†These authors contributed equally.
Active compounds and corresponding targets of the traditional Chinese herb, jiaweisinisan, were obtained from systems pharmacological database and placed into ClueGO for gene ontology analysis. The targets of depression were obtained from the Online Mendelian Inheritance in Man, the Therapeutic Target Database, and the Pharmacogenomics Knowledge Base. Compound-target and target-pathway networks were constructed using Cytoscape, and then their topological parameters were analyzed. The targets of jiaweisinisan and depression were mapped to pathways, thereby constructing antidepressant pathways of jiaweisinisan. It was found that jiaweisinisan has 82 different active compounds and 306 relevant potential targets. Also, 107 unrepeatable targets related to depression were found. In all, 26 common targets were found to be the direct anti-depression targets of jiaweisinisan and 9 pathways of jiaweisinisan related to depression were divided into three modules (synaptic transmission, cell apoptosis, and immune-inflammatory). The jiaweisinisan formula was found to have synergistic antidepressant effects due to aspects of its herb composition and the active compounds therein, giving rise to potential targets and signaling pathways related to depression. Its antidepressant mechanisms were found to mainly involve the regulation of synaptic transmission, cell apoptosis, and immune-mediated inflammation.
Depression is a common chronic mental illness worldwide. Its core symptoms include low mood, diminished interest, loss of pleasure, feelings of guilt, worthlessness, low self-esteem, and lack of energy, along with other problematic changes such as disorders of sleep and appetite, and disturbance in body weight (Battle, 2013). According to the World Health Organization, depression is set to replace cardiovascular disorders as the leading cause of disability by the year 2030. It will account for 13% of the total burden of disease (Levav and Rutz, 2002). The pathogenesis of depression is understood to be complex, influenced by both genes and the environment. Various hypotheses have sought to encapsulate the biological aspect of the disorder, centering around the monoaminergic neurotransmitter system, neurotrophic factors, and hippocampal neurogenesis, among others. However, while several biomarkers have been identified in support of leading theories, current understanding suggests that depression is not a single disease. Instead, it seems to be related to multiple risk genes interacting with environmental risk factors, yielding a spectrum of outcomes ranging from depressive symptoms to full-blown major depressive disorder (Kennis et al., 2020; Serafini et al., 2015). Despite a variety of antidepressant drugs currently on the market, there remains around 50% of patients who fail to respond to a single target antidepressant clinically.
Furthermore, many of the antidepressants used in the clinical setting are problematic in terms of both effectiveness and tolerability, with issues including delayed onset and severe adverse reactions and side effects (Papakostas, 2010). Thus, exploring alternative and complementary therapeutic options has been a topic of growing research interest. Traditional Chinese medicine (TCM) is one such area providing the potential for novel clinical therapies of depression.
TCM is well known as a system of treating a variety of diseases that have been employed for many thousands of years. It remains one of the primary approaches to healthcare today internationally, as well as continuing to garner considerable research interest (Tang et al., 2009). Natural products, including TCM herbs, have the characteristics of being both multi-component and multi-targeting. As such, each biochemically active component possesses low activity, but also low toxic or side effects, relative to synthetically created single-component drugs, which typically act predominantly on a single target. Hence, TCM can also reasonably be used as long-term therapies, such as in the treatment of chronic diseases. Given the growing awareness that depression is a multifactorial disease with heterogeneous characteristics, multi-targeting TCM could play a better antidepressant role than single-component therapies. Also, some TCM formulae are very well characterized clinically, because of the treatment experience that has been accumulated over thousands of years. For example, Sinisan, which forms the basis of jiaweisinisan (JWSNS), is a classic anti-mental illness formula commonly used to treat depression (see, e.g., Ng et al., 2017a,b).
Jiaweisinisan comprises seven commonly used Chinese herbs, namely, Bupleurum chinense DC (Radix bupleuri, RB), Paeonia lactiflora Pall (Paeoniae radix alba, PRA), Citrus × aurantium L (Aurantii fructus, AF), Gardenia jasminoides J. Ellis (Gardeniae fructus, GF), Lycium afrum L (Lycii fructus, LF), Rehmannia glutinosa (Gaertn.), DC (Rhizoma rehmanniae, RR) and Haliotis diversicolor Reeve (Concha haliotidis, CH). JWSNS combines RR, LF, GF, and CH with a Sinisan base. Sinisan is a classic TCM formula used in the treatment of emotional diseases. The use of JWSNS in the prevention and treatment of depression has been documented clinically, described as alleviating the clinical symptoms of patients, as well as reducing the frequency of illness exacerbation and side effects (Zhong et al., 2008). Also, our group has conducted pre-clinical research into the efficacy of JWSNS, providing evidence that it can improve pessimistic and desperate behaviors in a rat model of depression, as well as influencing the hypothalamic-pituitary-adrenal (HPA) axis, known to play an essential role in the pathogenesis of several mood disorders, including depression (Shi et al., 2008; Wu et al., 2007). Moreover, JWSNS has been shown to improve hippocampal neuron damage caused by excitatory amino acid toxicity, as well as regulating brain-derived neurotrophic factor (BDNF) and promoting hippocampal neurogenesis, thereby preventing and treating hippocampal neurogenesis disorders of depression (Wu et al., 2013; Yan et al., 2016, 2009). The antidepressant effect of JWSNS has been verified in both clinical and animal experiments. Yet, the pharmacological mechanisms of action and active components of JWSNS remain poorly understood, therefore needing further investigation.
It is generally known that TCM possesses diverse compounds. The structure and biological activities components within any single herb are distinct, and different herbs possess distinct compound profiles. During the past decade, numerous pharmacological studies have been carried out with the aim of TCM identification, and to determine the principal bioactive components of TCM. This process has proven to be resource-intensive and time-consuming. As a synergistic system, the multi-ingredient and multi-target nature of TCM formulae pose a considerable challenge to the study of mechanisms of action. Yet the use and development of TCM drugs have been hampered by the lack of systematic studies. Therefore, a novel strategy is urgently needed to identify the active compounds, therapeutic targets, mechanisms, and the synergistic effects of herbal drugs more effectively. Systems pharmacology, based on systems biology, polypharmacology, and molecular network analysis, provides a possible strategy to elucidate the mechanism of action of multi-ingredient medicines. Using a systems pharmacology approach could not only identify compound-compound, compound-target, and target-disease interactions but could also more generally improve the understanding of the synergistic effects of herbs (Azmi, 2012; Liang et al., 2014).
This approach has been used in clarifying the roles of numerous TCM formulas in many complex diseases, such as the Radix Curcuma formula Xiao-Chai-hu-Decoction and Da-Chaihu-Decoction in cardiovascular diseases, or the Danshen Formula for cardiovascular disease treatment (Li et al., 2014a). Such results appear to support the rationality of the “Jun-Chen-Zuo-Shi” theory of complex TCM formulation, as well as promoting the understanding of the multi-ingredient interactions in these formulations. Moreover, they demonstrate that the systems pharmacology approach applies to the study of the synergistic effects of complex herbal formulations.
All compounds originating from constituents of the JWSNS formula were obtained from (1) literature sources; (2) T.C.M. Database@Taiwan (http://tcm.cmu.edu.tw; updated in 28th June 2012); (3) Chinese Academy of Sciences Chemistry Database (http://www.organchem.csdb.cn); and (4) Traditional Chinese Medicine Systems Pharmacology platform (TCMSP), see http://tcmspnw.com, which is a unique platform of systems pharmacology designed for herbal drugs. The TCMSP system provides up-to-date, detailed, and accurate data on pharmacokinetic absorption, distribution, metabolism, and excretion (ADME), as well as structural properties such as oral bioavailability (OB), molecular weight, intestinal epithelial permeability, drug-likeness (DL) and water solubility. It also provides drug targets and their associations with diseases (Ru et al., 2014). The information collected from these four databases was eventually put into TCMSP to search OB and DL values.
2.2.1 OB screening
Since OB describes the potential for a compound to be delivered into the systemic circulation, it is one of the most crucial pharmacokinetic parameters for drugs that are administered orally. OB is defined by the US Food and Drug Administration as the rate and extent to which an active moiety or ingredient is absorbed from a drug and rendered available at the action site (Barnes et al., 2008). Herein, ingredients with OB of 30% were selected as candidate molecules (Luo et al., 2016).
2.2.2 Calculation of DL
As a qualitative concept for drug screening, DL is estimated at the early stage of drug discovery to evaluate the structural similarity between drugs and compounds from the Drug Bank database (Ma et al., 2011). This model was established based on the Tanimoto similarity, defined below:
where a represents a new compound, and b is the average DL of all 6511 molecules from the Drug Bank database (https://www.drugbank.ca/) based on Dragon software descriptors. Compounds were selected if they met the threshold of DL ≥ 0.18 (Luo et al., 2016).
2.2.3 Literature
Employing the above screening criteria omits several candidate ingredients that were identified through the literature search. These candidate compounds were also included in the present study (Németh et al., 2003).
2.3.1 Targets of active compounds in jiaweisinisan
To predict multiple targets of potential active drugs, Yu et al. (2012) constructed a robust in silico model that integrates much genomic, chemical, and pharmacological data. TCMSP was used to identify the targets of active compounds. TCMSP depends on the systematic drug targeting strategy to identify possible targets, with a support vector machine score of 0.7 and a random forest score of 0.8 as thresholds. Then, by using the BLAST tool in the UniProt database (http://www.uniprot.org/blast), target-related genes were standardized, and targets from human were screened.
2.3.2 GO analysis for candidate targets of jiaweisinisan
We then employed ClueGO, a Cytoscape plugin (Bindea et al., 2009), to analyze the roles of the candidate targets as well as the associations of the functional groups with their scientific annotations in bionetworks. The obtained results were after that classified into biological processes/molecular functions and pathways. P ≤ 0.05 was considered statistically significant, and the hypergeometric test was performed to identify gene ontology (GO) terms that had been enriched.
2.3.3 Targets related to depression
Known targets related to depression were obtained from (1) OMIM Database (Online Mendelian Inheritance in Man; http://www.omim.org), (2) TTD Database (http://database.idrb.cqu.edu.cn/TTD), (3) PharmGKB Database (https://www.pharmgkb.org/index.jsp).
2.3.4 Direct antidepressant targets of jiaweisinisan
Targets of JWSNS were used to map to depression-related targets, from which the direct antidepressant targets of JWSNS were obtained.
To unravel the synergic effects and therapeutic mechanisms for JWSNS with multiple components, the active ingredients predicted above and corresponding targets were studied by (1) compound-target (C-T) network, where active ingredients are all associated with their targets; and (2) target-pathway (T-P) network, where compound-targets are related to pathways. C-T and T-P networks were established with Cytoscape software 3.2.1 (Shannon et al., 2003).
Based on current findings of depression, the pathways related to depression were screened out in JWSNS pathways. The targets of depression and JWSNS, as well as the direct antidepressant targets of JWSNS, were mapped to the pathways. Pathways extracted from the database of KEGG were manually graphed by Adobe Illustrator (Adobe Inc.).
A total of 834 compounds from seven herbs in JWSNS were collected and subjected to ADME screening (DL ≥ 0.18, OB ≥ 30), of which 98 potentially active compounds were identified. A further 22 compounds had sub-threshold DL or OB values but were also included in our analysis as active candidates, as they were cited as pertinent components in the literature. As such, a total of 120 compounds were identified as “active ingredients” (Table S1, Supplementary Information), including 19 from RB, 15 from PRA, 45 from LF, 8 from RR, 6 from Concha CH, 19 from GF and 8 from AF.
Among these active ingredients, five compounds - kaempferol, beta-sitosterol, quercetin, mandenol, and stigmasterol - were shared by various herbs of JWSNS (kaempferol in PRA, GF and RB; beta-sitosterol in PRA, GF and AF, LF; quercetin in GF, RB, and LF; mandenol in GF and LF; stigmasterol in GF, RB, and LF). Ultimately, 110 different active compounds were obtained.
3.2.1 Targets of active compounds in jiaweisinisan
In general, the effect of TCM in treating complex diseases is characterized by a synergy of multiple components and multiple targets. Therefore, not only the active components but also the therapeutic targets of JWSNS should be explored. TCMSP was used to predict potential targets of active compounds.
Because 28 out of the total of 110 active compounds did not have corresponding characterized targets (Table S2, Supplementary Information), it was only possible to include the remaining 82 candidate compounds in this analysis. Also, it was found that targets 117, 341, 142, 358, 374, 52, and 88 were connected with PRA, RB, AF, GF, LF, RR, and CH, respectively (Table S3, Supplementary Information). While the number of targets of compounds belonging to each herb varied, they significantly overlapped, and ultimately 306 non-repeating targets were finally obtained.
3.2.2 GO analysis for candidate targets of jiaweisinisan
To explore the biological processes and signaling pathways involved in the therapeutic effects of JWSNS, ClueGO was first applied to the GO analysis of the 306 candidate targets (Fig. 1; Tables S4 and S5, Supplementary Information). Biological processes analysis showed that there were 29 significantly enriched terms (P-value ≤ 0.05; Fig. 1A). Meanwhile, pathway analysis showed that 306 targets were classified into 22 pathways (Fig. 1B). According to the current knowledge of depression, 9 of these pathways (amine ligand-binding receptors, cellular senescence, transmission across chemical synapses, interleukin (IL) 4 and 13 signaling, intrinsic pathway for apoptosis, muscarinic acetylcholine receptors, neurotransmitter clearance, interleukin-10 signaling, and dopamine receptors) are considered to be tightly relevant to depression (Barre et al., 2016; Heuser, 2002) (Table S6, Supplementary Information).
 Figure 1.
Figure 1.Biological process (BP) and pathways of jiaweisinisan in GO analysis. Candidate jiaweisinisan targets enriched in the representative biological process are shown in A and pathway are shown in B. The Y-axis shows significantly enriched categories in GO relative to the target genes, and the X-axis shows the enrichment scores of these terms (P-value ≤ 0.05).
3.2.3 Targets related to depression
A total of 118 depression-related targets from databases were found: 23 in TTD Database, 33 in PharmGKB Database, and 62 in the OMIM Database (Table S7, Supplementary Information). And all targets were integrated into 107 unrepeatable targets.
3.2.4 Direct antidepressant targets of jiaweisinisan
By mapping to depression targets, 26 direct targets of JWSNS against depression were identified (Table S8, Supplementary Information).
In network topology analysis, degree, as an important topological parameter, represents the number of nodes from one node to others. Active components and potential targets can be assessed using this parameter. The higher the degree of the component, the more targets it is attached to. Moreover, the target with the higher degree plays a more critical role in the mechanisms.
3.3.1 C-T network
To systematically clarify the complicated interactions of active compounds with targets, a C-T network was established based on active compounds and targets of JWSNS. The C-T network included 395 nodes (7 herbs, 82 active compounds, and 306 potential targets) and 1571 C-T edges (Fig. 2). Kaempferol, quercetin, and beta-sitosterol possess high degrees of 186, 441, and 140, respectively. This indicated that they were important active compounds in JWSNS. In contrast, the average degree (number of associated compounds) of targets was 5.13. PTGS2/COX2 (Prostaglandin G/H synthase 2; degree = 47), ADRB2 (beta-2 adrenergic receptor; degree = 18), and BCL2 (B-cell lymphoma-2; degree = 15) were found to be the important targets of JWSNS.
 Figure 2.
Figure 2.C-T network. Orange nodes, green nodes, and blue nodes represent herbs, candidate compounds, and targets of jiaweisinisan, respectively. RB, Radix Bupleuri; P.R.A., Paeoniae Radix Alba; LF, Lycii Fructus; RR, Rhizoma Rehmanniae; CH, Concha Haliotidis; GF, Gardeniae Fructus; AF, Aurantii Fructus.
3.3.2 T-P network
According to the current research of depression mechanisms, 9 pathways were selected from 22 pathways obtained by the GO analysis for JWSNS. To explore the underlying antidepressant mechanisms of JWSNS, all targets of active compounds were mapped to 9 pathways to establish the T-P network.
As shown in Fig. 3, the T-P network contained 181 nodes (172 targets and 9 pathways) and 291 edges. The average degree of targets and pathways was 1.69 and 32.3, respectively. This indicated that the targets of JWSNS were located in multiple pathways. In addition, the selected 9 pathways had enriched multiple targets, including IL-4 and IL-13 signaling (degree = 102), amine ligand-binding receptors (degree = 71), transmission across chemical synapses (degree = 53), and cellular senescence (degree = 35).
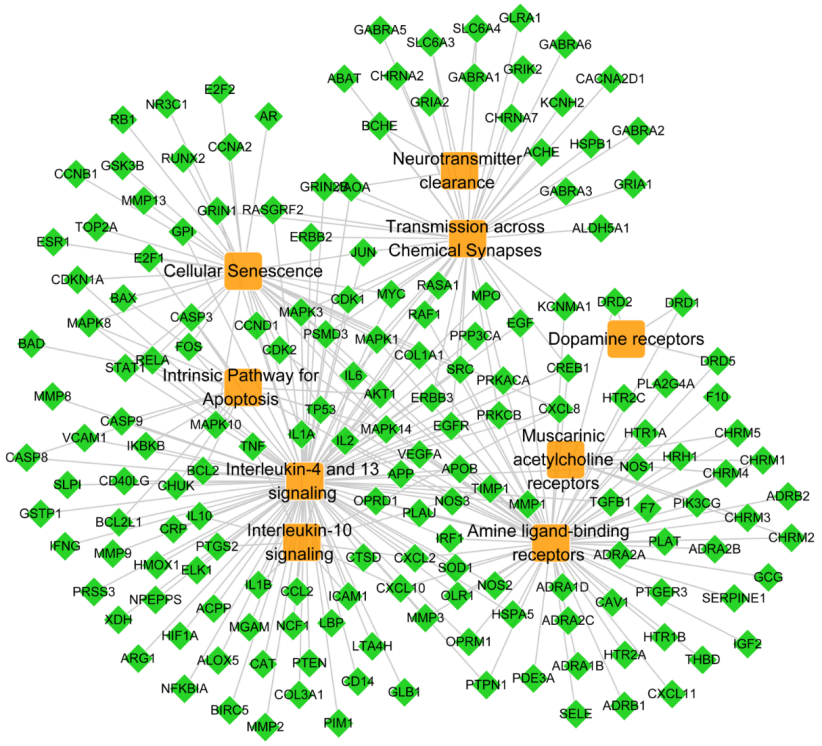 Figure 3.
Figure 3.T-P network. The green nodes represent the targets of jiaweisinisan, and the orange nodes represent the antidepressant pathways of jiaweisinisan.
In accordance with the current pathophysiological understanding of depression, 9 pathways were integrated and divided into three pathological modules; 5 pathways (amine ligand-binding receptors, transmission across chemical synapses, muscarinic acetylcholine receptors, neurotransmitter clearance, and dopamine receptors) were integrated into a synaptic transmission module (Fig. 4); 2 pathways (cellular senescence and intrinsic pathway for apoptosis) were integrated into a cell apoptosis module (Fig. 5). Lastly, 2 pathways (interleukin-4 and 13 signaling and interleukin-10 signaling) were integrated into an immune-inflammatory module.
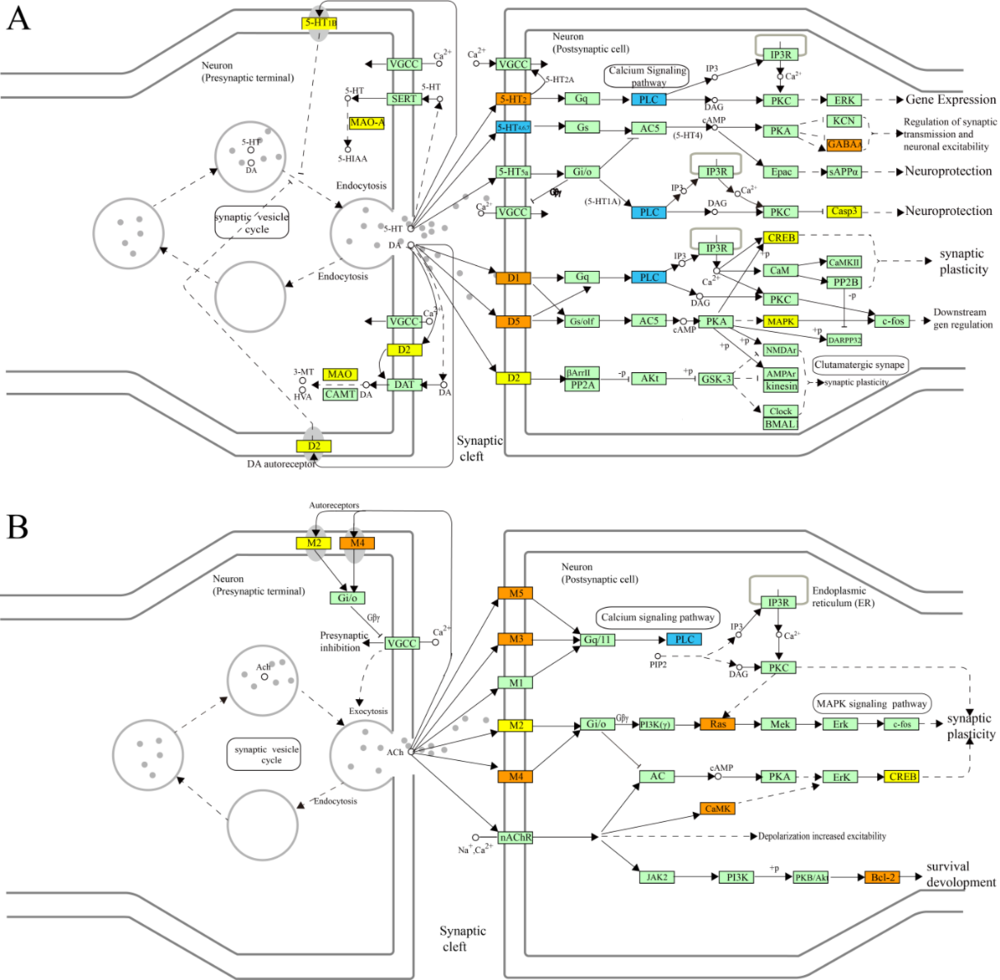 Figure 4.
Figure 4.Regulation of synaptic transmission module of a depression-relating pathway. A represents the pathway of serotonergic receptors and dopamine receptors; B represents the pathway of acetylcholine receptors. The orange nodes are targets of jiaweisinisan, and the blue nodes are targets of depression, the yellow nodes represent the frequent targets of jiaweisinisan and depression, and the light green nodes are relevant targets in the pathway. The arrows indicate activation, and the T-arrows indicate inhibition. The solid line represents the direct action, and the dotted line represents the indirect action.
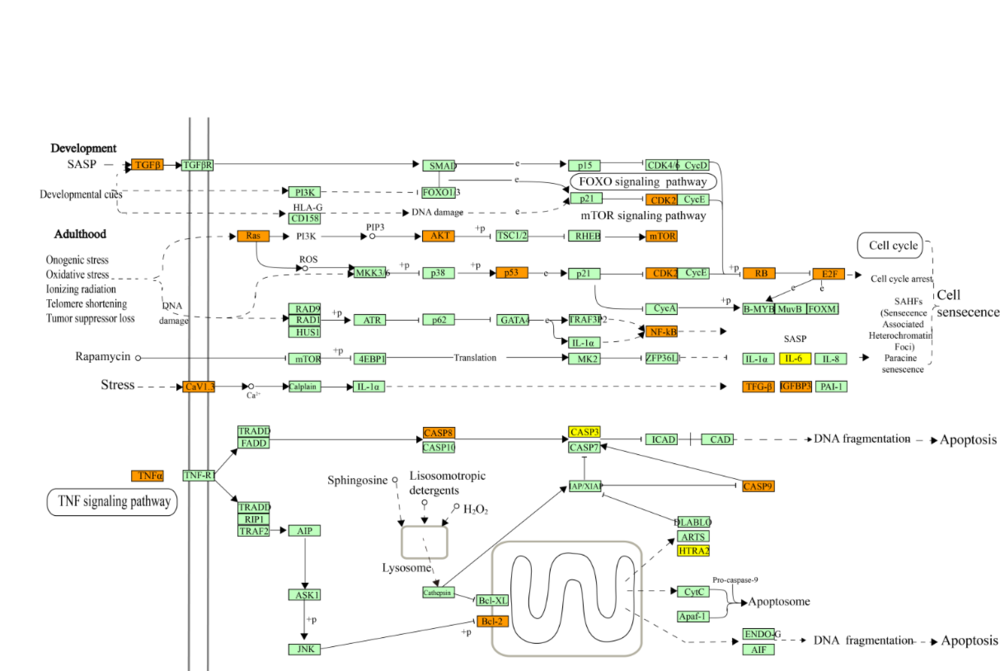 Figure 5.
Figure 5.Cell apoptosis module of a depression-relating pathway. The orange nodes are targets of jiaweisinisan, the yellow nodes represent the frequent targets of jiaweisinisan and depression, and the light green nodes are relevant targets in the pathway. The arrows indicate activation, and the T-arrows indicate inhibition. The solid line represents the direct action, and the dotted line represents the indirect action.
As a synergistic multiple-ingredient system, TCM formulas are thought to act by holistically regulating multiple biological functions and processes. Nevertheless, the utilization of TCM drugs has been constrained by a lack of systematic study. The antidepressant effect of JWSNS has previously been shown in clinical and animal experiments (Shi et al., 2008; Wu et al., 2013, 2007; Yan et al., 2016; Zhong et al., 2008). However, its mechanisms of action are as yet unclear. Thus, the systematic pharmacology strategy was employed to screen candidate active ingredients, predict candidate targets, and investigate the antidepressant mechanisms of JWSNS.
It was found that 82 different bioactive ingredients are contained in the seven herbs of JWSNS. Therein, 306 targets were obtained, providing a fundamental basis for further, more comprehensive study of the antidepressant mechanisms of JWSNS. Kaempferol, quercetin, and beta-sitosterol were found to be vital active compounds of JWSNS in C-T network mapping, possibly representing the principal antidepressant compounds of JWSNS. Indeed, previous research has identified the role of kaempferol in inhibiting neuronal apoptosis and promoting nerve regeneration (Park et al., 2010). Quercetin has been found to inhibit the hyperactivity of the HPA axis significantly by inhibiting the expression of corticotropin-releasing factor (CRF) mRNA (Kawabata et al., 2010). Beta-sitosterol has been shown to increase serotonin (5-HT) and norepinephrine (NE) levels of the central nervous system (Zhao et al., 2016). Important targets of JWSNS were also revealed, including PTGS2/COX2, ADRB2, and BCL2. These are considered potential targets for antidepressants (Galecki et al., 2014; Zhang et al., 2013, 2009), indicating that JWSNS may exert antidepressant effects by acting on PTGS2/COX2, ADRB2, and BCL2.
Hypotheses around monoaminergic neurotransmitters, neurotrophic factors/neuroplasticity, neuroinflammation, and hippocampal neurogenesis are implicated in the pathogenesis of depression. Our study has shown that the antidepressant pathways of JWSNS are mainly linked with monoamine transmitters and their receptors, neurotrophic factors, and cytokines. These pathways were divided into three modules, the regulation of synaptic transmission, cell apoptosis, and immunoregulation.
Synaptic transmission of neurotransmitters plays a fundamental role in the function of the brain, and structural and functional changes therein serve as important factors in the development of a range of psychiatric disorder phenotypes. Chronic stress, via the HPA axis, is a known contributor to the dysregulation of synaptic plasticity that leads to altered brain region connectivity related to a psychiatric disorder. Compounds of JWSNS, as illustrated in Fig. 4, may exert neuroprotective functions that mitigate the effects of chronic stress or ameliorate the structural and functional changes associated with depression
Many antidepressants effectively increase the levels of the monoamine 5HT in the synaptic cleft, while others additionally influence the monoamines norepinephrine and dopamine - thus implicating the monoamine system in depression. As shown in Fig. 4A, 5HT released into the synaptic cleft is subsequently taken up by the serotonin transporter (SERT). It may then be stored in synaptic vesicles or undergo deamination via the monoamine oxidase (MAO) enzyme. Another group of powerful antidepressants, MAO. Inhibitors are known to act by inhibiting the action of MAO. Enzymes that would otherwise deaminate 5HT, norepinephrine, and dopamine; thereby, MAO. Inhibitors increase their availability in the synaptic cleft: both selective serotonin reuptake inhibitors (SSRIs) and MAO.
Inhibitors are known to give rise to structural synaptic changes (involving up- or down-regulation of particular subtypes pre- and post-synaptic receptors, changes that occur over weeks) that are known to ameliorate specific symptoms of depression in a subset of the depression population - although precise mechanisms that result in these emergent effects are as yet unknown (Blier, 2003). As can be seen in Fig. 4A, within the monoamine system, JWSNS shares common targets with depression, including 5HT1B autoreceptors and the MAO enzyme, indicating that they are important anti-depression targets of JWSNS. Also, 5-HT receptors can mediate the 3’, 5’-cyclic AMP (cAMP)-protein kinase A (PKA)- cAMP response element-binding (CREB) signaling pathway and regulates the phosphorylation of CREB. The previous study of the acute fasting produces found it to exert antidepressant-like effects via enhancement of the p-CREB/CREB ratio, and additive antidepressant-like effects of fasting with imipramine may be related to modulating 5-HT2 receptors (Li et al., 2014b).
In the dopamine receptors pathway (Fig. 4A), the cAMP/PKA signaling pathway is also closely related to depression and the antidepressant action of SSRIs and MAO inhibitors. The downregulation of signaling via cAMP seems to play a role in the pathophysiology and pharmacology of depression, and some antidepressants have been shown to indirectly increase both the activity of cAMP-dependent PKA and the expression and function of CREB (Fig. 4A). The previous study has demonstrated that the TCM Helicid can improve depressive behavior and enhance hippocampal neurogenesis in a rat model of depression and that this may be achieved by the regulation of SERTs, activating cAMK/PKA/CREB signal pathway, and upregulating p-CREB levels in the hippocampus (Li et al., 2019). Such findings indicate that JWSNS may similarly exert anti-depression, given the role of the cAMP/PKA signaling pathway indicated in our study (Fig. 4A).
In the pathway of acetylcholine receptors (Fig. 4B), MAPK and CaMK signaling pathways are involved in modulating synaptic plasticity and are thought to be involved in the pathogenesis of depression. More specifically, in the MAPK signaling pathway, M2 (Muscarinic acetylcholine m2 receptor) activates the Ras (G protein), MAPK kinase (MEK) and phosphorylated extracellular signal-regulated kinase (ERK), followed by the activation of a c-fos to complete the whole MAPK signaling pathway (Fig. 4B). The MAPK signaling pathway is associated with rapid and sustained antidepressant-like effects of ketamine (Humo et al., 2020). An increase in both of the ACh levels and the AChRs number in the brain of patients with depression have been shown in human tomography studies (Hannestad et al., 2013). Depressive symptoms are induced by blocking acetylcholine degradation (Mineur et al., 2013). In terms of the CaMK signaling pathway, Ca2+ releases and binds with nAChR, and then activates CaMK and ERK, thus finally activating CREB (Fig. 4B). It has been verified in our previous study that JWSNS can exert neuroprotective effects by regulating the cAMP-CREB signaling pathway (Yan et al., 2009). As a target of JWSNS, CREB serves as an important target in the regulation of synaptic transmission and has recently been the topic of considerable research interest. Activated CREB may directly regulate brain-derived neurotrophic factors (BDNF) expression, known for its association with neuroprotection and neurogenesis. p-CREB is now considered to be a biomarker for the efficacy of antidepressant therapy (Koch et al., 2002), and its expression may be enhanced by antidepressant therapies (Ren et al., 2011). As such, CREB may represent a crucial facet of the antidepressant action of JWSNS.
Cell senescence and apoptosis have emerged as fields of interest in depression research over recent years. Cell senescence involves the regulation of the oncogene-induced senescence (OIS) signaling pathway, and the molecular mechanism of OIS induction has been gradually revealed. Besides this, excessive expression of the oncogene Ras in the pathway of cell senescence can cause DNA damage, which is accepted by p53, thereby transcribing and activating P21, leading to the activation of the retinoblastoma protein (Rb), which then arrests the cell cycle and produce the phenotype of senescence (Fig. 5). Cell senescence is closely related to late-onset depression (LOD). The number of hippocampal nerve cells tends to decrease with advancing age, gradually resulting in hippocampal atrophy, the neuroanatomical marker of LOD patients (Lloyd et al., 2004). Interestingly, P53, a target of JWSNS, plays a significant role in the initiation and maintenance of cellular senescence.
Regarding cell apoptosis, tumor necrosis factor α (TNF-α) binding with its receptor TNFR1 can activate the F-associated protein with death domain (FADD)/caspase-8 pathway, ultimately inducing apoptosis, and this is termed the external apoptotic pathway mediated by death receptors (Fig. 5). The apoptosis of hippocampal nerve cells is a crucial aspect of depression. Chronic stress can increase the apoptosis of hippocampal neurons, changing hippocampal structure and function, and causing depression (Anacker et al., 2013). In this pathway, CASP3, a member of the caspase family, serves as a promotion gene of apoptosis (Jahanbazi Jahan-Abad et al., 2017). Bcl-2 may realize a neuroprotective effect by antagonizing or promoting apoptosis (Kosten et al., 2007). In short, GO analysis demonstrated that JWSNS targets like Ras, P53, CASP3, and Bcl-2, participated in cell senescence and apoptosis pathways, suggesting that JWSNS may exert antidepressant effects by regulating cell senescence and apoptosis.
It is generally acknowledged that proinflammatory cytokines, acting as neuromodulators, can mediate the pathogenesis of the neuropsychopharmacological features of depression (Ng et al., 2017b). Thus, there is a relationship between immune activity and depression. It has been shown in a meta-analysis that peripheral levels of cytokines IL-6, TNF-α, IL-10, IL-13, IL-18, IL-12 are elevated in patients with depression (Kohler et al., 2017), while antidepressants could decrease the peripheral levels of IL-6, TNF-α, IL-10, and CCL-2 (Kohler et al., 2018). These findings suggest a two-way relationship between inflammation and depression. There are multiple targets of JWSNS participating in the IL-4, IL-13 signaling pathway, and the IL-10 pathway, suggesting that JWSNS may play an antidepressant role by regulating immune inflammation. As an inflammatory marker target of JWSNS, IL-6 exists in both the IL-4 and-13 signaling pathway and the IL-10 pathway. IL-6 activates indoleamine 2, 3 dioxygenases (IDO), while the latter converts the precursor tryptophan of 5-HT into kynurenine, significantly reducing the synthesis of 5-HT and playing a synergistic role in depression. IL-6 gene knocks out mice are resistant to stress-induced depression, suggesting that IL-6 may be an essential gene in the development of depression (Chourbaji et al., 2006; Wiener et al., 2019). As such, JWSNS may exert an antidepressant effect by regulating immune-inflammatory reactions via IL-6 modulation.
What has been discussed heretofore demonstrates how a single active component could act upon multiple targets, as much as multiple components of JWSNS could also act upon a single target. Similarly, multiple targets seemingly connect multiple pathways, giving rise to synergistic antidepressant effects. For instance, quercetin could act on 441 targets (degree = 441); PTGS2/COX2 was derived from 18 active compounds (degree = 18). In the regulation module of synaptic transmission, serotonin and dopamine receptors indirectly activate CREB by acting on the CAMP, MAPK, and CaMK pathways. This could further influence the expression of BDNF, which is known to be involved in neuronal survival as well as the differentiation and growth of new neurons and synapses, thus giving rise to the putative link between BDNF and brain changes related to depression.
Databases adopted in this network pharmacology analysis would benefit from improvement. There are many databases identifying ingredients of TCM herbs. However, we used only three of them (mainly TCMSP), which may not have been sufficient to obtain comprehensive data on JWSNS compounds. At the time of this study, TCMSP contained no information relating to RR or CH, and as such, does not fully cover all herbs of JWSNS. Therefore, we used other databases, as well as literature sources, to supplement knowledge pertaining to the compounds of JWSNS. Given that there are many disease target databases, we may have obtained complete information accessing a greater range of them. Generally, the results of this study would benefit from further experimental verification.
In this work, we used systems pharmacology as a tool to provide information about the compounds, targets, and mechanisms of JWSNS, to improve the current understanding of the efficacy of JWSNS for the prevention and treatment of depression. The systems pharmacology approach framework for the present work is shown in Fig. 6. The multi-component, multi-target, and multi-pathway features of the synergistic effects of JWSNS against depression were effectively elucidated with a systems pharmacology approach. 82 different bioactive ingredients and 306 targets were identified from JWSNS. Specifically, kaempferol, quercetin, and beta-sitosterol were considered as the key active compounds of JWSNS, while PTGS2/COX2, ADRB2, and BCL2 were found to be important targets of JWSNS. In all, 26 common targets, which may be the direct targets of JWSNS in treating depression, were obtained by mapping the targets of JWSNS and depression. Finally, 9 pathways were selected to probe into the underlying antidepressant mechanisms of JWSNS, mainly related to the regulation of synaptic transmission (involving 5 pathways, including amine ligand-binding receptors, transmission across chemical synapses, muscarinic acetylcholine receptors, neurotransmitter clearance, and dopamine receptors), cell apoptosis (involving 2 pathways, including cellular senescence and intrinsic pathway for apoptosis) and immunoregulatory (involving 2 pathways, including interleukin-4,13 signaling and interleukin-10 signaling).
 Figure 6.
Figure 6.A systems pharmacological approach. The schematic diagram of the research methodology and the proposed model of jiaweisinisan acting on depression are shown.
JC, YH: drew the relevant pictures and wrote the paper. LL, JN: searched the database to obtain relevant data. WY, YW: consulted literature and analyzed data.CY, LW: determined the network pharmacology methods of action and general director of the project.
The study was supported by grants from the National Natural Science Foundation of China (no. 81573858), Natural Science Foundation of Guangdong Province, P. R. China (no.2016A030313648), and Major Basic Research Project of Educational Commission of Guangdong Province, P. R. China (no. 2017KZDXM020).
All the authors do not have any conflicts of interest.
Supplementary material associated with this article can be found, in the online version, at https://jin.imrpress.com/EN/10.31083/j.jin.2020.02.1246.
