† These authors contributed equally.
Sepsis associated encephalopathy is a common complication of sepsis, but its pathogenesis of sepsis-associated encephalopathy remains unclear. Astrocytes are the most abundant brain glial cells, and reactive astrogliosis, a pathological response to central nervous system diseases, has a clear disease and disease-stage specificity. Functional changes of astrocytes are of great significance for the detection and prognosis of sepsis-associated encephalopathy. The pathogenesis of sepsis-associated encephalopathy was explored at the cellular level by examining astrogliosis in an in vitro model of sepsis-associated encephalopathy. Astrocytes of Wistar neonatal rats were incubated with different concentrations of lipopolysaccharide combined with interferon-γ. Cell viability was assessed by levels of tumor necrosis factor-α, interleukin-6, nitric oxide, reactive oxygen species, glial fibrillary acidic protein, changes of astrocyte morphology, and prevalence of apoptosis and necrosis. Compared with the control group, the cell viability of treated groups was decreased. The levels of tumor necrosis factor-α, interleukin-6, nitric oxide, reactive oxygen species, and glial fibrillary acidic protein were increased, hypertrophy of astrocytes was observed, and apoptosis was increased. The pathogenic outcomes of astrogliosis in sepsis-associated encephalopathy is discussed and a new tool provided to explore the pathogenesis of sepsis-associated encephalopathy at the cellular level.
Sepsis is defined as life-threatening organ dysfunction caused by a dysfunctional host response to infection. Sepsis-associated encephalopathy (SAE) is widely understood to be a diffuse brain disorder that occurs with sepsis but in the absence of central nervous system infection or other forms of encephalopathy (Singer et al., 2016). It is usually characterized by a disturbance in the sleep-wake cycle, impaired consciousness, mild cognitive impairment, apparent delirium, or coma (Sonneville et al., 2013). The prevalence of SAE is difficult to define due to inconsistencies in naming and the uncertainty of diagnostic methods. However, in patients with severe sepsis, the incidence of SAE is approximately 9%-71% (Molnar et al., 2018). Brain injury is one of the most common and early outcomes of multiple organ dysfunction syndromes (MODS) in sepsis (Dal-Pizzol et al., 2014). SAE is generally considered the primary factor determining the clinical evolution and prognosis of sepsis (Shulyatnikova and Verkhratsky, 2019). It is, therefore, of great importance to study the pathogenesis of SAE to identify effective treatments, thereby improving prognosis and reducing the social and economic burden of patients diagnosed with sepsis.
The complete pathophysiology of SAE remains unclear. There is an urgent need to improve the understanding of how specific cellular interactions respond to central nervous system insults and how these insults develop and are controlled by complex molecular signaling mechanisms. However, many mechanisms have been identified as potential factors for pathogenesis, including changes in blood-brain barrier permeability (Cervos-Navarro et al., 1983), mitochondrial and oxidative stress (Berg et al., 2011), direct nerve injury, increased levels of cytokines and pro-inflammatory factors (Jacob et al., 2011), vascular endothelial dysfunction (Jeger et al., 2013), neurotransmitter disorders and changes in amino acid content (Basler et al., 2002). As the basic unit of an organism, the maintenance of cells and their function greatly affects the occurrence and development of cerebral diseases. Glial cells, in particular, are responsible for central nervous system homeostasis (Verkhratsky et al., 2013). Astrocytes, the most abundant glial cells in the brain, have many functions, including maintenance of ion and fluid balance, neurotransmitter metabolism, neurogenesis, maintenance of synaptic connections, and synaptic plasticity (Shulyatnikova and Verkhratsky, 2019). The defensive function of astrocytes is characterized by astrogliosis, which is a multi-component and complex remodeling of astrocytes caused by damage to the central nervous system (Brosius et al., 2014).
Pathological changes of astrocytes depend on the nature and severity of the damage and are regulated by specific and complex intracellular and intercellular signals (Sofroniew and Vinters, 2010). Astrogliosis is associated with beneficial effects but may be harmful in certain situations. Recently, research into the role of astrocytes in central nervous system diseases has gradually increased (Pekny et al., 2016), for example, neurotrauma, stroke, Alzheimer’s and Huntington disease, yet there are few studies on astrogliosis in SAE. Studying the pathological characteristics of astrocytes in SAE should help understand the associated pathophysiological changes. In this study, primary cultured astrocytes were incubated with lipopolysaccharide (LPS), and interferon-γ (IFN-γ) to construct an in vitro model of SAE (Zhao et al., 2017), observe the pathological changes of reactive astrogliosis and provide a new tool for the exploration of SAE pathogenesis.
All animals were obtained from the Center of Experimental Animals at Xinjiang Medical University (Xinjiang, P. R. China). Animal procedures were strictly under the National Institutes of Health guidelines and were approved by the ethics committee of the First Affiliated Hospital of Xinjiang Medical University (IACUC20180411-03).
Neonatal Wistar rats (within three days of birth) were disinfected with alcohol and meninges, and blood vessels stripped immediately. The cerebral cortex was rapidly separated and digested with 0.125% trypsin (HyClone, Logan, Utah, USA) at 37 °C for 20 minutes. Complete medium containing 10% fetal bovine serum (FBS (fetal bovine serum); Gibco, Carlsbad, USA), 1% antibiotics (penicillin and streptomycin, Gibco, Carlsbad, USA) and 89% DMEM (Dulbecco's modified eagle medium)/high glucose medium (HyClone, Logan, Utah, USA) was used to terminate digestion. Cells were incubated with complete medium at 37 °C in a humidified atmosphere under 5% CO2. After about four weeks, anti-glial fibrillary acidic protein antibody (anti-GFAP (glial fibrillary acidic protein); Abcam, Cambridge, UK) was used to determine the purity of astrocytes by immunocytochemistry.
Different concentrations of LPS (BOSTER, Beijing, P. R. China) and IFN-γ (Abcam, Cambridge, UK) were used to treat primary cultured astrocytes to establish the SAE model and were prepared in DMEM without fetal bovine serum and antibiotics. Control cells were cultured in DMEM without FBS or antibiotics, while LPS and IFN-γ were included for 12 hours in the treated conditions. The treated conditions included three groups defined by LPS concentration: 50 group (LPS 50 ng/mL + IFN-γ 20 ng/mL), 100 group (LPS 100 ng/mL + IFN-γ 20 ng/mL), 200 group (LPS 200 ng/mL + IFN-γ 20 ng/mL).
Astrocytes were seeded to give three replicates in 96-well plates at a density of 104 cells per well. The different treatments were applied for 12 hours. The control group was treated with DMEM/high glucose medium. CCK-8 (BOSTER, Beijing, P. R. China) was used following the manufacturer’s instructions, and the 96-well plates were incubated at 37 °C for 1.5 hours. Cell viability was reported as the ratio of optical density values of experimental wells to control wells.
Cultured medium concentrations of TNF-α (tumor necrosis factor-α), IL-6 (interleukin 6), NO (nitric oxide), and cell homogenate concentrations of ROS (reactive oxygen species) obtained from the above groups were assayed by the ELISA kit (Andy, Beijing, P. R. China). All procedures were performed according to the manufacturer’s instructions.
Total protein from primary cultured astrocyte was extracted, and concentrations were determined with a Bradford Protein Assay Kit (Thermo Scientific, USA). Subsequently, 15 μg/lane of protein was used for SDS-PAGE, which was conducted according to the SDS-PAGE (sodium dodecyl sulfonate-polyacrylamide gel electrophoresis) gel preparation kit instructions (Solarbio, Beijing, P. R. China), and the proteins were then transferred to polyvinylidene fluoride membranes (PVDF (polyvinylidene difluoride); Solarbio, Beijing, P. R. China). Next, 5% non-fat dried milk dissolved in TBST (Tris-buffered saline Tween-20) was used to block the membranes at room temperature for one hour. Membranes were then incubated using primary antibodies against GFAP (diluted 1 : 1000, Abcam, Cambridge, UK) overnight at 4 °C. Subsequently, membranes were incubated with secondary rabbit antibody at 37 °C for two hours. Finally, the bands were visualized with ECL (enhanced chemiluminescence) reagent and quantified by BIO-RAD (Gel Doc XR, USA).
Astrocytes were seeded in 24-well plates, and the cells were treated as described above. Cells were fixed at room temperature in 4% paraformaldehyde for 30 minutes, blocked with 0.1% Triton X-100 for 30 minutes, blocked with goat serum at room temperature for two hours, and incubated with primary antibody against GFAP (diluted 1 : 500; Abcam) overnight at 4 ℃. After washing with PBS (phosphate buffered saline), cells were incubated with Alexa Fluor 488-conjugated goat secondary antibody (1 : 200, Abcam, Cambridge, UK) at room temperature for two hours. Images were acquired with a fluorescence microscope.
Astrocytes were seeded in 24-well plates and treated as described above. The Fluorescence Hoechst 33342/propidium iodide (PI) double staining kit (Solarbio, Beijing, P. R. China) was used to visualize apoptosis and necrosis in each group. Cell staining buffer, the Hoechst staining solution, and PI staining solution were added to each well at a ratio of 1 : 5 : 5 and incubated at 4 ℃ for 20-30 minutes. Apoptosis was observed by fluorescence after washing with PBS. After double dyeing with the above two dyes, normal cells showed weak red fluorescence and weak blue fluorescence, whereas apoptotic cells showed weak red fluorescence and strong blue fluorescence and necrotic cells showed strong red fluorescence and strong blue fluorescence. Image J was used to measure the area of apoptosis and necrosis according to the corresponding threshold. The pathological outcome of each group was compared.
Data are presented as mean±standard deviation (mean±SD). SPSS 19.0 (SPSS, Chicago, USA) software was used to analyze the data. Each experiment was performed in triplicate. The statistical significance of differences for group comparisons was evaluated using a one-way ANOVA followed by a least significant difference test or Dunnett’s T3 test. Statistical significance was assumed at P < 0.05 for all tests.
To explore the effects of LPS and IFN-γ treatment, a CCK-8 assay was employed to detect the proliferation of primary cultured astrocytes. After incubation with LPS and IFN-γ, cell viability was decreased compared to control cells (Fig. 1).
 Figure 1.
Figure 1.LPS combined with IFN-γ decreased cell viability in primary cultured astrocytes. Cultures were treated with LPS (50 ng/mL, 100 ng/mL, or 200 ng/mL) and IFN-γ (20 ng/mL) for 12 hours. CCK-8 was used to assess cell viability. ** P < 0.01 versus control, *** P < 0.001 versus control; # P < 0.05 versus 50 group, ### P < 0.001 versus 50 group; +++P < 0.001 versus 100 group.
To determine whether LPS and IFN-γ mediated the increase of inflammatory cytokine release, the control, and treated groups were examined by ELISA. Cultured media concentrations of TNF-α and IL-6 obtained from the treated groups were elevated compared to those for the control group (Fig. 2).
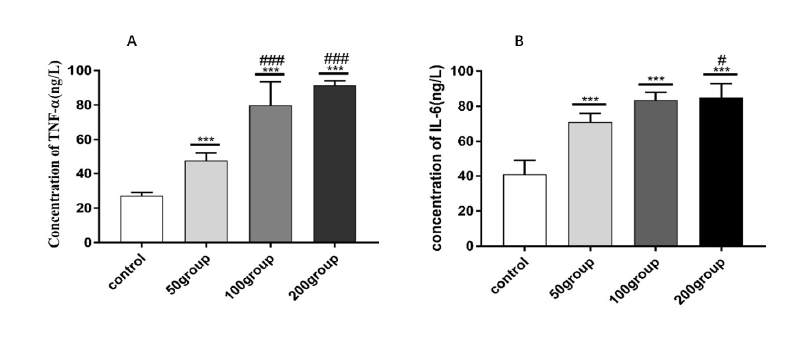 Figure 2.
Figure 2.Treatment with LPS and IFN-γ upregulated inflammatory cytokines in primary cultured astrocytes. Astrocytes were treated with DMEM, LPS (50 ng/mL, 100 ng/mL, or 200 ng/mL) and IFN-γ (20 ng/mL) for 12 hours. ELISA was used to determine the inflammatory cytokines released by the control and treated groups. (A) Cultured media concentrations of TNF-α; (B) Cultured media concentrations of IL-6. *** P < 0.001 versus control; # P < 0.05 versus 50 group; ### P < 0.001 versus 50 group.
Cell homogenate concentration of ROS obtained from the treated groups was elevated compared to that of the control group. To detect whether LPS and IFN-γ mediated the increase of ROS, the control, and treated groups were examined by ELISA. (Fig. 3).
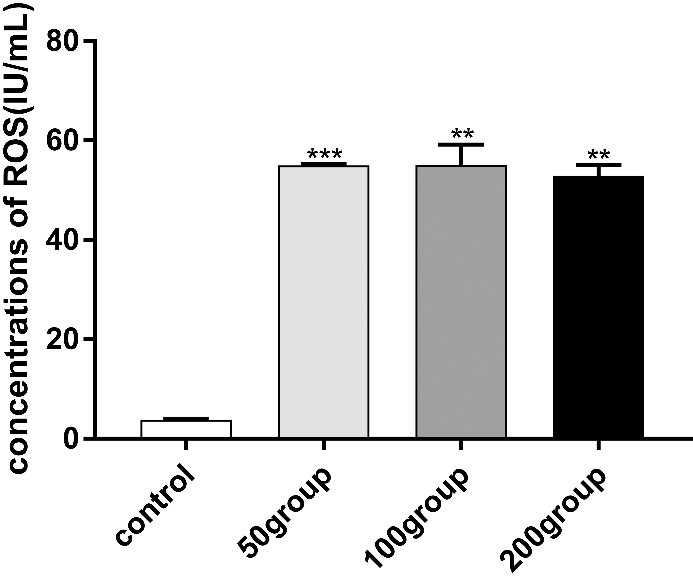 Figure 3.
Figure 3.LPS combined with IFN-γ upregulated ROS in primary cultured astrocytes. Astrocytes were treated with DMEM, LPS (50 ng/mL, 100 ng/mL, or 200 ng/mL) and IFN-γ (20 ng/mL) for 12 hours. ELISA was used to determine ROS concentrations. ***P < 0.001 versus control; **P < 0.01 versus control.
Cultured medium concentrations of NO obtained from the treated groups were increased compared to those of the control group. To detect whether LPS and IFN-γ induced increases in NO concentration, the control group, and treated groups were examined by ELISA. (Fig. 4).
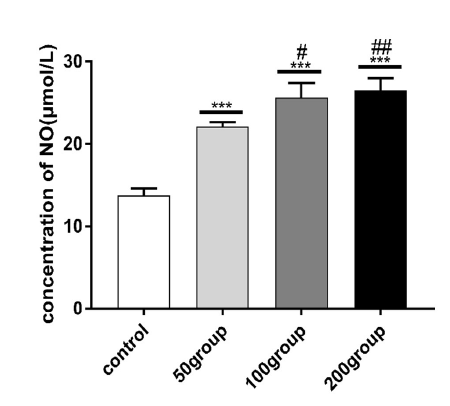 Figure 4.
Figure 4.LPS combined with IFN-γ upregulated NO in primary cultured astrocytes. Astrocytes were treated with DMEM, LPS (50 ng/mL, 100 ng/mL, or 200 ng/mL) and IFN-γ (20 ng/mL) for 12 hours. ELISA was used to determine NO levels. *** P < 0.001 versus control; # P < 0.05 versus 50 group; ## P < 0.01 versus 50 group.
Astrocyte hypertrophy is a morphological marker of reactive astrogliosis. Immunocytochemistry was used to observe the morphological changes of astrocytes under different conditions. Astrocytes were seeded in 24-well plates and subjected to various treatments, as described above. Compared with the control group, different degrees of astrocyte hypertrophy were observed in each treatment group (Fig. 5).
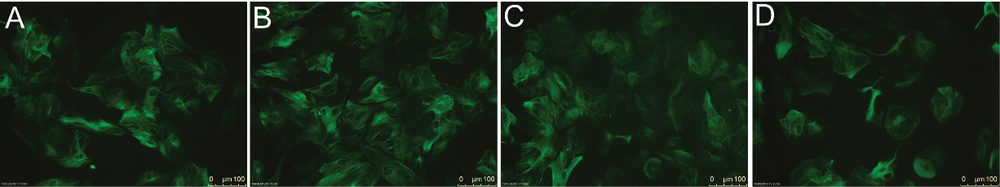 Figure 5.
Figure 5.LPS combined with IFN-γ induced astrocyte hypertrophy. Immunocytochemistry was used to determine the morphological changes of astrocytes. The control and treated groups were examined by fluorescence microscopy. (A): Control group. (B): 50 group (LPS 50 ng/mL + IFN-γ 20 ng/mL). (C): 100 group (LPS 100 ng/mL + IFN-γ 20 ng/mL). (D): 200 group (LPS 200 ng/mL + IFN-γ 20 ng/mL).
To further confirm the existence of astrogliosis in primary cultured astrocyte, the expression levels of GFAP were detected by Western blot analysis. After treatment with LPS and IFN-γ, the expression level of GFAP protein was found to be upregulated (Fig. 6).
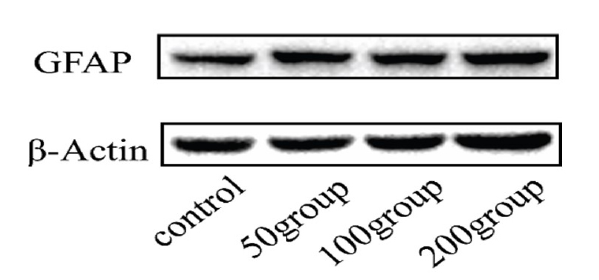 Figure 6.
Figure 6.LPS combined with IFN-γ upregulated GFAP levels in primary cultured astrocytes. Astrocytes were treated with DMEM, LPS (50 ng/mL, 100 ng/mL, or 200 ng/mL) and IFN-γ (20 ng/mL) for 12 hours. Western blot analysis was used to determine changes in the levels of GFAP.
To detect pathological outcomes in primary cultured astrocytes, LPS- and IFN-γ-induced apoptosis and necrosis were detected with the Apoptosis Fluorescence Hoechst 33342/PI double staining kit. Compared with the control group, the apoptosis proportion of the 100 groups and 200 groups was increased, compared with the 50 groups, the apoptosis proportion of 200 groups increased. Compared with the control group, the necrosis proportion of 200 groups increased, compared with 50 groups, the necrosis proportion of 200 groups increased, compared with 100 groups, the necrosis proportion of 200 groups increased. (Figs. 7, 8).
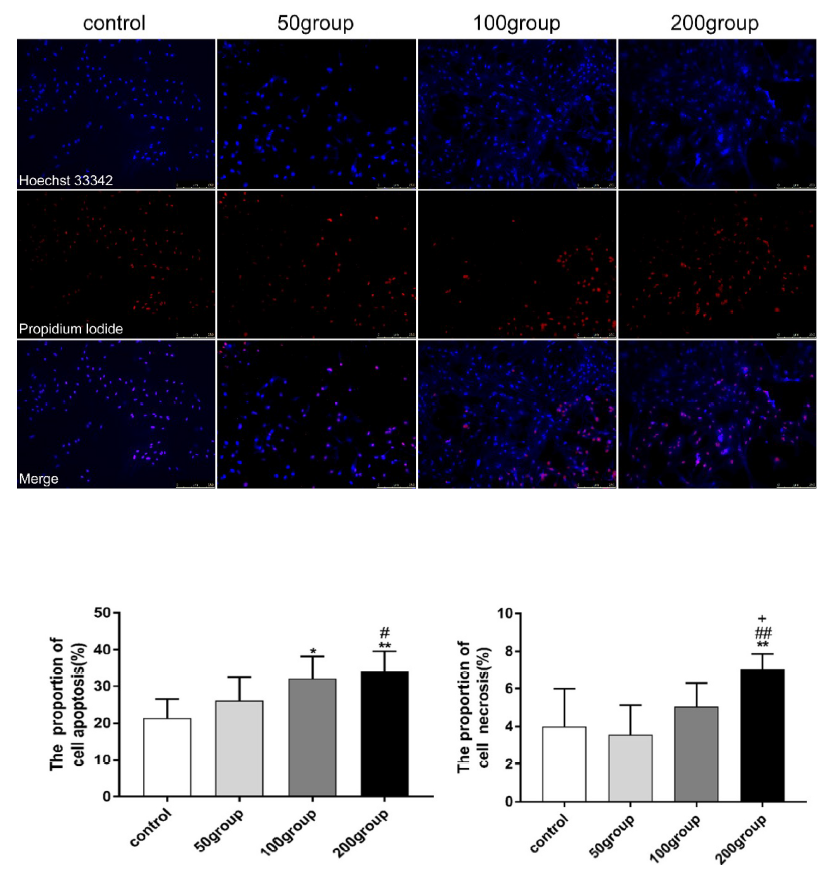 Figure 7.
Figure 7.Incubation of cells with LPS (100 ng/mL, or 200 ng/mL) and IFN-γ (20 ng/mL) changed the pathogenesis outcome of astrogliosis in SAE. Apoptosis and necrosis were detected by Apoptosis Fluorescence Hoechst 33342/PI double staining kit. Fluorescent images were analyzed with Image J software. (A): Representative photomicrographs are shown. (B): Proportion of apoptosis. (C): Proportion of necrosis.
 Figure 8.
Figure 8.Fluorescence images were analyzed with Image J software. (A): Image obtained from the fluorescence microscope. (B): Fluorescent image converted to an 8-bit grayscale image. (C): Grayscale image was inverted to black and white. (D): Threshold chosen to isolate necrotic cells in the image. (E): A threshold was chosen to isolate necrotic and apoptotic cells in the image. (F): Threshold has chosen such that all cells in an image were selected.
The pathogenesis of sepsis is complex, and there are many factors that influence prognosis. SAE is an independent risk factor for sepsis (Deng et al., 2013). It is necessary to study the pathogenesis of SAE and develop appropriate interventions to improve clinical outcomes for sepsis. Recently, research on central nervous system diseases has gradually shifted focus from neurons to the study of non-neuronal cells (Goritz et al., 2011). For example, glial cells play important roles in central nervous system diseases. Changes in glial cell function following injury may have significant effects on neuronal interactions and central nervous system function (Burda and Sofroniew, 2014). Systemic inflammation can damage both neurons and neuroglia in the brain and particularly affects activation of neuroglia, which is involved in the immune response of nerve tissues to inflammatory injury in SAE (Michels et al., 2017). As the most abundant glial cells, astrocytes provide the necessary support to neurons, mediate brain immunity, maintain the integrity of the blood-brain barrier and secrete inflammatory factors (Stogsdill et al., 2017). The pathological changes that occur in astrocytes during disease are multifaceted and characteristic of both the disease and disease stage (Verkhratsky et al., 2016, 2017). Reactive astrogliosis is an evolutionarily conserved process for the genetic, morphological and functional reconstruction of astrocytes following nerve tissue damage (Liddelow et al., 2017).
An in vitro model of SAE was established through combined treatment with LPS and IFN-γ (Wang et al., 2014). LPS is produced by gram-negative bacteria and induces inflammatory responses in cultured astrocytes through pathogen-associated molecular patterns. Also, primary cultured astrocytes do not secrete IFN-γ, so it was hypothesized that combined incubation with LPS and IFN-γ might better mimic the stimulatory effects of a septic environment in terms of inflammatory response compared with LPS or IFN-γ alone. CCK8 results show that cell viability decreased with LPS and IFN-γ incubation.
TNF-α and IL-6 are early indicators of sepsis (Kruttgen and Rose-John, 2012), and their concentrations are associated with outcomes in patients with sepsis. NO, and ROS play key roles in the pathogenesis of sepsis and also indicate oxidative stress and nitric stress in SAE, respectively (Kawakami et al., 2018; Neri et al., 2016). The results of this study showed that the concentrations of these indicators increased in the test groups compared with the control group. In this, in vitro model of SAE it was observed that astrocytes were hypertrophic, and GFAP expression increased after incubation with LPS and IFN-γ. This indicates the occurrence of reactive astrogliosis. Through the observations of pathological changes in astrocytes, it was found that the proportion of apoptotic astrocytes increased when astrocytes were incubated with LPS (100 ng/ml, or 200 ng/mL) and IFN-γ (20 ng/mL) compared with those of control group, while the proportion of apoptotic astrocytes in total astrocytes did not change when they were incubated with either LPS (50 ng/mL) or IFN-γ (20 ng/mL).
These findings suggest that reactive astrogliosis may provide protective effects for astrocytes incubated with LPS and IFN-γ. Reactive astrocytes help protect and limit the transmission of pathologic drugs through nerve tissue by establishing physical and molecular barriers to the spread of these factors. In essence, astrogliosis is a defensive response designed to protect nerve function and limit damage to nerve tissue. However, in some cases, astrogliosis may have neurotoxic and harmful effects (Liddelow et al., 2017). The significance of reactive astrocytes in diseases of the central nervous system is dependent on the specific disease environment and presents a two-fold activity (Pekny et al., 2014) as reactive astrogliosis has been found to have protective effects in the acute phase, but neural regeneration and plasticity responses may be inhibited if it persists (Wilhelmsson et al., 2004).
Further, astrogliosis does not occur in isolation but is part of a coordinated multicellular response in the central nervous system. This includes damage to a variety of glia, neurons, and different types of non-neuronal cells that are intrinsic to the central nervous system or enter from the bloodstream (Burda and Sofroniew, 2014). It is difficult to determine whether astrocyte pathology is the main cause of SAE in the current study. The pathological changes of astrocytes cannot be treated independently without considering overall changes in tissues and organs, especially with the complicated pathological mechanisms of sepsis, which involves almost the entire body. However, given the pathological changes of astrocytes associated with sepsis, including changes in the levels of GFAP, therapies can be developed to improve the outcome of SAE by targeting the pathological changes of astrocytes.
Reactive astrocytes play important roles in response to central nervous system injury. Without these functions, tissue damage and dysfunction in neuroplasticity and CNS regeneration will increase, but tissue repair will not occur. However, excessive activity of reactive astrocytes can, in some cases, result in increased tissue damage. Interventions to block such activity must target specific molecular events rather than trying to stop the process itself, and maybe a useful strategy for future therapeutic interventions. Currently, there is no clear scheme to regulate reactive astrocytes. Studying their characteristics in SAE for understanding the regulation of these changes in the rat model allows for further in vivo experimentation and provides a new tool for further understanding of SAE pathogenesis.
XH and XY designed the research study. XH wrote the manuscript. YW, CY and RC analyzed the data. XL, GT, and PP provided help and advice on the ELISA, WB experiments.
This work is partly supported by the University Research Program of Xinjiang Province: XJEDU2018I011.
The authors declare no competing interests.
