Electrophysiological monitoring of saccadic eye movements in patients with hypoxic-ischemic encephalopathy was carried out. Externally guided saccades (prosaccades) were recorded using a patented hardware-software complex for studying a subject's physical activity. Recordings were performed in two independent experimental procedures - for saccades separately and when they were coordinated with movement of the head and hand. In both cases statistically significant differences of latent period and duration of saccadic eye movement were detected for hypoxic-ischemic encephalopathy subjects in comparison with healthy controls of the same age (p < 0.05). Jerking and deviation of eyes after gaze fixation on a target were often present in hypoxic-ischemic encephalopathy subjects. In some cases saccades of these subjects were asymmetrical among themselves. Hypoxic-ischemic encephalopathy induced changes in the parameters of autosaccades were also found They were expressed through instability of gaze fixation periods, sometimes asymmetric eye movements, slow gaze shift from one target to another, and disturbance of gaze stabilization (jerking of eyeballs during the saccadic period).
Hypoxic-ischemic encephalopathy (HIE) or brain ischemia, is a condition characterized by blood flow to the brain insufficient to meet metabolic demand [1]. This leads to poor oxygen supply or cerebral hypoxia and thus to the loss of brain tissue or cerebral infarction/ischemic stroke [2]. Ischemia leads to alterations in brain metabolism, reduction in metabolic rates, and energy crisis [3]. HIE is considered to be a slowly progressing brain disease, found mainly among elderly patients. It leads to diffuse structural changes of the brain and, consequently, multifocal damage of function emerge [4]. Pathomorphological changes of cerebral vessels during acute brain ischemia induce degradation of individual neurons, gliacytes and fibers of white matter, and often leads to infarctions of various size and localization in the central nervous system (CNS). Ischemic changes in neurons are usually followed by the formation of glial scars (not total necrosis) and damage axon fibers and the myelin that form the white matter [5]. There are two types of ischemia: focal ischemia is confined to a specific region of the brain while global ischemia encompasses wide areas of brain tissue [5]. Serious ischemia causing focal multifunctional abnormalities (disorders) often lead to lacunar infarcts [6].
Several stages of HIE have been clinically discriminated: the first stage is light or compensatory without significant change in neurological status; the second stage is light or compensatory with characteristic neurological symptoms; while the third stage is very evident with significant focal changes in neurological status (decompensating stage) [7]. The main symptoms involve impairments of vision, body movement and speech. Symptoms of brain ischemia may include unconsciousness, blindness, problems with coordination, and general body weakness [6]. HIE induced pathological changes in the psychomotor system are often followed by significant disorders of movement programming. At the initial stages of the disease they are easily compensated for, dueto increased voluntary control. Disorders of voluntary control caused by disturbances of attention and speech increase with further progression of pathological processes in the brain circulatory system. The development of memory and knowledge dysfunctions result in more evident defects of movement programming. CT scan and MRI (magnetic resonance imaging) in typical cases show multiple microfocal changes mostly in brain cortex, subcortical formations, brain stem, and periventricular areas, which are often followed by atrophy of the brain cortex, increasing volume of brain ventricles (hydrocephalic syndrome), and white matter thinning (leykoareozis) as a consequence of demyelination [7]. It should be noted that changes of brain tissue condition (on CT and MRI) are not always accompanied by clinical manifestations [3].
Disturbances of oculomotor function occur at the earliest stages of various neurological diseases. This is due to the fact that the oculomotor system is the most sensitive to changes occurring in the brain. Normal functioning of eye movements is implemented by a complicated multilevel eye-movement system characterized by a hierarchical structure [8]. The first hierarchical level is represented by a complete efferent structure providing eye movements, and involves nuclei of the 3rd, 4th and 6th pairs of cranial nerves. The second level is organized by the brainstem structures underlying supranuclear control of eye movements (back longitudinal fascicle, mesencephalon and pons cerebelli). The brainstem saccadic generator is an important structure responsible for eye movements. For horizontal eye movements it is located in the paramedial mesencephalon [9]. The third level of the eye-movement system is comprised of structures controlling the saccadic brainstem generator. This subsystem, responsible for associated eye movements is comprised of the thalamus, basal ganglions, interior capsule, superior colliculus of corpora quadrigemina, external geniculatus body, and the mesolobus. The fourth level consists of different zones of hemispheric brain cortex: frontal, rear parietal and occipital lobes, prestriar cortex, additional eye movement fields, dorsolateral frontal eye field (Brodmann's area 8) and the posterior cingulate. Disorders in the oculomotor system have been investigated in patients with different syndromes: schizophrenia [10,11,12], attention-deficit/hyperactivity disorder [13,14,15], Parkinson's disease [16,17,18,19], and Alzheimer’s disease [20]. However, data on HIE influence of saccadic eye movements are rather scarce. The main aim of the present study was to evaluate properties of externally guided saccades in HIE patients. Externally guided saccades assessed through visually guided saccadic tasks, where subjects are instructed to look at a visual stimulus as soon as it appears, are usually referred as prosaccades [13,21]. In the prosaccade task designed for the present study the subjects (HIE patients and healthy controls) were instructed to fixate on a central stimulus, until a new flashing point appeared to the left or right. Saccadic movements towards the emerging stimuli were analyzed.
Recordings of prosaccades were made during two independent experimental procedures - for eye movements separately (Test 1) and when eye movements were coordinated with head and hand motion (Test 2). Latent structural and functional changes arising at the beginning of a pathological process, that underlie the mechanisms of each simple motion are not detectable at the level of clinical symptoms. However, movements made during a joint coordinated movement superimpose on each other and as a result manifest themselves in the form of significant changes. This suggests that due to superimposition of these pathological changes the likelihood of detecting motor disorders at the earliest stages of a disease is significantly increased during implementation of coordinated movement. In a former study performed on patients in the early stages of Parkinson's disease, saccade impairment not detected by Test 1 were significantly expressed during the coordinated test [18,19].
Accordingly, in the present study it was decided to apply the coordinated test to HIE subjects given that the saccade disorders recorded may be more pronounced when compared with a simple test (Test 1) where only saccadic movements were recorded (head and hand of a remained motionless). Attention was focussed on latent periods and the durations of recorded prosaccades. HIE induced saccade disorders observed during the study are discussed.
Present multidisciplinary clinical and neurophysiological study was carried out at the laboratory of neurocybernetics of the Research Center of Neurology, and followed contemporary ethical norms and rules for biomedical researches in accordance with the World Medical Association's 1964 Helsinki Agreement. All subjects signed up the inform consent on participation in the study.
24 patients (age range 52-73 years) with the first and second clinical stages of HIE were investigated and the results compared with a control group (norm) (20 healthy persons, age range 54-71 years).
As diagnosed by brain MRI all patients had symptoms of multifocal vascular cerebropathy with focal damage (in subcortical parts of frontal, temporal and parietal lobes, subcortical nuclei and radiating crown, semioval centre, and pons). Patients complained of unstable walking, periodic indistinct eyesight and diplopia, impairment of memory, disorder of sleep, headaches, vertigo, periodic hand tremor, bradykinesia, weakness in an arm or a leg. The complaints were very diverse. In the majority of subjects neurological examination frequently revealed asymmetric pyramidal syndrome. This was more obviously expressed in the legs. Pathological reflexes such as the Rossolimo symptom, symptoms of oral automatism on one or two sides, patient wobbling in the Romberg pose, insignificant disorder of movement coordination, and postural and intentional tremor of arms were also present. HIE induced disorders of cerebral innervation caused asymmetry of the nasolabial fold and light deviation of the tongue in nine patients. Four patients had amiostatic syndrome, which clinically resembles Parkinsonism (hypokinesias). It is expressed as a disorder of movement initiation and slight muscle stiffness.
Prosaccades were examined either separately or when coordinated with head and hand motion. Purposeful movements were performed in a horizontal direction in response to a visual stimulus by both HIE and control subjects. Electrophysiological recordings of eye, head, and hand movements were obtained by means of a complex patented hardware-software unit for studying human physical activity [22,23] (Fig. 1A). Electrophysiological monitoring was carried out in a light proof screened room. Constant illumination (40 lux) was maintained inside the room during examination of the subjects. During the study a subject was seated about 70 cm from a horizontal support. The subject’s head was fixed in a plastic helmet that could move horizontally. Stimulation was controlled by the investigator. It consisted of visual targets provided by 10 light-emitting diodes (LEDs) placed at different positions along the horizontal support (five each for right and left saccades). The task for subjects was to fixate on LEDs that were alternately switched on, each of which presented a single visual target. Eye movement, evoked by the location of the LED target along the the horizontal support was recorded from both eyes simultaneously by means of electrooculography (EOG). Recording electrodes were placed at the lateral margins of the eye-pits (orbits) while the referent electrode was attached to the ear [16]. The EOG signal, was amplified and low-pass filtered by a DC amplifier (band pass 0.1 Hz-150 Hz) and subsequently digitized by an A/D converter (512 Hz sampling rate). A software package (computer program "Neocortex") was used for storage and subsequent off-line processing of the digitized data.
 Fig. 1.
Fig. 1.Hardware-software complex unit for studying physical activity of a subject. (A) A photo of the equipment: 1 - horizontal support; 2 - plastic helmet; 3 - hand lever used for movement of the cursor (5); 4 - hand movement indicator (detector); 6 - head movement indicator (detector); 7 - light emitting LEDs located at different positions along the horizontal support; each LED provided a single visual target. Detailed description of the equipment is given in the text. (B) A photo made during the coordinated test: The subject visually fixates on the far right and simultaneously moves the hand lever ($ {40^\circ} $ gaze shift to the right; rightward hand movement - 54 cm). Detailed explanation of the coordinated test (Test 2) is given in the text.
As mentioned above, recordings of prosaccades were performed during two independent experimental procedures - for eye movements separately and then when coordinated with the motion of head and hand. In the first procedure the subject was instructed to keep their head motionless during recordings and to fixate on the visual target, an illuminated LED, solely by using eye movement. If, during the visually-guided saccade task, the subject accidentally moved their head, which could be determined from a monitor, any associated saccadic movements were disregarded. A subject was initially positioned in front of the far left LED. They were then instructed to fixate on other LEDS that switched on and off sequentially and horizontally across the right half of the visual field i.e. right saccades were evoked. Distances between LEDs were arranged such that the rightward appearing peripheral visual targets were placed 10, 20, 30 and 40$^\circ$ from the far left stimulus. Prior to the start of measurement, saccade amplitudes (10-40$^\circ$) were individually calibrated for each subject. The peripheral target always switched on simultaneously with the central target switching-off, i.e. without any temporal gap or overlap. Before the presentation of the succeeding peripheral target the subject had to return their gaze to the control position. In other words, any switching-off of the peripheral target was followed by the switching-on of the control LED. Peripheral LEDs were illuminated in a quasi-random sequence in order to avoid adaptation to saccades of any particular amplitude. In a series of 50-60 stimulus presentations peripheral LEDs of different angle values were used. However, only 40$^\circ$ saccades were recorded for subsequent processing (among all presentations of stimuli 40$^\circ$ peripheral targets were shown on 20-30 occasions). Further, saccade data was only collected in the case of exact fixation of the peripheral stimulus - saccades that exhibited eye jerking or multisaccades were rejected. In the second half of the procedure a subject was placed in front of the far right LED diode and 40$^\circ$ left saccades were recorded using the equivalent experimental procedure.
During the second procedure the head was fixed in the plastic helmet, but could move horizontally in coordination with the eyes. Movements of the eyes and head were additionally coordinated with the motion of the hand. The task for a subject in this procedure was to simultaneously fixate on a target visually and by hand (coordinately). Fixation by hand was as follows - the subject had to move a cursor by means of a hand lever along the support for the LEDs, to the position of the visual target (a LED) was illuminated (Fig. 1A). The helmet and the lever had indicators joined to potentiometers which detected the position of the head and hand. It should be noted that this second procedure was also performed independently for right- and left-directed saccades. Similarly to the first test, peripheral visual targets of different angle values were illuminated in a quasi-random sequence, but only prosaccades evoked by 40$^\circ$ stimuli were recorded and afterwards processed and analysed.
The essential characteristic of the method employed here that distinguishes it from other studies was that only the saccades evoked by the 40$\circ$ stimulus were analyzed. This was contrary to strong recommendations to use the conventional prosaccadic step task. That task does not support recording eye movements with amplitudes greater than 10$^\circ$ as under natural conditions they are accompanied by head movement [24]. To adequately compare the saccades recorded in both simple and coordinated tests, it is necessary that the movements in both tests are evoked by a stimulus that is moved to the same angle. To force subjects to move their heads in the coordinated test, the stimulus must be moved to an angle of exactly 40$^\circ$. Further, the requirement for reflex head movement suppression during Test 1 is an additional complicating factor for a subject that also increases the probability of detection of saccade disorders.
As mentioned above, in a series of presentations of peripheral stimuli of 40$^\circ$, visual targets were shown on 20-30 occasions. In both tests single saccade responses to 40$^\circ$ stimuli were recorded during 2-second temporal periods (see Fig. 3 and Fig. 6). Latent periods and durations were measured for all accurate saccades recorded in the series (the ones with eye jerking or multisaccades were rejected) and averaged values of these two parameters were calculated for each subject tested in the study.
In short, the "prosaccades" of HIE subjects and controls were investigated using two independent tests:
Test 1 - subjects had to perform saccadic movements in response to the transfer of targets to the right or to the left from the control position, subsequently stabilizing their gaze on the peripheral target, to finally return their gaze to the initial position after reillumination of the control LED (motionless head; only the 40$^\circ$ saccades were recorded, subsequently processed, and analyzed - latency and duration of saccadic movement was measured and statistically analysed);
Test 2 - a subject had to perform a joint coordinated and purposeful move of eyes, head, and hand in response to the transfer of a target from the control position to the right or to the left, subsequently stabilizing the gaze and hand on the peripheral target, finally to return the gaze and the hand to the initial position after the control visual target was reilluminated (coordinated movements were recorded only for the 40$^\circ$ gaze shifts - latency and duration of saccadic movement was measured and submitted to statistical analysis).
Finally, in the independent test (Test 3) "autosaccades" were investigated, both for HIE subjects and controls. The far left and the far right LED were both switched on throughout the procedure used for Test 3. A subject was placed in front of the central LED, head motionless. During the entire procedure which lasted approximately 1 min they had to spontaneously transfer their gaze from the far left to the far right LED and back (using only eye movements). Rhythmic eye movements that occurred when the gaze was alternately moved from the left to the right target and back are referred as autosaccades. Recorded autosaccades were divided into 4-second durations which were separately saved for further analysis (see Fig. 7).
The statistical analyses of results employed Statistica 7.0 software (StatSoft, Inc.). Statistical differences between healthy controls and HIE subjects were evaluated by means of the nonparametric two-tailed Mann-Whitney U-test, and significance levels were adjusted in accordance with the overall number of comparisons. Statistical differences between Test 1 and Test 2 (HIE patients) were performed according to the Wilcoxon matched pairs test (nonparametric alternative to the t-test for dependent samples). For all tests the significance level was set at p = 0.05.
The analysis of saccade trajectories in control subjects (norm) and subjects with HIE were conducted by means of Test 1. Ten patients had disorders of attention and difficulties in understanding the task while performing the required test. The growth of the latent period of saccadic eye movements and increased saccadic duration were observed for a majority of subjects. Statistically significant differences in latent period and duration were found between HIE subjects and controls of the same age group (Mann-Whitney U-test; p < 0.05) (Fig. 2). HIE induced increase of saccade latency and duration is clearly shown in Fig. 3A and 3B, where the saccadic movements recorded in one control and one HIE subject are shown (single responses from a series of 30 recorded saccades). In the majority of tested subjects only a single saccade was required for the fixation of a target. However, in some cases multisaccades were elicited, a few more in HIE subjects (4% of all recordings in controls and 6.6% in HIE subjects). In these particular cases the target was acquired by both the main and a subsequent second saccade (Fig. 3C). As a rule, the amplitude of the second saccade was lower. Moreover, for some HIE subjects separate saccades were asymmetric among themselves (Fig. 3C).
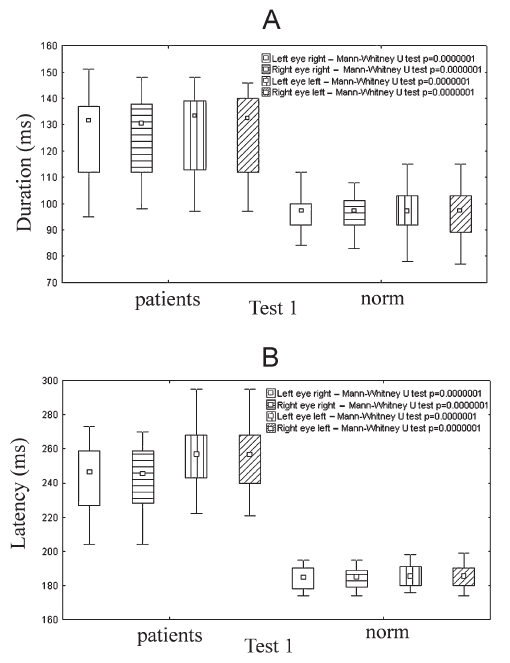 Fig. 2.
Fig. 2.Differences in the mean value of saccade durations (A) and latent periods (B) between HIE subjects and healthy controls (norm) of the same age group in the Test 1 (Mann-Whitney U-test: small inner "$\Box $" - median; large outside "$\Box$" - 25%-75%; "I" - non outlier range (max and min value); p < 0.05). P-values for comparisons between the data of right and left saccades of two groups of subjects are given in the top right corners. A participant (HIE patient or healthy person) was initially positioned in front of the far left diode (control position). They were instructed to fixate on other LEDs as they illuminated sequentially in a quasi random sequence within the right half of the visual field i.e. right saccades were thus evoked (left eye right, right eye right). In the second part of the procedure a subject was placed in the front of the far right LED and left saccades were recorded (left eye left, right eye left). Distances between LEDs were arranged such that the peripheral visual targets were located 10, 20, 30 and 40 40$ {^\circ} $ apart from the control stimulus (far left or far right LED). Only 40 40$ {^\circ} $ saccades were recorded and afterwards processed and analysed. Detailed explanation of Test 1 is given in the text.
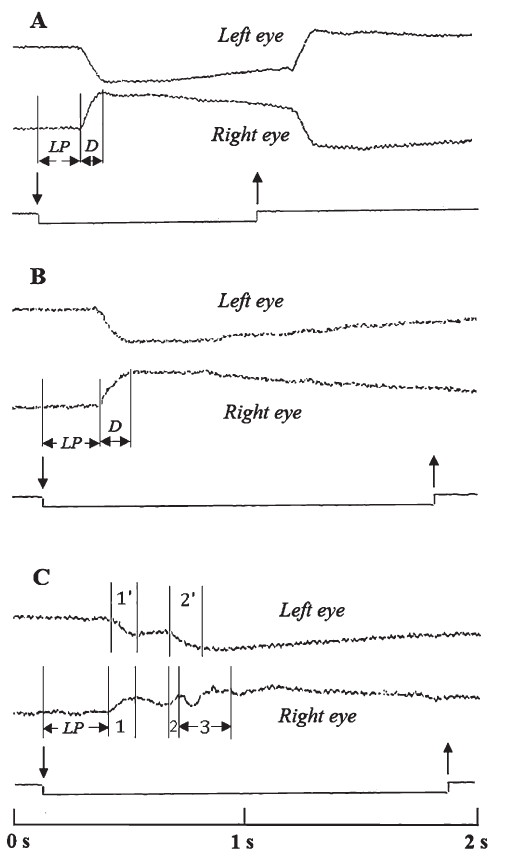 Fig. 3.
Fig. 3.Saccadic movements to the right (Test 1; single responses from the series of 30 recorded prosaccades). (A) control; (B) HIE subject (with increased latent period and duration of saccadic eye movements); (C) multisaccades recorded in one HIE subject: 1, 2 - durations of the first and the second right eye saccades, respectively; 1', 2' - durations of the first and the second left eye saccades, respectively; note that second right eye saccade was interrupted by transient eye jerking (3)subsequently right eye stabilized on the lateral target (detailed explanation in the text). LP - saccadic latent periods, D - duration of saccades. Downward oriented vertical arrows indicate beginning of stimulation i.e. moment of target relocation from the control to peripheral position; upward oriented vertical arrows indicate moment of return of target from the peripheral to the control position. Saccades amplitudes - 40$ {^\circ} $. Time scale given at bottom.
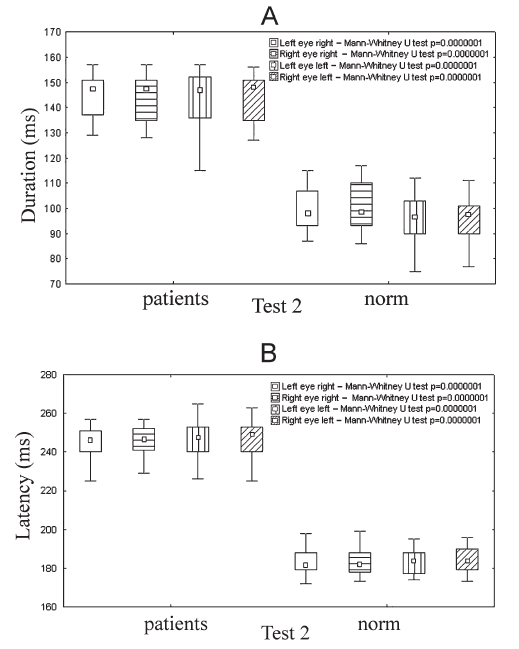 Fig. 4.
Fig. 4.Comparison of saccadic eye movement for HIE subjects and controls (norm) in same age group in coordinated test (Mann-Whitney U-test; $ p < $ 0.05). $p$-values for comparisons between the averaged values of saccade durations (A) and the latencies (B) of the two subject groups are given in top right corners. Latency and duration of saccadic movement was independently recorded at 40 $ \mathrm{{}^\circ} $ for gaze shifts of right and left saccades independently. Other conditions identical to Fig. 2. Detailed explanation of coordinated test (Test 2) given in the text.
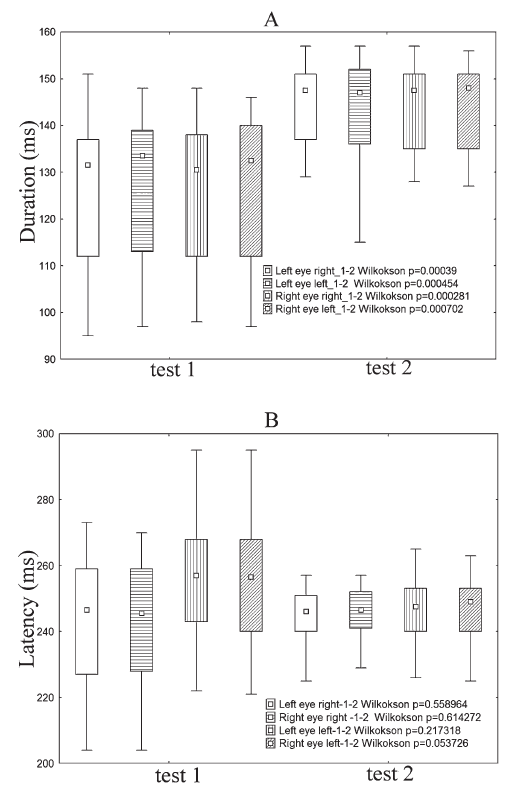 Fig. 5.
Fig. 5.Differences between Test 1 and Test 2 in averaged value of saccade durations (A) and latencies (B) for HIE subjects (Wilcoxon matched pairs test; $ p < $ 0.05). P-values for comparisons between the saccade parameters in the two tests are given in the right bottom corner. Other conditions identical to Fig. 2.
 Fig. 6.
Fig. 6.Coordinated movements of eyes, head and hand for 40 $ \mathrm{{}^\circ} $ gaze shift to the left. (A) control (a single response from 30 recorded prosaccades); note sequence of eye, head and hand movements typical for controls - initially eyes moved towards the target, subsequently a slightly delayed, head turn was initiated, followed by initiation of hand movement; (B) HIE subject (an inadequate single response from 25 recorded prosaccades). Other conditions identical to Fig. 3.
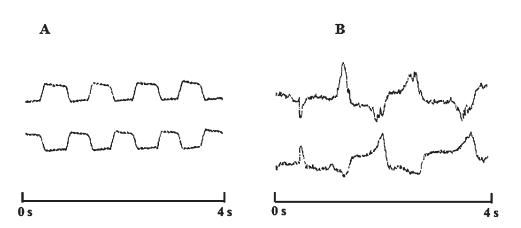 Fig. 7.
Fig. 7.(A) control autosaccades; (B) HIE subject autosaccades. Time scales given at bottom.
While making a coordinate test (Test 2) considerable change in the electrophysiological parameters of eye movement were detected in HIE subjects. It must be emphasized that latent period and saccade duration increased in nearly all HIE subjects when compared to controls (with the exception of two HIE subjects). Comparative characteristics of saccadic movement for controls and HIE subjects in the coordinate test are shown in Fig. 4 (Mann-Whitney U-test; p < 0.05). Despite saccades observed in the coordinate test being of lesser amplitude than in Test 1 their duration was significantly increased relative to saccade duration recorded in the same patients during Test 1 (Wilcoxon matched pairs test; p < 0.05) (Fig. 5A). On the other hand, average values of saccade latencies did not differ significantly between the two tests (Fig. 5B). However, the range of saccade latencies (the differences between minimum and maximum values recorded during each series of stimuli) in Test 2 were considerably larger than in Test 1 for each tested subject (Table 1). Among other saccade abnormalities expressed by HIE subjects during Test 2, the most severe dysfunctions were associated with disorders of gaze-holding. Disturbances of gaze stabilization were often observed in the coordinate test for HIE subjects after saccade movements in either direction. Also, multisaccades occurred more frequently during Test 2 than during Test 1. While the percentage of multisaccades in controls remained similar (6% from all recordings), in HIE subjectss the number of multisaccades significantly increased in the Test 2, up to 23% of all recorded saccadic movements. In two patients a severe deviation of the eyeballs during and after termination of saccadic movement was detected. Some HIE subjects were not always able to perform adequate saccades (three subjects). An example of such functional disorder is presented in Fig. 6. Saccade disorder was followed by a short inadequate head movement the. It was easily seen that the head of the patient returned to the initial position much earlier than the stimulus returned to the central point (Fig. 6B). The same happened with the patient's hand - it returned to the initial position prior to the stimulus recentering (Fig. 6B).
During Test 3 (in which "autosaccades" were investigated), changes were found in different parameters that showed an instability of gaze fixation periods, sometimes asymmetry of saccades, slow gaze shift from one target to another and a disturbance of gaze stabilization (seen as jerking of the eyeballs during the saccadic period). Examples of "autosaccades" in a control and a HIE subject are shown in Fig. 7.
| Patient # | Left eye right | Right eye right | Left eye left | Right eye left | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test-1 | Test-2 | Test-1 | Test-2 | Test-1 | Test-2 | Test-1 | Test-2 | |||||||||||||||||
| mean | min | max | mean | min | max | mean | min | max | mean | min | max | mean | min | max | mean | min | max | mean | min | max | mean | min | max | |
| 1 | 204 | 198 | 226 | 232 | 204 | 247 | 204 | 198 | 216 | 229 | 201 | 247 | 222 | 212 | 232 | 226 | 200 | 251 | 221 | 212 | 230 | 225 | 200 | 250 |
| 2 | 266 | 251 | 273 | 251 | 234 | 281 | 266 | 250 | 271 | 251 | 232 | 280 | 295 | 286 | 304 | 276 | 251 | 301 | 295 | 286 | 304 | 276 | 250 | 301 |
| 3 | 259 | 245 | 275 | 251 | 226 | 280 | 258 | 244 | 272 | 250 | 225 | 278 | 268 | 257 | 278 | 249 | 226 | 278 | 267 | 257 | 277 | 251 | 226 | 282 |
| 4 | 237 | 224 | 250 | 249 | 223 | 273 | 238 | 226 | 250 | 247 | 223 | 271 | 243 | 231 | 256 | 244 | 220 | 269 | 245 | 231 | 259 | 245 | 221 | 269 |
| 5 | 215 | 205 | 226 | 225 | 202 | 246 | 215 | 205 | 226 | 224 | 202 | 244 | 239 | 229 | 248 | 235 | 210 | 265 | 237 | 227 | 248 | 230 | 207 | 263 |
| 6 | 263 | 246 | 274 | 250 | 233 | 283 | 263 | 246 | 272 | 255 | 236 | 287 | 276 | 265 | 290 | 273 | 245 | 300 | 279 | 269 | 290 | 273 | 245 | 300 |
| 7 | 227 | 216 | 230 | 244 | 219 | 257 | 228 | 215 | 231 | 242 | 219 | 255 | 241 | 227 | 253 | 238 | 214 | 262 | 239 | 225 | 253 | 238 | 214 | 262 |
| 8 | 213 | 208 | 230 | 236 | 211 | 259 | 219 | 208 | 230 | 234 | 211 | 257 | 234 | 222 | 243 | 235 | 212 | 262 | 231 | 219 | 243 | 235 | 212 | 262 |
| 9 | 253 | 240 | 268 | 253 | 231 | 288 | 254 | 241 | 267 | 252 | 230 | 286 | 266 | 255 | 277 | 252 | 226 | 284 | 266 | 255 | 277 | 252 | 226 | 284 |
| 10 | 221 | 211 | 236 | 238 | 213 | 261 | 222 | 211 | 234 | 238 | 213 | 264 | 243 | 228 | 255 | 238 | 211 | 264 | 240 | 226 | 254 | 236 | 211 | 262 |
| 11 | 245 | 231 | 259 | 246 | 221 | 268 | 244 | 231 | 257 | 244 | 220 | 268 | 259 | 246 | 273 | 241 | 222 | 268 | 259 | 246 | 273 | 247 | 222 | 272 |
| 12 | 261 | 248 | 276 | 253 | 236 | 287 | 263 | 250 | 276 | 253 | 236 | 286 | 273 | 261 | 284 | 263 | 242 | 290 | 276 | 265 | 287 | 263 | 242 | 290 |
| 13 | 234 | 221 | 247 | 240 | 218 | 268 | 234 | 223 | 245 | 243 | 220 | 267 | 247 | 231 | 257 | 258 | 235 | 286 | 245 | 231 | 255 | 260 | 235 | 286 |
| 14 | 273 | 250 | 278 | 255 | 232 | 290 | 270 | 248 | 278 | 254 | 231 | 290 | 279 | 270 | 294 | 265 | 240 | 294 | 284 | 274 | 294 | 263 | 240 | 292 |
| 15 | 226 | 214 | 232 | 241 | 218 | 256 | 226 | 214 | 232 | 241 | 218 | 257 | 231 | 220 | 244 | 240 | 216 | 264 | 233 | 220 | 246 | 240 | 216 | 264 |
| 16 | 248 | 235 | 263 | 245 | 223 | 275 | 247 | 235 | 262 | 248 | 225 | 275 | 253 | 240 | 266 | 246 | 228 | 276 | 254 | 242 | 266 | 246 | 228 | 276 |
| 17 | 231 | 216 | 244 | 246 | 221 | 271 | 229 | 216 | 242 | 246 | 221 | 271 | 243 | 229 | 256 | 251 | 225 | 277 | 243 | 229 | 256 | 250 | 225 | 275 |
| 18 | 258 | 243 | 268 | 257 | 236 | 286 | 259 | 245 | 267 | 257 | 236 | 288 | 267 | 256 | 278 | 250 | 223 | 275 | 268 | 258 | 278 | 253 | 221 | 277 |
| 19 | 251 | 239 | 263 | 248 | 220 | 276 | 250 | 237 | 263 | 248 | 220 | 275 | 259 | 248 | 272 | 250 | 223 | 277 | 260 | 248 | 274 | 251 | 225 | 277 |
| 20 | 248 | 235 | 262 | 246 | 222 | 270 | 247 | 235 | 260 | 245 | 222 | 268 | 260 | 246 | 272 | 246 | 222 | 272 | 258 | 244 | 272 | 248 | 224 | 272 |
| 21 | 262 | 248 | 274 | 251 | 228 | 284 | 261 | 247 | 275 | 252 | 228 | 282 | 273 | 261 | 279 | 253 | 226 | 278 | 269 | 261 | 279 | 251 | 223 | 279 |
| 22 | 240 | 232 | 254 | 238 | 223 | 275 | 239 | 230 | 256 | 238 | 221 | 273 | 255 | 245 | 266 | 245 | 226 | 278 | 255 | 245 | 266 | 248 | 226 | 280 |
Pathological changes in eye movements observed during this study in HIE subjects, such as increased saccade latency and duration, jerking of eyeballs during the saccadic period and following their termination, and finally, dissonance of autosaccades, could be a consequence of the disintegration of brain networks that ensure general neuroplasticity in the CNS [25]. It was shown that, during various neurogeriatric diseases including the HIE brain, neurotransmission is seriously affected. As was shown in a number of previous studies, neurotransmission in the CNS depends upon the integrity of the brain's inter-areal connectivity, which in turn, is functionally dependent on the condition of myelinated fibers [3]. Degradation of myelinated fibers during HIE can effect qualitative and quantitative properties of nerve impulses and cause substantial changes of inter-areal connectivity [25]. the frontal lobes, the destination of neuropathological, and structural disturbances, are subjected to the most significant changes [26]. Significant functional and structural changes affected by demyelination were also observed in the subcortical formations, periventricular region, and damage in brainstem structures often leads to the "disconnection syndrome" underlying clinical symptoms and cognitive dysfunction [27]. From all of the abovementioned, it can be concluded that HIE induces morphological changes of the brain white matter which in turn causes degradation of its functional properties and consequently, seriously effects functional integrity of the CNS. Thus, rates and synchronism of neural processes should be significantly affected which, in this view, caused the movement and cognitive disorders of the HIE subjects that were expressed as impairments of saccade programming, increased saccade latency and duration, disturbance of attention, and lack of understanding of assigned tasks. The neurobiological bases of prosaccades have been investigated in a number of studies (for reviews, see Pierrot [28,29,30,31]. The oculomotor system ensures efficient and effective goal-directed saccades and comprises different cortical and subcortical structures. The paramedian pontine reticular formation (PPRF) is involved in the coordination of eye movements, particularly horizontal gaze and saccades. The rostral PPRF probably coordinates vertical saccades, whereas the caudal PPRF may be the generator of horizontal saccades. It receives excitatory inputs from the superior colliculus (SC) and from the frontal eye fields (FEFs). The SC in turn receives excitatory and inhibitory inputs from different cortical structures involved in oculomotor control. Direct excitatory pathways to the SC come from the FEFs, dorsolateral prefrontal cortex, parietal eye fields, and supplementary eye fields. Indirect cortical inputs to SC from the dorsolateral prefrontal cortex and FEFs are sent through the caudate nucleus, which inhibits the substantia nigra pars reticulata, which in turn inhibits the SC. Patients suffering from HIE have disorders in brain inter-areal connections due to ischemic damage in distinct brain areas such as the PPRF, different subcortical structures, and the brain cortex (to a larger degree in FEF - Brodman's area 8) [9]. As a consequence, HIE induced disorders of excitatory and inhibitory influences in the oculomotor system may induce slow functioning of the saccade generator. Parkinsonian syndrome is an example of how dysfunction of inhibition, which occurs on the level of subcortical structures, can induce saccadic disorders.1(1 The motor symptoms of Parkinson disease result from reduced activity of inhibitory dopaminergic neurons in the substantia nigra [32].) In a number of studies of patients with Parkinson disease saccadic disorders similar to those described in the present study were observed [16,17,18,19] It should be emphasized that in some cases HIE induced disorders of saccadic electrophysiological parameters were even more pronounced than in Parkinson's patients (in particular three HIE subjects with clearly expressed hypokinetic syndrome). In comparison with Parkinson's patients HIE subjects with hypokinetic syndrome were characterized by increased instability of the latent period of saccades in the coordinated test and a pronounced disturbance of gaze stabilization after termination of saccades that was manifested by tremor eye movement of different amplitudes.
Finally, it is necessary to point out that in two subjects with considerable focal damage diagnosed from their brain MRI it was not possible to find significant deviations of electrophysiological parameters related to eye movement. This provides an opportunity in these cases to describe the functioning of cortical-subcortical and subcortical-brainstem interactions as close to normal and attests to sufficient maintenance of compensatory brain mechanisms in these two HIE subjects (they were not included in the statistical analysis).
Results of this study give additional information on impairments in the programming and implementation of saccadic eye movements (including autosaccades) in HIE subjects. HIE-induced saccade dysfunctions were demonstrated by two independent experimental procedures - for eye movements alone and when coordinated with head and hand motion. Controls successfully managed the task of reflex of head movement for 40$^\circ$ saccades in the first test. On the contrary, for HIE subjects, this caused considerable difficulty, which resulted in increased saccade latencies and durations. The complication of the task by coordinated movements in Test 2 led to a further deterioration of the physiological parameters of saccades in subjects. First, saccade duration in the coordinated test was statistically significantly increased relative to saccade duration recorded in the same patients during Test 1 (Fig. 5A). Another significant HIE induced impairment of saccadic function observed in Test 2 was the larger range of differences between minimum and maximum values of saccade latencies in comparison with those recorded in the same patients during the first test. It is considered here that with sufficient will-power, subjects initially increased their concentration to perform a complex task in the coordinated test. However, due to cognitive impairment they were unable to maintain concentration at a sufficiently raised level and, consequentially, the range of saccade latencies increased considerably (Table 1). Significant increase in the percentage of multisaccades was also observed in subjects during Test 2. Numerous disturbances of gaze stabilization were observed that considerably increased eye tremor. All impairments of saccadic function in the coordinated test when compared with Test 1 argue for the abovementioned hypothesis that the pathological functional changes underlying the mechanisms of each simple motion superimpose during joint coordinated movement. The hypothetical changes described in the motor system arising during the coordination of movements should negatively affect the implementation of the visual task in Test 2, which is actually what was demonstrated in this study.
Patterns of saccadic eye movement disorder provide additional information about the degree of HIE induced cortico-subcortical and brainstem dysfunction. In clinical practice observing saccade dysfunctions can help with the performance of functional HIE diagnostics and estimations of the effectiveness of vascular, metabolic, and nootropic therapy. Finally, recorded saccadic disorders enable more precise evaluation of the degree of damage of compensatory processes involved in the brain functions of a particular patient.
We are grateful to Luka Ga$\check{c}$ić who provided improvements to our English.
All authors declare no conflicts of interest.
