# These authors contributed equally
Hippocampal neurogenesis plays an important role in the onset and treatment of depressive disorders. Previous studies suggest that paeoniflorin could be used as an antidepressant for treating rats subjected to chronic unpredictable stress. In this study, the effects of paeoniflorin on neurogenesis in the hippocampus dentate gyrus and potential mechanism of action are further investigated in chronic unpredictable stress-induced rat. Results suggest that paeoniflorin markedly increased both sucrose consumption and the number of 5-bromo-2-deoxyuridine-positive cells in the dentate gyrus of chronic unpredictable stress-induced rats, and the ratio of co-expressed 5-bromo-2-deoxyuridine and glial fibrillary acidic protein-positive cells, but exerted no significant effect on the ratio of co-expressed 5-bromo-2-deoxyuridine and neuronal nuclei-positive cells. Compared with the vehicle group, a significant increase was detected in the number of brain-derived neurotrophic factor-positive cells and the expression of brain-derived neurotrophic factor mRNA in the hippocampus of the paeoniflorin-treated group. According to the results, paeoniflorin promoted neural stem cell proliferation, their differentiation into astrocytes, and neurogenesis in the hippocampal dentate gyrus of chronic unpredictable stress-induced rats. Apart from enhancing the protein expression and gene transcription of brain-derived neurotrophic factor, it also activated the expression of tropomyosin receptor kinase B (a high-affinity receptor of brain-derived neurotrophic factor). This suggests that paeoniflorin might promote neurogenesis in the hippocampus dentate gyrus of chronic unpredictable stress-induced rats and act as an antidepressant by regulating the brain-derived neurotrophic factor-tropomyosin receptor kinase B signaling pathway.
Globally, over 300 million people (equivalent to 4.4% of the world’s population in 2015) are estimated to suffer from depression. Depression is ranked by the Word Health Organisation as the single largest contributor to global disability (7.5% of all years lived with disability in 2015) (World Health Organization:2017). Numerous preclinical and clinical studies have reported that adult neurogenesis in dentate gyrus (DG) is closely related to depression (Dranovsky:2006, Cameron:2018). Hippocampal atrophy is observed in patients with depressive disorders and changes in hippocampal volume are possibly caused by lowered DG neurogenesis (Malberg:2004, Alfonso:2004). Santarelli et al and others (Santarelli:2003, Bulmash:2009) have reported that the behavioral effects of antidepressants are dependent upon hippocampal neurogenesis in animal models of depression.
Hippocampal neurogenesis is regulated by multiple factors in vivo and in vitro, among which brain derived neutrophic factor (BDNF), with high-affinity binding to tropomyosin receptor kinase B (TrkB), is critical for hippocampal neurogenesis in patients with depressive disorders (Castrén:2017, Begni:2017). Its expression reflects the severity of lesions in neurodegenerative diseases (Begni:2017) and its deficiency reduces proliferation of newborn granule cells. Additionly, injected exogenous BDNF increases neurogenesis of granule cells (Hu:2010, Zheng:2012, Wang:2017). Currently, antidepressants are reported to activate the functions of BDNF and TrkB in several neuronal processes, such as excitation, development, apoptosis, and synaptic plasticity (Leal:2017, Li:2018).
Paeoniflorin is a major active ingredient in the root of Paeonia lactiflora Pall. (Ranunculaceae), a Chinese medicinal herb widely used for treating depression-like disorders in many prescriptions of traditional Chinese medicine, including “Danggui Shaoyao San” and “Xiaoyao powder” (Qiu:2013). In many studies, paeoniflorin is found to be effective in treating several types of animal models of depression. Some animal experiments related to depression have shown paeoniflorin to significantly shorten swimming immobility time and tail suspension of mice, and blepharoptosis induced by reserpine antagonist (Qiu:2013a, Cui:2009). It also markedly ameliorates depressive-like behaviors in interferon-$\alpha$(IFN-$\alpha$)-induced mice, ovariectomized rats under stress, and chronic unpredictable stress (CUS) induced rats (Qiu:2013, Huang:2015, Li:2017). This suggests that paeoniflorin may be useful in the prevention of depression. In in vitro experiments paeoniflorin has been observed to be highly effective in protecting PC12 cells or neurons from damage by some drugs, leading to a significant increase in viable cell number, mortality reduction, and protection of these cells (Mao:2012, Mao:2011, Mao:2010). However, its role in adult neurogenesis have neither been studied nor reported.
This study further investigated the impact of paeoniflorin on neurogenesis in the hippocampus DG of CUS-induced rat and its possible mechanisms of action.
Paeoniflorin (purity > 95%, 201110) was obtained from Guizhou Dida Technology Co., Ltd; imipramine (090M14881V), 5-bromo-2-deoxyuridine (BrdU) (HMBC4217V) and mouse monoclonal BrdU antibody (D32N4389) were obtained from Sigma-Aldrich (Shanghai) Trading Co,.Ltd; both rabbit polyclonal glial fibrillary acidic protein (GFAP) antibody (2013929) and rabbit monoclonal neuronal nuclei (NeuN) antibody (2011151) were obtained from Millipore. Tetramethylrhodamine (TRITC)-labelled goat anti-mouse antibody (97860), anti-fade mounting medium (K127719E), diaminobenzidine (DAB) (K126915E), fluorescein isothiocyanate (FITC)-labelled goat anti-rabbit antibody (101272) and rabbit anti-rat BDNF polyclonal antibody (E0112) were obtained from Santa Cruz Biotechnology, Inc. Rabbit anti-rat TrkB polyclonal antibody (GR45120-6) was obtained from Abcam. 2 $\times$ SYBR Premix Ex Taq (B704-1), Taq enzyme (DR100B), M-MLV RTase (M-MLV Reverse Transcriptase, CK3401E), Oligo d (T) 18 Primers (B1801-1), ribonuclease (RNase) inhibitor (CK6301B) and dNTP (Deoxynucleotide, B1801-1) were obtained from Takara Biotechnology (Dalian) Co., Ltd. Gene sequences were obtained from Genbank. Primers were designed using Prime premier 5 and synthesized by Sangon Biotech (Shanghai) Co., Ltd. Chloral hydrate was dissolved in normal saline to give a 10% chloral hydrate solution.
Male Sprague Dawley rats (140-160 g, four weeks old) were supplied by the Animal Center of Zhejiang Chinese Medical University (license number SCXK (Zhejiang) 2008-0115). Subjects were housed at room temperature (24 $\pm$ 1 $^{\circ}$C, humidity 50 $\pm$ 10%) with a 12:12 hour light/dark cycle (light on 8:00 am) and fed and watered ad libitum for 7 days. Experimental procedures were approved by the Animal Experimentation Ethics Committee of Zhejiang Traditional Medical University (ZSLL-2012-057), and were conducted as specified by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
A full chronological description of the experimental protocol is given in Fig. 1.
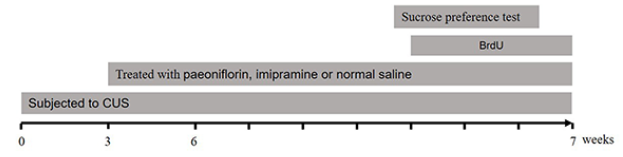 Figure 1.
Figure 1.Chronological description of experiment protocols.
Subjects were randomly divided into four groups, including control, vehicle, paeoniflorin-treated (60 mg/kg (Qiu:2013) and imipramine-treated (10 mg/kg (Qiu:2013)), each of which had eight subjects. The CUS animal depression model employed here was modified from a previously reported model (Qiu:2013). Subjects in stressed groups were exposed for seven weeks to daily varying stressors (9:00 am): 24 hours of food deprivation, 24 hours of water deprivation, 24 hours of cage tilt (45$^{\circ}$), one minute of tail pinch (1 cm from tail end), two hours of restraint stress, four minutes of exposure to 45 or 4$^{\circ}\mathrm{C}$, and overnight illumination. The control group was not stimulated. Three weeks after exposure to stressors, subjects were intraperitoneally injected with paeoniflorin and imipramine at 2 mL/kg (8:30 am) one hour before stress stimuli every day for four weeks. The same volume of normal saline was administered to the control and the vehicle groups.
The sucrose preference test was performed after seven weeks of CUS (10:30 am) and subjects were adapted by training to sucrose before the test, which was performed as previously described (Qiu:2013). Briefly, subjects were adapted by training to 1% sucrose solution (w/v): two bottles of solution were placed in each cage, and 24 hours later the solution in one bottle was replaced with tap water for 24 hours. After adaptation, subjects were deprived of water and food for 12 hours and then exposed to the sucrose preference test, where they were free to access either bottle. After two hours, the volumes of sucrose solution and water consumed were recorded and sucrose preference calculated.
Five subjects were randomly taken from each group. On the 4$^{th}$ day of the 7$^{th}$ week of CUS, BrdU (50 mg/kg) was given to the subjects six times via intraperitoneal injection at intervals of 12 hours (Lee:2013). After seven weeks of CUS, subjects received a BrdU injection, were anethetized, and fixed (4% polyformaldehyde) by perfusion through the left ventricle. Subjects were then immediately decapitated and the brains were fixed overnight (4% polyformaldehyde) for immunofluorescent staining and immunohistochemical tests. Finally, brains were collected 24 hours after dosing so as to test proliferation and differentiation of hippocampus neural stem cells in CUS subjects. Subjects that did not receive a BrdU injection were anesthetized by intraperitoneal injection (10% chloral hydrate), and decapitated. Brains and hippocampi were immediately split on a glacial table, weighed, frozen in liquid nitrogen, and used to measure the expression of hippocampus-related mRNA.
BrdU, GFAP, and NeuN expression were measured by immunofluorescent staining, araffin followed by embedding (Takahashi:1991). Coronal sections (5 $\mu$m) were continuously taken by paraffin slicing machine (HM 340E Rotary Microtome, Thermo Scientific Co,.Ltd) with one slice chosen from every six slices. After dewaxing (Takahashi:1991), antigens were retrieved in 0.01 mol/L sodium citrate buffer (pH = 6.0) and soaked in PBS containing 3% H2O2 to suppress the activity of endogenous peroxidase. Following incubation in 2 mol/L HCl at 37 $^{\circ}\mathrm{C}$ for 50 minutes, BrdU antigens of DNA were unmasked. After placement in 0.1 mol/L NaB4O3 for 10 minutes, slices were incubated in goat serum blocking solution for 20 minutes. Anti-mouse BrdU primary antibody (1:500) was dripped into the solution, stored overnight at 4$^{\circ}\mathrm{C}$ and rewarmed to 37$^{\circ}\mathrm{C}$ for 45 minutes. Goat anti-mouse TRITC secondary antibody (1:200) was added, slices were then incubated at 37 $^{\circ}\mathrm{C}$ for 45 minutes and reacted without exposure to light. Anti-rabbit NeuN or GFAP primary antibody (1:500) was added and incubated at 37$^{\circ}\mathrm{C}$ for one hour. Goat anti-goat FITC secondary antibody (1:200) was added and slices were incubated at 37$^{\circ}\mathrm{C}$ for 45 minutes. Antifade mounting medium was added and the flask was covered with a glass slide. Observations (559 nm) were conducted by Fluo ViewTM FV1000 laser scanning confocal microscope (Olympus Co,.Ltd). Positive cells in hippocampus DG were counted with Image-Pro Plus.
BDNF and TrkB protein expression was measured by immunohistochemical test. Methods for material collection, paraffin embedding, slicing, dewaxing (Takahashi:1991), high-pressure antigen retrieval and suppression of endogenous peroxidase activity were the same as for immunofluorescence staining. Either anti-mouse BDNF (1:200) or TrkB (1:200) primary antibody was added and the solution cultured overnight (4$^{\circ}\mathrm{C}$). Rabbit secondary antibody was incubated for one hour (37$^{\circ}\mathrm{C}$) and DAB color development then performed (1:200, 2-10 min). Hematoxylin staining (2 min) was followed by cells differentiation (10 s chlorhydric acid). Cells were kept transparent by routine dehydration procedures and mounted in neutral balsams. All immunohistochemical positive cells were counted using Image-Pro Plus.
The expression of BDNF mRNA was detected by qRT-PCR. The hippocampus was homogenized with liquid nitrogen and Trizol solution added. After addition of chloroform, oscillation, and even mixing, the supernatant layer was taken (0.4 mL). Isopropanol (0.4 ml) was added and the solution evenly mixed, the mixture was then stood still at room temperature. Sediments were removed after centrifugation and cleaning (500 $\mu$L 70% ethanol). The sediment was dissolved (50 $\mu$L DEPC-H2O) to obtain the total tissue RNA. Total RNA was converted to cDNA using MMLV reverse transcriptase. For real-time PCR, the SYBR qPCR Real-Time kit was used according to the instructions and the product amplified by Bio-Rad Mini Optico real-time PCR. Amplification conditions were set to a 30-cycle program (94$^{\circ}\mathrm{C}$, 55$^{\circ}\mathrm{C}$, 72$^{\circ}\mathrm{C}$, each for 30 s). GAPDH was used as an internal control for the PCR amplification. Primer sequences for BDNF were: F: 5'-CAGTGGCTGGCTCTCATACC-3' and R: 5'-CGGAAACAGAACGAACAGAA-3'. Primer sequences for GAPDH weres: F: 5'-CCCACGGCAAGTTCAACGGCA-3' and R: 5'- TGGCAGGTTTCTCCAGGCGGC-3'. The Ct value of each sample was determined. The relative expression (2$^{-\triangle \triangle Ct}$) of target genes in each sample was calculated by comparing them with those of the control group and using GAPDH as an internal control.
Data were statistically processed using SPSS (Windows v.13.0) and reported as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was performed, and intergroup differences were measured by Dunnett’s test, where differences were considered statistically significant for a p-value < 0.05.
The effect of paeoniflorin on sucrose consumption by CUS subjects is given in Fig. 2. One-way ANOVA revealed that the percentage of sucrose consumption significantly differed between groups [F($_{3, 31}$) = 11.5, p = 0.0]. Results suggest the vehicle group exhibited a lower sucrose preference than the control group (p = 0.041) and the paeoniflorin group consumed more sucrose than the vehicle group (p = 0.041). Long-term imipramine treatment led to recovery of sucrose preference in CUS subjects (p = 0.005 vs. CUS-saline group).
 Figure 2.
Figure 2.Effect of paeoniflorin and imipramine on percentage of sucrose consumption in CUS-exposed rats (mean $\pm$ SEM, eight rats per group). *p < 0.05 vs. Control-saline group. $^{\#}$p < 0.05, $^{\#\#}$p < 0.01 vs. CUS-saline group.
The effect of paeoniflorin on BrdU expression in hippocampus DG of CUS-treated subjects is shown in Fig. 3. One-way ANOVA showed BrdU expression to be significantly different between groups [F($_{3, 19}$) = 8.7, p = 0.001]. BrdU-positive cells in the DG appeared to have red nuclei. They were either round or oval-shaped and were detected in isolated cells or clusters (Fig. 2). Compared with the control group, significantly fewer BrdU-positive cells were detected in the hippocampus DG of CUS subjects (p = 0.012). The number of BrdU-positive cells in hippocampus DG was significantly increased in paeoniflorin and imipramine treated groups when compared with the vehicle group (p = 0.002, p = 0.0, respectively) (Fig. 3E).
 Figure 3.
Figure 3.Effect of paeoniflorin and imipramine on BrdU immunofluoroscent staining in DG regions of CUS subjects (mean $\pm$ SEM, five rats per group). (A) Control-Saline, (B) CUS-Saline, (C) CUS-Paeoniflorin, (D) CUS-Imipramine. BrdU (red). Scale bar 50 $\mu$m. (E) Number of BrdU-labelled cells in DG region of CUS subjects. *p < 0.05 vs. Control-saline group. $^{\#\#}$p < 0.01 vs. CUS-saline group.
The impact of paeoniflorin on the ratio of co-expressed BrdU/GFAP positive cells in the hippocampus DG of CUS subjects is illustrated in {Fig. 4. Co-expressed BrdU/GFAP positive cells were mature GFAP-immunoreactive astrocytes that differentiated from BrdU-positive cells. Somas of GFAP-immunoreactive cells (green) were swollen and fat and had many dendrite-like protrusions. The protrusions were thickened and heavily stained. Positive cells were individually or collectively aggregated into clusters. In co-expressed BrdU/GFAP-positive cells, BrdU was expressed inside the nucleus (red), and GFAP was expressed in the cytoplasm (green) and protruded like dendrites. In the control group, GFAP-immunoactive cells were remarkably extended and long. However, they rarely protruded and were short in the hippocampus of CUS subjects (Fig. 4). One-way ANOVA results suggested that the ratio of co-expressed BrdU/GFAP positive cells significantly differed among groups [F($_{3, 19}$) = 3.2, p = 0.054]. Compared with the control group, the ratio of co-expressed BrdU/GFAP positive cells was significantly smaller in the hippocampus DG of CUS subjects (p = 0.026). Compared with the vehicle group, a significant increase was detected in the ratio of co-expressed BrdU/GFAP positive cells in hippocampus DG of paeoniflorin and imipramine treated groups (p = 0.044, p = 0.025, respectively), as shown in Fig. 4E.
 Figure 4.
Figure 4.Effect of paeoniflorin and imipramine on BrdU/GFAP double immunofluoroscent staining in the DG region of hippocampus in CUS subjects (mean $\pm$ SEM, five rats per group). (A) CUS-Imipramine, (B) CUS-Saline, (C) CUS-Paeoniflorin, (D) Control-Saline in the corresponding areas of the hippocampus. BrdU (red), GFAP (green). White arrow: New astrocyte-like cell. Scale bar 20 $\mu$m. (E) Percentage of BrdU+/GFAP+ cells in the DG region of CUS subjects. *p < 0.05 vs. Control-saline group. $^{\#}$p < 0.05 vs. CUS-saline group.
The effect of paeoniflorin on the ratio of co-expressed BrdU/NeuN positive cells in hippocampus DG of CUS subjects is shown in Fig. 5. Changes to the number of co-expressed BrdU/NeuN positive cells in the hippocampus DG of subjects were measured by double immunofluorescence staining. Co-expressed BrdU/NeuN positive cells were mature NeuN-immunoreactive neural cells into which BrdU-positive cells were differentiated. Observed within a field of view amplified $\times$ 10-20 times, NeuN positive cells (green) are a kind of mature neuron with green nuclei. The nuclei positively co-expressed by BrdU/NeuN were a tangerine yellow color (Fig. 5). One-way ANOVA suggested that the ratio of co-expressed BrdU/NeuN positive cells significantly differed among groups [F$_{(3, 19)}$ = 0.9, p = 0.448]. Under a fluorescence microscope ($\times$ 10-20) no significant difference was observed in the BrdU/NeuN positive co-expression rate between the vehicle group and other groups (p = 0.374, p = 0.457, p = 0.133, respectively) (Fig. 5E).
 Figure 5.
Figure 5.Effect of paeoniflorin and imipramine on BrdU/ NeuN double immunofluoroscent staining in DG region of hippocampus in CUS subjects (mean ± SEM, five rats per group). (A) Control-Saline, (B) CUS-Saline, (C) CUS-Paeoniflorin, (D) CUS-Imipramine in corresponding areas of hippocampus. BrdU (red), NeuN (green). White arrow: Newly granular neuron-like cell. Scale bar 50 $\mu$m. (E) Percentage of BrdU+/NeuN+ cells in the DG region of CUS subjets. There were no significant differences vs. the CUS-saline group.
The effect of paeoniflorin on BDNF protein and mRNA expression in hippocampus DG of CUS subjects is given in Fig. 6 and Fig. 7, respectively. Changes in the number of BDNF positive cells in the hippocampus DG of subjects were measured by immunohistochemical techniques. BDNF, a neurotrophic factor, was mostly expressed in the cytoplasm or on cell membranes. Observed within a field of view amplified ×10-20, the BDNF positive cytoplasm or cell membranes were brownish yellow (Fig. 6). According to one-way ANOVA, BDNF protein expression differed significantly between groups [F$_{(3, 19)}$ = 3.7, p = 0.035]. Compared with the control group, a significant decrease in BDNF positive cells was detected in hippocampus DG of CUS subjects (p = 0.019). Compared with the vehicle group, there was a significant increase in the number of BDNF positive cells in hippocampus DG of paeoniflorin and imipramine treated groups (p = 0.013, p = 0.043) (Fig. 6E). The expression of BDNF mRNA in the hippocampus was detected by qRT-PCR and as indicated by one-way ANOVA significantly differed between groups [F$_{(3, 11)}$ = 4.8, p = 0.033] Compared with the control group, BDNF mRNA expression was significantly downregulated IN CUS subjects (p = 0.009), whereas it was significantly upregulated in CUS subjects treated with paeoniflorin and imipramine treated groups compared with the vehicle group (p = 0.026, p = 0.038, respectively) (Fig. 7).
 Figure 6.
Figure 6.Effect of paeoniflorin and imipramine on level of BDNF protein in hippocampus of CUS subjects (mean ± SEM, five rats per group). (A) Control-Saline, (B) CUS-Saline, (C) CUS-Paeoniflorin, (D) CUS-Imipramine. Scale bar 20 $\mu$m. (E) Effect of paeoniflorin and imipramine on hippocampal BDNF positive cells in CUS subjects. White arrow: BDNF positive cell. *p < 0.05 vs. Control-saline group. $^{\#}$p < 0.05 vs. CUS-saline group.
 Figure 7.
Figure 7.Effect of paeoniflorin and imipramine on BDNF mRNA level in hippocampus of CUS subjects (mean ± SEM, three rats per group). **p < 0.01 vs. Control-saline group. $^{\#}$p < 0.05 vs. CUS-saline group.
The effect of paeoniflorin on TrkB protein expression in hippocampus DG of CUS subjects is illustrated in Fig. 8. Changes to the number of TrkB positive cells in hippocampus DG of subjects were measured by immunohistochemical techniques. Observed within a field of view amplified by $\times$ 10-20, TrkB positive cells appeared as granules and their membranes were brownish yellow. ANOVA results suggested that TrkB positive cells significantly differed among groups [F($_{3, 19}$) = 6.1, p = 0.006]. A significant reduction was detected in TrkB positive cells in hippocampus DG of CUS subjects, with significant differences from the control group (p = 0.001). Compared with the vehicle group, there was a significant increase in the number of TrkB positive cells (p = 0.020) in hippocampus DG of paeoniflorin treated groups, but no significant difference was obtained for imipramine treated groups (p = 0.191) (Fig. 8E).
 Figure 8.
Figure 8.Effect of paeoniflorin and imipramine on TrkB immunohistochemical staining in DG region of CUS subjects (mean ± SEM, five rats per group). (A) Control-Saline, (B) CUS-Saline, (C) CUS-Paeoniflorin, (D) CUS-Imipramine. Scale bar 20 $\mu$m. (E) Effect of paeoniflorin and imipramine on hippocampal TrkB level in CUS subjects. White arrow: TrkB positive cell. *p < 0.05 vs. Control-saline group. $^{\#}$p < 0.05 vs. CUS-saline group.
Extensive studies suggest that most chronic stress inhibits neurogenesis (Dranovsky:2006, Sousa:2000, Malberg:2004). Stress suppresses proliferation of precursor cells in hippocampus DG. It causes morphological changes of neurons and dendritic atrophy of hippocampal pyramidal neurons, thus resulting in DG nerve injuries (Dranovsky:2006, Sousa:2000). After modelling with stressors in this experiment, a significant reduction was observed in newborn neural stem cells expressed by BrdU positive cells in hippocampus DG. These results suggest that CUS reduced proliferation of hippocampal neural stem cells in CUS-induced rat with depressive-like behaviors and suppressed neurogenesis.
According to the results, paeoniflorin enhanced the expression of BrdU positive neural cells in the hippocampus of rats, the proliferation of hippocampal neural stem cells and the transformation of hippocampal neural stem cells of CUS rats into astrocytes (the ratio of co-expressed BrdU/GFAP positive cells), but had no significant effect on the transformation of hippocampal neural stem cells into mature neurons in CUS-induced rats. This suggests that paeoniflorin was effective for promoting neurogenesis and transformation of hippocampal neural stem cells into astrocytes. It has previously been reported that astrocytes promote neurogenesis of hippocampal neural stem cells in adult rats (Guillamón-Vivancos:2015). Astrocytes synthesize and release several neurotrophic factors such as BDNF, glial-derived neurotrophic factors, nerve growth factors, and neurotrophin-3, which are critical for preserving normal neuronal function (Nibuya:1995). Chronic social/psychological stress and on-going treatment of depressive disorders play a role in changing the normal levels of plasticity in neurons, while their hypofunction might be associated with onset of depressive disorders (Guillamón-Vivancos:2015). Results reported here demonstrate that paeoniflorin is effective in promoting the transformation of hippocampal neural stem cells into astrocytes. This suggests that paeoniflorin possibly stimulates hippocampal neurogenesis in rats by secreting neurotrophic factors from transformed mature astrocytes. Thus, BDNF protein and gene expression in hippocampus DG was measured in this study to better determine the exact mechanisms of action.
According to the results, BDNF expression was significantly upregulated in hippocampus DG of CUS rats after administration of paeoniflorin, which could greatly enhance the expression of BDNF mRNA in the hippocampus. Therefore, paeoniflorin should promote hippocampal neurogenesis by enhancing the expression and transcriptional activity of BDNF in the hippocampus of CUS-induced rats. As a high-affinity receptor of BDNF, TrkB mediates biological effects. It is critical for activation of the downstream signal transduction pathway. TrkB exhibits low expression in animal models of depression and antidepressants are effective for treating depression by promoting the expression of TrkB (Nibuya:1995, Tsai:2007, Vigneswara:2012, Koike:2013). A significant increase was observed in the expression of TrkB in the hippocampus DG of CUS rats after they were administered paeoniflorin. According to this result, paeoniflorin enhanced the expression of BDNF and activated the expression of both TrkB (a high-affinity receptor of BDNF) and the BDNF-TrkB signal transduction pathway.
In summary, paeoniflorin stimulates hippocampal neurogenesis, enhances the expression of BDNF and activates the expression of TrkB. This demonstrates that paeoniflorin plays a role in promoting neurogenesis in hippocampus DG of CUS subjects and function as an antidepressant through the BDNF-TrkB signaling pathway. The mechanism of action of paeoniflorin against depression has been further clarified in this study, of which the findings for the further development and utilization of paeoniflorin.
LC contributed to data collection and drafted the article. FQ contributed to preparation of animal models and medicine administration. XZ contributed to coordination of data collection and statistical analysis. CH revised the style of reference. ZH designed the study and revised the manuscript for important intellectual content. All authors contributed to and approved the final manuscript, and agreed to be accountable for all aspects of the work.
This study was carried out in accordance with the principles of the Basel Declaration and recommendations of the Ethical Committee on Laboratory Animals, Zhejiang Chinese Medical University and Principles for protection of Laboratory Animals. The protocol was approved by the Ethical Committee on Laboratory Animals, Zhejiang Chinese Medical University.
This project was supported by the Zhejiang Education Department Research Project (Y201328806 to FQ), Zhejiang Provincial Natural Science Foundation (LQ14H280005 to FQ, Q14H280023 to HZ) and National Natural Science Foundation of China (81573643 to HZ, 81503274 to FQ).
Authors report no confict of interest.
