), are responsible for the cognitive impairments seen in MS patients (Nickel and Gu, 2018). Also, demyelination of the axons that connect the PFC with the hippocampus leads to memory dysfunction (Nickel and Gu, 2018).
Cuprizone (biscyclohexanone oxaldihydrazone) is a copper chelator that is commonly used to induce artificial demyelination in different animal species through oligodendrocyte depletion, microglial activation, and astrocytosis (Abo Taleb and Alghamdi, 2019). Memory impairments are one of the most noticeable effects of cuprizone toxicity (Nickel and Gu, 2018). Other toxic effects of cuprizone include weight gain reduction, a significant decrease in motor activity, oxidative stress, and neuroinflammation (Abo Taleb and Alghamdi, 2019).
Melatonin (N-acetyl-5-methoxytryptamine) is a neurohormone that is mostly produced by the pineal gland (Hevia et al., 2015). It is a small, amphiphilic, nonpolar molecule, that has broad regulatory and neuroprotective functions (Alghamdi, 2018a; Hardeland and Pandi-Perumal, 2005). Melatonin receptors one and two (MT1 and MT2) are present at varying levels in different layers of the cerebral cortex. MT1 receptors are abundant in layers II, III, and V, while a small amount of the MT2 receptor is found in layers V and VI (Ng et al., 2017). Although the physiological function of melatonin in these layers remains unknown, some evidence indicates a role in protecting cognition and preserving memory processes in neurodegenerative diseases (Ng et al., 2017). The biological functions of melatonin are not limited to actions mediated by its receptors. They are not defined by a single pathway because various targets are involved, such as transduction pathways (Shah et al., 2019). Receptor-independent neuroprotective functions of melatonin have been demonstrated, involving antioxidant and anti-inflammatory properties. It acts as a scavenger of free radicals, reduces the production of reactive oxygen species, stimulates the formation and activity of antioxidant enzymes, including catalase, superoxide dismutase, and glutathione peroxidase, and decreases lipid peroxidase levels (Hevia et al., 2015).
Furthermore, melatonin inhibits cyclooxygenase enzyme function and reduces the production of inflammatory cytokines (Alghamdi, 2018a; de la Rocha et al., 2007; Hevia et al., 2015). Moreover, melatonin enhances synaptic growth and protects the neural and glial structures (Alghamdi, 2018a). Recent animal studies have revealed that melatonin reduces memory impairments and neuroplasticity deficits by upregulating Notch1 signaling (Zhang et al., 2016), regulates cognitive function by upregulating the levels of synaptic proteins (Rehman et al., 2019), enhances synaptic integrity by ameliorating stress-activated protein kinase expression, oxidative brain damage, neuroinflammation, and neurodegeneration (Muhammad et al., 2019), attenuates long-term memory defects by calcium channel modulation (Ran et al., 2018), and enhances cognitive deficits by the restoration of myelin and elevating oligodendrocyte function (Chen et al., 2018).
The PFC and hippocampus are the main regions involved in memory processes and are associated with cognitive impairments in MS patients. While some studies have investigated the effect of melatonin on protein levels in the hippocampus, the effect of melatonin on protein levels in the PFC remains poorly understood. We, therefore, designed this study to assess the effect of melatonin in a cuprizone model of MS using a memory test and expression analysis of Creb, Syp, and Psd95.
SWR/J mice (male, 20-25 g, King Fahad Medical Research Center, Jeddah, SA) with a total number of 30 were housed in standard laboratory conditions at 20 to 22 °C with a 12-12 hour light/dark cycle. The mice were given free access to chow and water. The animals in each group were marked with different numbers and colors for identification. Male mice were used to exclude any effect of fluctuating hormones (Beery and Zucker, 2011). All animal experiments followed the regulations of the Animal House committee of the King Fahad Medical Research Center, Jeddah, SA.
Carboxymethyl cellulose (CMC) with a medium viscosity, cuprizone, and crystalline melatonin were purchased from Sigma Aldrich (St. Louis, Missouri, U.S.). A 1% CMC solution was prepared by adding 1 g CMC powder to 100 ml of hot water (60 °C) (Lan et al., 2018). Each day, cuprizone powder (400 mg/kg) was solubilized with CMC solution (cuprizone-CMC) for oral gavage. We administered cuprizone orally to ensure dose consistency among mice to limit the variation of toxic effects (Zhen et al., 2017). Melatonin with a dose of 80 mg/kg was solubilized in normal saline, and intraperitoneally (IP) administrated to mice in a volume of 0.5 ml at around 11:00 a.m. daily, thereby limiting variations in circadian rhythm among the experimental animals. The dose of melatonin was selected as a higher protective dose (Álvarez-Sánchez et al., 2015), and the route of administration was chosen because melatonin is well absorbed after IP injection (Gul et al., 2018).
The total duration of the study was 5 weeks. The 30 mice were randomly assigned into three groups, ten mice in each. Group 1. Control: mice were administrated 0.2 ml CMC orally and IP-injected with 0.5 ml normal saline. Group 2. Cuprizone (CPZ): mice received 0.2 ml cuprizone-CMC orally and were IP-injected with 0.5 ml normal saline. Group 3. Cuprizone + melatonin (CPZ + MLT): mice received 0.2 ml cuprizone-CMC orally and were IP-injected with 0.5 ml freshly prepared melatonin. These were administered once daily for the duration of the experiment.
2.4.1 Tail flick test
The tail-flick test is a widely used technique to measure nociception induced by a thermal stimulus. We used a tail-flick operator (code: 37360, Ugo Basile, Italy), which generates high-temperature stimulus focused on the tail and automatically records the reaction time of the tail-flick. Each mouse was individually was placed in the restrainer at 45 degrees with the tail over the beam source, and the latency of tail-flick was recorded. A cut-off point of 10 s was adopted to avoid tissue injury (Hole and Tjølsen, 1993; Vakilzadeh et al., 2016; Yarushkina et al., 2018).
2.4.2 Locomotor activity (velocity, total distance moved)
An open field test (45 × 45 cm) and EthoVision system XT8A (Noldus, Wageningen, The Netherlands) were utilized for automated calculation of velocity (cm/s), and total distance moved (cm) for each mouse during 3 minutes (Abo Taleb and Alghamdi, 2019; Vakilzadeh et al., 2016).
2.4.3 Novel object recognition test
The novel object recognition test (NORT) has been widely used to assess short-term memory deficits in mice without stress or rewards (Antunes and Biala, 2012). During the experiment, we ensured that the environment was noise-free and that the objects (novel and familiar) could be easily cleaned. Before the experiment, two habituation trials were performed in an empty arena for 10 min per mouse per day. The test was composed of two phases: the sample phase and the test phase (Fig. 2a). In the sample phase, the mouse was placed in the arena for 3 minutes to explore two identical objects placed in the arena (both are familiar objects; FOs). During the test phase, one of the FOs was replaced by a new different object (novel object; NO). The exploration time for each object and the frequency of nose-pointing (sniffing frequency) in the familiar and novel zones were automatically calculated by the EthoVision tracking system. Memory was estimated by measuring the discrimination index (DI), which measures the ability of the mouse to discriminate against the NO as a new object. DI is calculated from the equation (TN - TF)/(TN + TF), where TF is the exploration time for FOs, and TN is the exploration time for NO (Alghamdi, 2018b; Moosavirad et al., 2016).
Five weeks after cuprizone and melatonin administration, mice were decapitated, and brain tissues were quickly removed and dissected on a dry cold plate. PFC samples were isolated and rinsed with ice-cold saline then kept in -80 °C for gene expression analysis.
To detect the expression of Creb, Syp, and Psd95 in the PFC, reverse transcription-polymerase chain reaction (RT-PCR) was performed. The primers for these genes and a housekeeping gene were obtained from the Macrogen Company (Seoul, South Korea) and used following the company’s instructions (Table 1).
| Gene Symbol | Primer | Sequence | Length | Amplicon Size |
|---|---|---|---|---|
| Creb | Forward | CCAAACTAGCAGTGGGCAGT | 20 | 166 bp |
| Reverse | TCCATCAGTGGTCTGTGCAT | 20 | ||
| Syp | Forward | ACCTCGGTGGTGTTTGGCTT | 20 | 127 bp |
| Reverse | CTGGTTGCTTTTCTGGGGCG | 20 | ||
| Psd95 | Forward | GACAACCCACACATCGGTGA | 20 | 168 bp |
| Reverse | CTCTTTGAGGGCCTCCACTG | 20 |
2.6.1 RNA extraction from tissue
RNA was extracted from tissues in 600 µL lysis buffer (RLT) + 6 µL B-mercaptoethanol in 1.5 ml tubes. The samples were homogenized using an IKA homogenizer. 70% ethanol (600 µL) was then added, and the sample vortexed. RNA was isolated using an RNeasy mini kit (Qiagen-250; Lot number:74106) following the manufacturer’s protocols (Qiagen, Hilden, Germany).
2.6.2 Reverse transcription-polymerase chain reaction (RT-PCR)
A first PCR master mix was prepared in 5 μl (total volume) which included 2-4 µl experimental RNA, 0.5 µl primer [Oligo (dT) 15], 0.5 µl random primer, and 0-2 µl nuclease-free water. The samples were then put in a thermal cycler for 5 minutes at 70 °C. Then, a second PCR master mix was prepared in a total volume of 15 μl and included 4.0 µl ImProm-II™ 5X Reaction Buffer, 1.2 µl MgCl2 (final concentration 1.5-8.0 mM), 1.0 µl dNTP Mix (final concentration of 0.5 mM for each dNTP), 1.0 µl ImProm-II™ reverse transcriptase, and X µl of nuclease-free water (to a final volume of 15 µl). The transcriptase was activated at 25 °C for 5 min, then incubated at 42 °C for 120 min, followed by 15 min denaturation at 70 °C.
2.6.3 Calculation of relative expression level
Gene expression levels were evaluated by quantitative real-time PCR using the housekeeping gene, Actb (β-actin), as an internal control. Relative expression was calculated by the 2 -△△Ct method.(Rao et al., 2013)
All statistical values are stated as the mean ± standard error (SEM) for behavioral tests and the mean ± standard deviation (SD) for gene expression results. The data were analyzed by analysis of variance (ANOVA) followed by Tukey’s post hoc test using GraphPad Prism 8.3.0 software (GraphPad Inc., La Jolla, CA, USA). CPZ and control groups were compared, as were CPZ and CPZ + MLT groups. A significant difference was quantified when P values < 0.05.
Nociception was evaluated in all groups by the tail-flick test. Cuprizone-induced MS model mice compared with control mice showed no significant difference in nociception (P = 0.9249, n = 10), Fig. 1a. However, mice injected with melatonin had a significantly more extended latency period compared with the CPZ group (P = 0.0137, n = 10). Interestingly, mice treated with melatonin also had a significantly more extended latency period compared with the control group (P = 0.0054, n = 10).
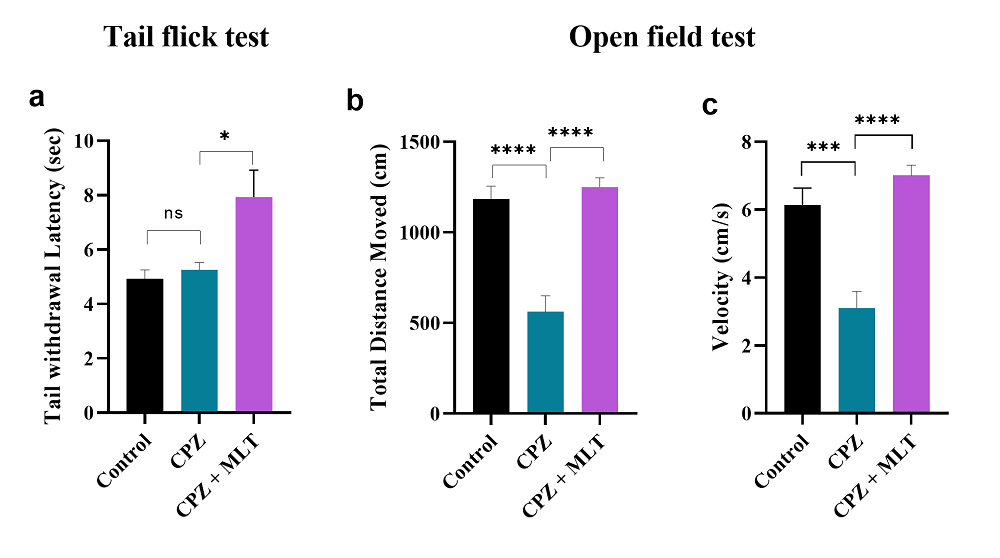 Figure 1.
Figure 1.Tail flick test and locomotor activity result at the end of the study. a. Tail flick test: melatonin significantly enhanced the tail-flick latency in the CPZ + MLT group compared with the CPZ group. b. Total distance moved: significant improvement was seen in the total distance movement measured by the open field test in the CPZ + MLT group compared with the CPZ group. c. Velocity: significant improvement was seen in the velocity measured by the open field test in the CPZ + MLT group compared with the CPZ group. One-way ANOVA, followed by Tukey’s test, was used. Data are present as mean ± SEM, ns = non-significant *P < 0.05, ***P < 0.001, ****P < 0.0001.
Locomotor activity was measured as total distance moved and velocity of movement. Mice in the CPZ group moved a significantly shorter distance (P < 0.0001, n = 10; Fig. 1b) and were slower (P = 0.0001, n = 10; Fig. 1c) compared with the control group. However, CPZ + MLT mice moved significantly further (P < 0.0001, n = 10) and faster (P < 0.0001, n = 10) compared with CPZ group mice.
Short-term memory was measured by the NORT (Fig. 2a). Mice in the CPZ group showed a significant deficit in the DI compared with the control group (P = 0.0001, n = 10; Fig. 2b) but no significant difference in visiting and sniffing the novel object versus the familiar object (P = 0.4691, n = 10; Fig. 2c). However, DI was higher significantly in the CPZ + MLT mice compared with the CPZ group (P = 0.0414, n = 10; Fig. 2b) and the mice in CPZ + MLT sniffed the novel object more than the familiar object significantly during the test phase (P = 0.0302, n = 10; Fig. 2c).
 Figure 2.
Figure 2.Memory assessment of mice by a novel object recognition test. The novel object recognition test was composed of two stages: a. sample phase, which is a familiar object exposure phase, and a test phase, the duration between two phases is 10 minutes. (N) represents the novel object, and (F) represents the familiar object. b. Discrimination index measured the ability of the mouse to discriminate the novel object as a new object. Melatonin improved the discrimination index in CPZ + MLT group significantly compared with the CPZ group. c. Frequency of sniffing each object. During the sample phase, no difference was detected between groups in sniffing both familiar objects. In the test phase, melatonin improved the ability of mice to recognize the novel object as a new object, this is determined by a significant increase in the frequency of sniffing new (novel) object compared with a familiar object in the CPZ + MLT group. One-way ANOVA followed by Tukey’s test (b) and Two-way ANOVA with Tukey’s test (c) were used. Data are present as the mean ± SEM, ns = non-significant, *P < 0.05, ***P < 0.001.
The expression level of Creb in the PFC was reduced significantly in the CPZ mice (0.200 ± 0.001) compared with the control mice (P < 0.0001, n = 10; Fig. 3a). Melatonin-treated mice had a significantly higher Creb expression level in the PFC (0.702 ± 0.006) in comparison to the CPZ group (P < 0.0001, n = 10; Fig. 3a).
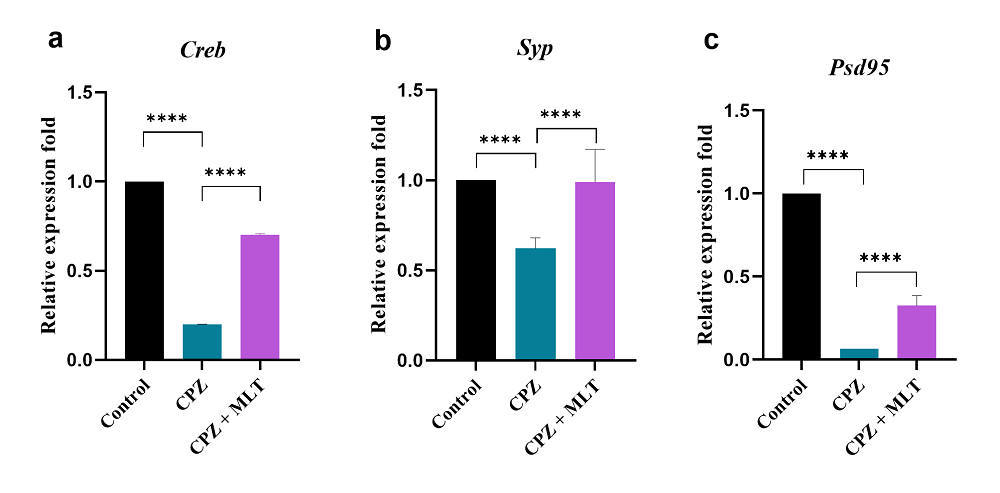 Figure 3.
Figure 3.Relative expression levels of Creb, Syp, and Psd95 in the prefrontal cortex of mice. Relative Creb expression levels in all groups. b. Relative Syp expression levels in all groups. c. Relative Psd95 expression levels in all groups. Cuprizone caused a significant reduction in relative expression levels of Creb, Syp, and Psd95 in the prefrontal cortex, while melatonin improved these levels significantly. All values were normalized against beta-actin. One-way ANOVA, followed by Tukey’s test, was used. Data are present as the mean ± SD, ****P < 0.0001.
Cuprizone administration induced significant reductions in the expression of Syp and Psd95 in the PFC (0.622 ± 0.059 and 0.065 ± 0.001; respectively) compared with the control mice (P < 0.0001, n = 10; Fig. 3b and 3c). CPZ + MLT group mice had significantly improved Syp and Psd95 expression levels in the PFC (0.989 ± 0.183 and 0.326 ± 0.060, respectively) in comparison to the CPZ group (P < 0.0001, n = 10, Fig. 3b and 3c).
Cognitive impairment is a major social challenge that affects around 65% of MS patients (Jongen et al., 2012). Many studies have identified cognitive impairment in MS patients in the form of loss of memory (40-50%), difficulty in maintaining attention (25%), decreased information processing speed (20-30%), and impaired executive functioning (19%). These symptoms are significantly correlated with their disability status (Guimarães and Sá, 2012). Specifically, long-term memory impairments and working memory loss are the most common memory dysfunctions seen in MS patients (Guimarães and Sá, 2012). Nevertheless, the pathophysiology of cognitive dysfunction in MS has not been fully clarified. However, several studies suggest a disconnection phenomenon between demyelinated and secondary brain regions as the main mechanism of cognitive impairment in MS. A correlation between cognitive disability and the location of specific demyelinated lesions was also revealed (Calabrese et al., 2013). Up to 30% of brain demyelination lesions in MS occur in the hippocampus, while 28% affect the temporal and PFC (Calabrese et al., 2013). Synaptic dysfunction resulting from inflammation is another cause of cognitive dysfunction in MS (Yuan et al., 2015).
We previously confirmed the proficiency of melatonin to decelerate demyelination, accelerate endogenous remyelination, and preserve motor behavior in a mouse model of MS through its anti-inflammatory and antioxidant properties (Abo Taleb and Alghamdi, 2019). In the present study, we have shown another neuroprotective effect of melatonin on cuprizone-caused memory deterioration. Melatonin is a promising supplement for the mild cognitive impairment stage of Alzheimer’s disease, and its benefits have been demonstrated in clinical trials (Cardinali et al., 2012). Moreover, melatonin attenuated spatial memory impairment (Tongjaroenbuangam et al., 2013) and has a positive effect on depressive and anxiety-like behavior (Ergenc et al., 2019).
However, there are contradictory results as to whether melatonin has any effect on cognitive decline in MS (Gholipour et al., 2014; Roostaei et al., 2015). In the present study, the memory impairment was ameliorated by upregulating expression of a presynaptic and postsynaptic associated proteins gene (Syp and Psd95), as well as by increasing Creb expression in the PFC.
Nociceptive pain is a common MS symptom that includes painful tonic spasms and musculoskeletal pain related to corticospinal tract involvement (Berra et al., 2019). In the current study, the pain threshold of the tail-flick test did not change by cuprizone administration, as reported previously (Vakilzadeh et al., 2016). Melatonin administration significantly increased the nociception latency, confirming the anti-nociceptive effect of melatonin observed in previous studies (Vakilzadeh et al., 2016). Melatonin appears to increase the anti-nociceptive effects through the brain and spinal cord MT1 and MT2 receptors as well as by interaction with other receptors, such as those of the N-methyl-D-aspartate receptor (NMDA), dopaminergic and GABAergic systems, and the nitric oxide-arginine pathway (Kuthati et al., 2019; Srinivasan et al., 2012). However, the importance of the PFC in pain processing was confirmed in several studies where the PFC was shown to arbitrate antinociceptive effects (Ong et al., 2019).
We also considered the motor activity outcomes to ensure the induction of the MS model and to investigate the parallel effects of melatonin on motor activity impairment and memory dysfunction, which are both common in MS patients (D'Orio et al., 2012). Our results demonstrated that the administration of cuprizone for 5 weeks significantly reduced the total distance moved and the velocity of movement, while melatonin antagonized the toxic effects of cuprizone on motor activity, which is in agreement with our previous study (Abo Taleb and Alghamdi, 2019).
We also used a recognition memory dependent task (the novel object recognition test) to examine the effects of cuprizone and melatonin on working memory. This test depends on the spontaneous exploratory behavior of mice to evaluate novelty preference and has been carried out to assess prefrontal dependent recognition memory (Antunes and Biala, 2012; Barker et al., 2007). Mice with an intact PFC region are expected to have a greater preference for novel over familiar objects, while prefrontal aberrations result in no preference (Pezze et al., 2017; Reid et al., 2013; Warburton and Brown, 2015). The present findings reveal that cuprizone diminished recognition memory by causing a significant decrease in DI without any difference in exploratory times between familiar and novel objects, which is consistent with results from previous studies (Kondo et al., 2016; Serra-de-Oliveira et al., 2015). However, recognition of memory defects induced by cuprizone was reversed by melatonin administration. Melatonin significantly increased the DI and improved the ability of mice to recognize novel objects in our model, which confirmed the positive effects of melatonin therapy on memory outcomes (He et al., 2013; Osier and Dixon, 2013).
Activity-dependent synaptic plasticity generated at appropriate synapses is important in the encoding, formation, and storage of memories. This complex activity that occurs at individual synapses is dependent on the type of memory required, as well as on the brain area responsible for such activation. Many cellular events, mediators, proteins, neurotransmitters, receptors, and ions are involved in optimizing the potency of connections between neurons, which underlies the ability of the brain to store information (Takeuchi et al., 2013). The PFC and hippocampus are the most important brain regions involved in cognition and memory; the PFC controls cognitive function, non-declarative long term memory, and working memory (Funahashi, 2017; Lara and Wallis, 2015), while the hippocampus mediates declarative long term memory (Eichenbaum, 2001). Moreover, the connections between the PFC, hippocampus, and parahippocampal regions are involved in encoding working memory (Barrett et al., 2010).
In our study, we examined the relative expression levels of Creb, Syp, and Psd95 in the PFC because few studies have investigated synaptic plasticity in the PFC after cuprizone and melatonin administration. Creb is an intranuclear memory modulator protein that participates in neuronal plasticity and neurogenesis (Ortega-Martínez, 2015). Moreover, Creb is a transcription factor that plays a central role in the formation and consolidation of new memories (Ortega-Martínez, 2015) and is a key control protein for long term memory formation. At the synapse, the biochemical response to external stimuli produces varied signaling strength from one neuron to another, which is called synaptic plasticity. This response (if sufficient, for example, by repeated stimulation) reaches the cell nucleus. It activates transcription factors such as Creb leading to synaptic growth, synaptic efficacy, and maintenance of optimal synaptic connectivity between neurons in defined brain circuits, thereby leading to the development of long-term memory (Tully et al., 2003). In our study, cuprizone decreased the expression level of Creb as previously shown (Kim et al., 2019), and melatonin significantly reversed this effect. However, in previous studies, melatonin increased Creb levels in the hippocampus (Ali and Kim, 2015; Kim et al., 2019; Lee et al., 2018; Peng, 2015; Yoo et al., 2012). To our knowledge, no studies have demonstrated a relationship between melatonin administration and Creb levels in the PFC of MS model mice.
We also investigated the expression of Syp in the PFC; Syp is a presynaptic glycoprotein present in the membranes of neurotransmitter-containing presynaptic vesicles and has an important role in synaptic plasticity as well as in the regulation of neurotransmitter release (Frick and Fernandez, 2003; Reddy et al., 2005). It is commonly used as a protein marker for synaptic plasticity (Counts et al., 2006; Reddy et al., 2005). Increased Syp levels are associated with long-term potentiation, learning, and memory (Wheeler et al., 2002). Conversely, downregulation of Syp in the hippocampus and frontal cortex is associated with cognitive impairments (Hajjar et al., 2013; Reddy et al., 2005). In our study, cuprizone significantly decreased Syp expression, as previously shown (Chami et al., 2017). Melatonin significantly increased the expression level of Syp in the PFC, which is consistent with results from previous studies confirming the beneficial effect of melatonin on Syp levels in multiple brain regions (Ali and Kim, 2015; Peng, 2015; Shah et al., 2019; Tongjaroenbuangam et al., 2013; Yoo et al., 2012).
Lastly, we assessed the PFC expression of Psd95, which is a member of the membrane-associated guanylate kinase family that is proposed to regulate the synaptic structure, transmission, stability, and plasticity as well as the formation of long-term memory (Cały et al., 2019). Psd95 is a major regulator of α-amino-3-hydroxy-5-methyl-4-isox-azoleproprionic acid (AMPA) and NMDA receptors (Coley and Gao, 2018) which are present on the surface of the postsynaptic neuron. AMPA and NMDA receptors initiate many forms of synaptic plasticity which strengthen the synapse and, therefore, control memory function (Li and Tsien, 2009). In our study, cuprizone significantly decreased Psd95 expression, as previously shown (Araújo et al., 2017). Melatonin significantly increased the relative expression of Psd95 in the PFC, which was consistent with a previous study showing the beneficial effect of melatonin on Psd95 levels in the hippocampus (Ali and Kim, 2015; Peng, 2015), cortex, and striatum (Shah et al., 2019).
Overall, this and previous studies confirm a protective role of melatonin against cognitive impairment and provide evidence that melatonin can be used as a protective supplement for memory decline in MS through its ability to increase synaptic plasticity.
This study used a limited number of synapse markers to evaluate the effect of melatonin on the PFC. Additional markers for oxidative stress and inflammation and immunohistochemistry in the PFC may be useful for assessing the exact pathophysiology of memory defects in the PFC in MS and for investigating the effect of melatonin in this specific region. The dose of melatonin used in this study, and several previous animal studies (80 mg/kg) needs to be qualified with a proper human supplementation dose to be used in the future human study.
The present findings propose that melatonin supplementation could provide a protective impact against memory impairments in MS. This effect may be facilitated by the upregulation of Creb and increased levels of synapse-associated proteins in the PFC.
All mouse experiments followed the protocols and guidelines of the Animal Unit Department at King Fahad Medical Research Center, Jeddah, SA. The study was approved by the Unit of Biomedical Ethics, Faculty of Medicine, King Abdul-Aziz University, Jeddah, SA (Reference No. 613-18).
This study was conducted at the Pre-Clinical Research Unit at King Fahad Medical Research Center and was funded by King Abdul-Aziz University, Jeddah, SA. We thank Jeremy Allen, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
The authors declare no conflict of interest.
