White matter asymmetries of the human brain have been well documented using diffusion tensor imaging. The purpose of this study was to investigate white matter asymmetry across the whole brain in cerebral small vessel disease patients and evaluate the relation between the factors which often represent disease existence and white matter asymmetry. A total of 105 nondemented elderly subjects with cerebral small vessel disease aged between 60 and 85 years were included in this study. All subjects underwent T1 MPRAGE, fluid-attenuated inversion recovery, and diffusion tensor imaging scanning. With tract-based spatial statistics in diffusion tensor imaging, this study examined the white matter asymmetries and the correlations between white matter asymmetries and four distinct factors such as deep white matter hyperintensities score, periventricular hyperintensities score, cerebral microbleed number and lacune number. The study suggested the asymmetric microstructural change in small vessel disease patients involving the right side being more injured than the left. The four factors jointly affect right brain anisotropy decrease in the middle cerebellar peduncle, the cerebral peduncle, the pontine crossing tract, the corticospinal tract, the medial lemniscus, the posterior limb of internal capsule, and the frontal pattern of white matter. Results of the study demonstrated the lost right white matter may be the main origin of dysfunction in small vessel disease patients. This asymmetry should help with the evaluation of prognostic indicators of disease progression in lesion-based neuropathology.
Anatomical asymmetries of the brain can be well identified by diffusion tensor imaging (DTI), which is a magnetic resonance (MR) imaging technique that probes the architecture of biological tissues with great sensitivity. Previous studies have reported that DTI is more sensitive to structural changes of white matter in the human brain. Meanwhile, it is less sensitive in differentiating T2-lesion volume from brain volume [1]. Fractional anisotropy (FA) and mean diffusivity (MD), which are the most common diffusion parameters, were widely used to estimate white matter asymmetry in previous studies. The white matter FA asymmetries of the healthy group were most frequently reported in previous studies. For example, the right-handed group had higher FA values in the left precentral gyrus [2]. The FA values of left internal capsule (posterior limb) [3], inferior longitudinal fasciculus [4], cingulum [5,6], and superior longitudinal fasciculus [7] were greater than the FA values of the right arcuate fasciculus [4,8] and the right internal capsule (anterior limb) [9]. However, the hemispheric asymmetry of FA mostly existed in healthy juveniles. Hemispheric asymmetry was much less in healthy middle-aged people [3]. All these studies provided an important insight into the neuroanatomical basis of lateralized brain functions, such as language learning and handedness [10,11].
Cerebral small vessel disease (SVD) is a leading cause of cognitive decline, and it commonly occurs in aged brains. It is an important cause of vascular cognitive impairment, incident dementia [12], Parkinsonism [13], and Alzheimer's disease [14]. Dyslexia (Beaton, 1997), Friedreich ataxia [15], Alzheimer's disease, dystonia [16], multiple sclerosis [11], and Parkinson's disease are the neurological ailments that cause lateralized pathologies between the two hemispheres of the human brain. Recently, many studies have reported about the occurrence of cognitive dysfunction in patients with SVD [17,18]. Most DTI studies of white matter have reported about reduced FA and increased ‘apparent' diffusivity in patients with SVD; however, there is a lack of white matter asymmetry inpatients with SVD. In addition, cerebral SVD is considered as a relatively homogeneous disease process. In SVD patients, most adverse effects are observed in the brain parenchyma; lesions mostly develop in the subcortical structures of the brain. Lacunar infarcts, white matter lesions (WML), and microbleeds are commonly observed in the brain parenchyma of SVD patients [12]. Therefore, this study investigated structural asymmetry in the brain of SVD patients. Furthermore, it was also evaluated whether brain asymmetry was affected by deep white matter hyperintensity (DWMH) scores, periventricular hyperintensity (PVH) scores, cerebral microbleed(CMB) number, and lacune number.
Tract-based spatial statistics (TBSS) is a whole-brain analysis technique that can be performed in an unbiased, fully automated environment. Therefore, it is used to compare the diffusion tensor properties of multiple subjects and to investigate the integrity of white matter in clinical studies. The TBSS technique offers several advantages over the voxel-based statistical approaches conventionally used for brain analysis [19].Therefore, this technique may be used to further elucidate white matter pathways that are specifically impaired in SVD patients. The extent of changes in the microstructure of the major white matter tracts was determined with the TBSS technique. Furthermore, additional lateral information in SVD patients was assessed by the DTI technique. This information helps in diagnosing cerebral SVD that causes changes in the human brain.
In neuroimaging studies, cerebral SVD is characterized by the presence of WML, lacunar infarcts, and CMB. Transient ischaemic attacks (TIAs) are often one of the symptoms of SVD. In this study, 117 non-demented old people were included that showed mild symptoms of ischemic cerebral vascular disease. All participants satisfied the following conditions: (i) older than 60 years of age, (ii) right-handed, and (iii), able to carry out basic daily activities, such as going out, reading, shopping, etc. The exclusion criteria were: (i) patients with complicated neuropsychiatric disorders, such as dementia, intracerebral hemorrhage (ICH), Parkinson's disease (PD), head trauma, primary and metastatic tumors in the central nervous system (CNS), other complications that cause mental disorders or cognitive impairment, and mental illness, (ii) MRI contraindications, (iii) severe visual or hearing impairment that affects communication, and (iv) history of drug abuse.
The study was approved by the Xiangya Hospital Ethics Committee. A written consent form was obtained from each participant.
The detailed clinical information of subjects included, patient history, physical examination records, and diagnostic assessments. It included the details of macrovascular disease (stroke or myocardial infarction that required hospitalization, and surgical or endovascular treatment for carotid, peripheral arterial, or coronary disease) and small vessel disease (the highest tertile of WMH volume (see below) or a lacunar infarct in a brain MRI). Diabetes mellitus was diagnosed under the following conditions: fasting blood glucose $\ge$ 126 mg/dL (7.0 mmol/L), random blood glucose $\ge$ 200mg/dL (11.1 mmol/L), hemoglobin A1c $\ge$ 6.5 $\%$, or a history of anti-diabetic treatment. Before measuring the height, weight, and blood pressure of study participants, they were asked to rest for about five to ten minutes. To determine the global cognitive function of each subject, the mini-mental state examination (MMSE) [20] was performed prior to MRI scanning. A day before or after MRI scanning, both fasting blood glucose and fasting blood lipids were measured for each subject, a carotid ultrasound was also performed on each subject.
Brain MRI images were acquired on a 1.5 T scanner (Magnetom Avanto system, Siemens, Erlangen, Germany) by using a standardized protocol that consisted of: an axial T1 image (TR/TE: 500/14ms, 19 slices, slice thickness 5mm, slice gap 1.5mm), an axial T2-weighted image (TR/TE: 4000/99ms, 19 slices, slice thickness 5mm, slice gap 1.5mm), an axial T2 fluid-attenuated inverse recovery (TR/TE: 8500/99ms, 19 slices, slice thickness 5mm, slice gap 1.5mm), a susceptibility weighted image (SWI) sequence (TR/TE: 49/40ms, matrix 250$\times$177, slice thickness 2mm, flip angle 15$^\circ $), a sagittal T1-weighted 3D-magnetization prepared rapid acquisition gradient echo (MP-RAGE) sequence (TR/TE 1900/2.91ms, slice thickness 1mm, matrix 256$\times$177). For DTI imaging, the diffusion gradients were applied along 12 independent orientations under the following conditions: $ {b} = 1000 $ s/mm$ ^2 $ after the acquisition of $ {b} = 0 $ s/mm$ ^2 $ (b0) images. Four sets of each image were acquired and averaged.
All images were rated by two trained neuroradiologists, who were blind to clinical information and MMSE scores. For the 117 participating subjects, Cronbach indices were relatively higher (0.91-1.00) while Kappa indices varied from 0.87 to 1.00. The round foci of hypointensities, whose diameter was 2-10 $mm$ in SWI, were considered as cerebral microhemorrhages. After counting the number of cerebral microbleeds, their distribution pattern was carefullty studied. Symmetrical hypointensities of the basal ganglia and flow voids artifacts of the pial blood vessels were excluded. After separately assessing deep white matter hyperintensities (DWMH) and periventricular hyperintensities (PVH) on T2WI and FLAIR imaging, they were rated according to the Fazekas scale [21](For DWMH: grade 0, absent; grade 1, punctuate; grade 2, early confluence; and grade 3 confluency. For PVH: grade 0, absent; grade 1, caps or lining; grade 2, bands; and grade 3, irregular extension into the deep white matter.) The grades 0 $-$1 were considered as white matter lesion negative, whereas grades 2-3 were considered as white matter lesion positive. Lacunar infarct was defined as a small lesion ($>$3 $mm$ and $<$15 $ mm$ in diameter) [22] which produced a low signal in T1-weighted images, a high signal in T2-weighted images, and a perilesional halo on FLAIR images. 12 patients who showed no symptoms in conventional MRI analysis were excluded. The symptoms included DWMH, PVH, CMB, and lacune stroke. Table 1 presents the demographic and clinical data of all subjects included in this study.
| SVD group (n = 105) | |
|---|---|
| Age: years | 70.92 (6.81784) |
| Gender (male) | 48 (57) |
| Biochemical measures | |
| Triglyceride: mmol/L | 1.55 (0.1) |
| Cholesterol: mmol/L | 4.61 (0.161) |
| Fasting blood gluscose: mmol/L | 5.834 (0.222) |
| Body mass index | 23.14 (0.402) |
| Systolic blood pressure: mmHg | 142.4 (2.830) |
| Diastolic blood pressure: mmHg | 82.13 (1.444) |
| Carotidintimathickness: cm | 0.801 (0.145) |
The brain function MRI (FMRIB) software library (FSL) was used to preprocess the DTI dataset. The FMRIB Diffusion Toolbox (FDT) was used to correct DTI vortex distortion and motion artifacts t and to apply affine matrixment of each diffusion-weighted image to the b0 image. The blood brain barrier was then generated with a brain extraction tool that used a first volume (i.e. b0 image) of the diffusion data without applying a gradient. Finally, a tensor was fitted to the data with the DTIFIT tool, which generated FA and MD values from voxelwise maps of each subject.
Tract-based spatial statistics (TBSS as implemented in FSL) was used to detect the diversity in the left and right brain of SVD subjects. The FA native images of all subjects were non-linearly registered using FNIRT onto the FMRIB58 FA template, which used a b-spline representation of the registration warp field [23]. The mean cross-subject FA skeleton was created and used to generate a white matter tract skeleton, which was thresholded at FA $ > $ 0.2. Next, the symmetric mean-FA image of the derived skeleton, the mask, and the distance map were generated with the script "tbss$\_$sym." The pre-matrixment data was projected onto this symmetrical skeleton, creating an asymmetric image of the original reverse projection data, to the right of the zeroed image. Finally, the resulting image was included in the voxel wise statistical analysis.
The framework of the general linear model (5000 random permutations) was based on the white matter integrity of FA and MD data measurements. In this framework, non-parametric permutations were carried out to obtain an accurate inference, including comprehensive correction for multiple spatial comparisons. To determine the between-group differences in FA and MD, the significance threshold was set at p $ < $ 0.05 [the family-wise error rate (FWE) was corrected for multiple comparisons] by using the threshold-free cluster enhancement (TFCE) option in the "randomize" permutation-testing tool. Thus, an arbitrary threshold was not used for initial cluster-formation [19,24].
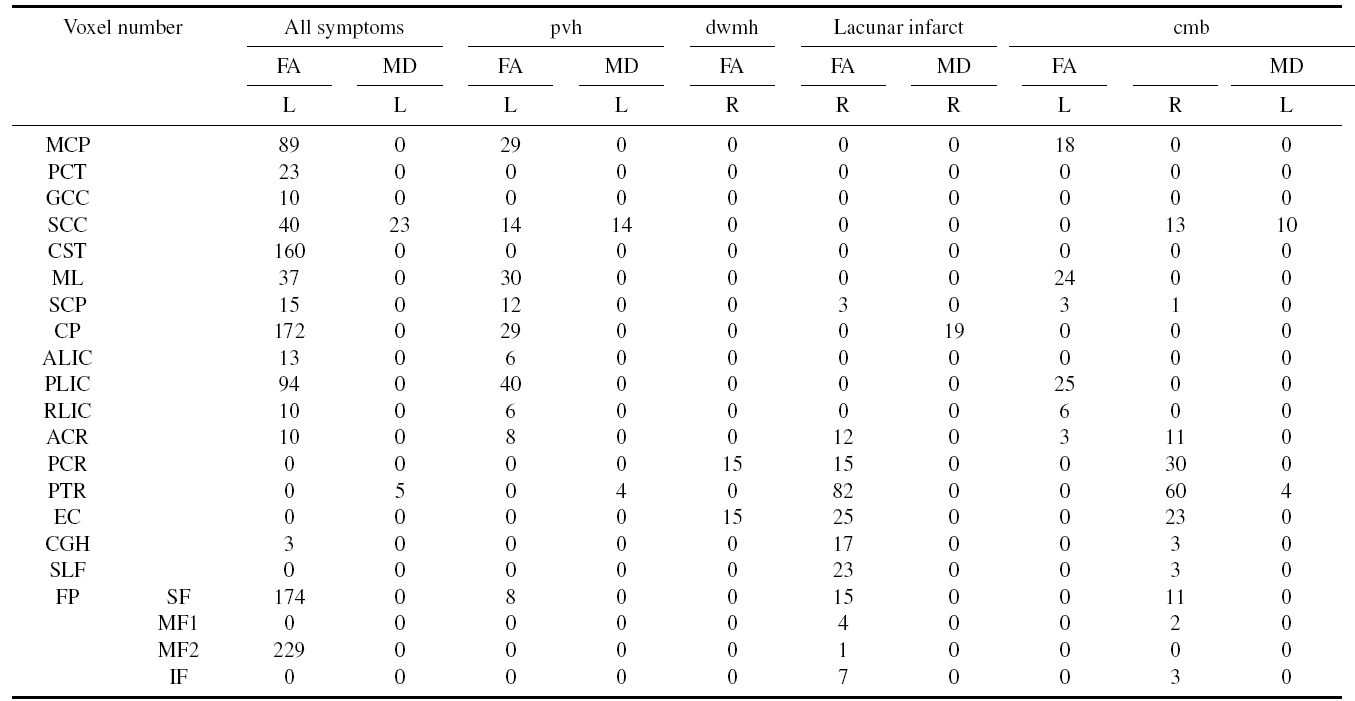
After identifying the asymmetric regions of FA and MD, the covariates of study participants (e.g. age and gender) were further analyzed. The areas where white matter hyperintensities, cerebral microbleed, orlacune number were significantly affected by the asymmetry of FA and MD were then determined. Table 2 presents the voxel numbers of every significant region. Only significant regions that were more than 20 voxels in size were investigated.
 |
Middle cerebellar peduncle (MCP), Pontine crossing tract (PCT, a part of MCP), Genu of corpus callosum (GCC), Splenium of corpus callosum (SCC), Corticospinal tract (CST), Medial lemniscus (ML), Superior cerebellar peduncle (SCP), Cerebral peduncle (CP), Anterior limb of internal capsule (ALIC), Posterior limb of internal capsule (PLIC), Retrolenticular part of internal capsule (RLIC), Anterior corona radiate (ACR), Posterior corona radiate (PCR), Posterior thalamic radiation (PTR, includes optic radiation), External capsule (EC), Cingulum (CGH, hippocampus), Superior longitudinal fasciculus (SLF), Frontal pattern (FP), Superior frontal gyrus (SF), Middle frontal gyrus (MF1), Medial frontal gyrus (MF2), Inferior front gyrus (IF).
Leftward FA asymmetry was seen in the following sections of human brain: the frontal pattern of white matter, the superior corona radiata, the posterior limb of the internal capsule, the genu of corpus callosum, the splenium of corpus callosum, the superior longitudinal fasciculus (temporal part), the posterior thalamic radiation (include optic radiation), and the external capsule. Rightward FA asymmetry was seen in the following sections of human brain: posterior thalamic radiation (including optic radiation), cingulum (hippocampus), external capsule, anterior corona radiata, and anterior limb of the internal capsule. A small number of MD asymmetries were observed in the white matter of subjects. Leftward MD asymmetry was seen in the following sections of human brain: splenium of corpus callosum and posterior thalamic radiation (include optic radiation). Rightward MD asymmetry was only seen in the body of corpus callosum (Fig.1).
 Fig. 1.
Fig. 1.TBSS analysis of white matter asymmetry in SVD subjects. (A) and (B) were FA asymmetry. (C) and (D) were MD asymmetry. (red, left asymmetry, blue, right asymmetry), corrected for multiple comparisons using the FWE rate ($ p < $ 0.05).
There were only left FA asymmetry in the corticospinal tract when age and gender were regressed out (Fig.2).
 Fig. 2.
Fig. 2.TBSS analysis of left FA asymmetry in the corticospinal tract of SVD subjects when age and gender were regressed out. Corrected for multiple comparisons using the FWE rate ( $ p< $ 0.05).
Several white matter asymmetries were related to DWMH score, CMB number, PVH score, and lacune number of study participants (Fig.3). In FAcontrast, only leftward asymmetrywas seen mainlyin the following sections of the human brain: the middle cerebellar peduncle, the splenium of corpus callosum, the pontine crossing tract, the medial lemniscus, the corticospinal tract, the posterior limb of the internal capsule, the cerebral peduncle, and the prefrontal cortex (superior frontal gyrus and medial frontal gyrus). In MD contrast, only leftward asymmetry was seen mainly in the splenium of corpus callosum.
 Fig. 3.
Fig. 3.Effect of all SVD symptoms on white matter asymmetry. (A)-(H) were left FA asymmetry which included the middle cerebellar peduncle, pontine crossing tract, splenium of corpus callosum, corticospinal tract, medial lemniscus, cerebral peduncle, posterior limb of the internal capsule, and forceps minor, respectively. Corrected for multiple comparisons using the FWE rate ($ p < $ 0.05).
White matter asymmetry was slightly affected by PVH (Fig.4). In FA contrast, only leftward asymmetry was seen mainly in the following sections of human brain: middle cerebellar peduncle, cerebral peduncle, medial lemniscus, and the posterior limb of the internal capsule.
 Fig. 4.
Fig. 4.Effect of PVH on white matter asymmetry. (A)-(D) were left FA asymmetry which represented the middle cerebellar peduncle, the medial lemniscus, the cerebral peduncle and the posterior limb of internal capsule respectively. Corrected for multiple comparisons using the FWE rate ($ p < $ 0.05).
White matter asymmetry was slightly affected by DWMH, minor effects were only observed in the right FA asymmetry of the human brain. No significant region was larger than 20 voxels.
The lacune number slightly affected the white matter asymmetry; minor effects were observed in the right FA asymmetry of the human brain (Fig.5). In FA contrast, the right section of the posterior thalamic radiation, external capsule, and the superior longitudinal fasciculus were significantly greater than the left.
 Fig. 5.
Fig. 5.Effect of on lacune number white matter asymmetry. (A)-(C) were right FA asymmetry which represented the thalamic radiation, the external capsule, the superior longitudinal fasciculus respectively. Corrected for multiple comparisons using the FWE rate ($ p < $ 0.05).
Left asymmetry was mainly seen in the medial lemniscus and the posterior limb of the internal capsule. In FA contrast, right asymmetry was mainly seen in the following sections of human brain: the posterior corona radiata, posterior thalamic radiation, and the external capsule (Fig.6).
 Fig. 6.
Fig. 6.Effect of CMB on white matter asymmetry. (A) and (B) were left FA asymmetry which represented the medial lemniscus and the posterior limb of internal capsule respectively. (C)-(E) were right FA asymmetry which represented the posterior corona radiate, the posterior thalamic radiation and the external capsule respectively. Corrected for multiple comparisons using the FWE rate ($ p < $ 0.05).
Compared to the value of MD, the value of FA was more closely related to the white matter asymmetry of SVD subjects. Left FA asymmetry was observed in several white matter regions, such as the frontal pattern of white matter, corticospinal tract, middle cerebellar peduncle, cerebral peduncle, medial lemniscus, and the posterior limb of the internal capsule. Moreover, left FA asymmetry of white matter was closely related to all the symptoms of SVD. Furthermore, right FA asymmetry of white matter was much less than left FA asymmetry. In addition, the location and distribution of DWMH, PVH, CMB, and the lacune number were symmetrical in SVD subjects. This indicates that axonal loss/damage is probably an important process underlying SVD and the location may not be as important as previously assumed [14, 18, 25,26,27].
It is well-known that leftward asymmetry co-exists with rightward asymmetry in healthy individuals. The hemispheric FA asymmetry was significant in healthy group of juveniles. However, hemispheric asymmetry was less significant in the middle-age healthy group. In similar age groups, the left FA asymmetry was not so obvious in healthy individuals [28,29]. In FA contrast, asymmetry of the right hemisphere was more prominent than that of the left hemisphere [30]. Disease processes are aggravated after interacting with existing brain asymmetries [25]. Many diseases progress asymmetrically in the brain. For example, in subjects with Alzheimer's disease, the right hemisphere regions of the brain are affected earlier and more severely than the left hemisphere regions [26]. Lower FA and higher MD values occur due to diminished myelinization, reducing the number of axons in fiber tracts or bundles. Therefore, left FA asymmetry indicates that the value of right brain is lower than the value of left brain. The results of this study indicate that the right hemisphere is more susceptible to neurodegeneration in SVD subjects. This is due to the right hemisphere receiving a larger blood supply than the left hemisphere [27]. Therefore, mortality is higher in SVD subjects with right-sided hemispheric lesions [31]. Interestingly, a previous study reported that it was the right brain of SVD subjects that was injured. Compared to controls, subjects with ischemic leukoaraiosis had lower values of right FA [32]. When age and gender of SVD subjects were regressed out to further analyze white matter asymmetry, only left FA asymmetry was observed in the corticospinal tract of SVD subjects. The corticospinal tract is arguably the most important descending tract in the central nervous system [33]. The asymmetry of corticospinal tract has been described in a previous study. In a group of healthy children and adolescents, 70% of subjects had higher FA in the left corticospinal tract [34]. However, there was no difference in the FA values of the corticospinal tract for the middle-age healthy group [35,36]. The asymmetry in CST was likely to increase in subjects with asymmetric neurological disorders, such as strokes, tumors, and demyelinating processes like multiple sclerosis (MS) [37]. The corticospinal tract's asymmetry was atypical in the amyotrophic lateral sclerosis group, which showed greater impairment in the right hemisphere [38].
Because the symptoms of SVD are more serious in older patients [1], the effect of SVD symptoms on white matter asymmetry was analysed without regressing out age and gender. All SVD symptoms mainly affect the white matter asymmetry in the following regions of the adult brain: the middle cerebellar peduncle, cerebral peduncle, pontine crossing tract, corticospinal tract, medial lemniscus, and the posterior limb of the internal capsule. In the high-definition fiber tracking technique, the strength of connectivity between the left corticospinal tract and the middle cerebellar peduncle, left cerebral peduncle, and the left posterior limb of the internal capsule was more intense than the strength of connectivity between the right corticospinal tract and the middle cerebellar peduncle, right cerebral peduncle, and the right posterior limb of the internal capsule. All regions were related to the sensorimotor function pathways. In a recent study, investigators studied the corticopontocerebellar tract(CPCT) in the human brain. Originating from the primary sensorimotor cortex, CPCT descends to the pontine nucleus through the posterior limb of the internal capsule and the cerebral peduncle. Furthermore, it enters the cerebellum through the middle cerebral peduncle and/or medial lemniscus. Interestingly, Nathan et al.[39] demonstrated that brain asymmetry was the result of more corticospinal fibers being present on the right side than those on the left in almost three quarters of asymmetric cords. Similarly, this study revealed that the right CPCT was injured in SVD patients. Cerebrovascular accidents usually damage the corticospinal tract in the motor cortex or the posterior limb of the internal capsule. The reduced values of FA indicate that the axonal integrity of the corticospinal tract changes after a stroke [40]. When the corticobulbar tract is massively disrupted in stroke patients, focal ischemia develops in the internal capsule and causes orofacial and laryngeal paresis [41]. Morphological changes have been observed in the cerebellar hemisphere of chronic stroke patients [42]. With the DTI technique, an altered corticocerebellar circuit was visualized in subjects with chronic stroke, but this was not confirmed by conventional MRI [43]. A previous study also reported asymmetry of these regions in the brain. It was found that the right middle cerebellar peduncle (MCP) was related to the sensorimotor and cognitive functions of the brain [44]. Longitudinal studies have been conducted on patients who suffered from a deep middle artery infarct. In these subjects, the right side of the corticospinal fibers showed lower values of FA. When the affected side of the cerebral peduncle was examined after 30 and 90 days, similar changes were found on the FA maps [44]. In a previous study, spasmodic dysphonia subjects were examined with the DTI technique. Highly significant FA abnormalities were found in the right-lateralized corticospinal tract [45]. The right cerebral peduncle and the posterior limb of the internal capsule are damaged in patients with Alzheimer's disease, which is indicated by reduced FA values [46]. The damage caused to right corticospinal tract may also injure the right posterior limb of the internal capsule. Neurological examinations have reported that severe bilateral sensory loss leads to left-side dominance. However, previous fiber tracking studies have presented a contrasting result after investigating gait disturbances and progressive motor loss in subjects. These studies reported that right-lateralized activity was highly significant and abnormal in corticospinal tracts and the medial lemniscus [47].
The frontal pattern of white matter variation, which is most frequently observed in patients with cerebral SVD [48], was also reported in a previous asymmetry study by the authors. In that study, it was found that the right frontal white matter was more susceptible when compared to the left in SVD patients. The frontal lobe FA was found to be greater in the right hemisphere, but the left temporal lobe FA was found to be greater than the right temporal lobe FA. This indicates that FA asymmetries are significant in the frontal regions of the brain [49]. The HAROLD model states that under similar conditions, prefrontal cortex activity tends to be less right-lateralized in older people than in young adults. In a cognitive aging study, the HAROLD model [50] showed that the function of the right brain declined while that of the left brain was compensatory. This finding completely agrees with the results of this study. Here it was found that the FA value of right frontal brain was lower than that of left frontal brain. Moreover, it was also shown that the left FA asymmetry was more distinct in SVD subjects than in healthy individuals. This indicates that cognitive aging was more significant in old subjects with SVD. Thus, SVD patients were shown to be more dysfunctional than healthy older people [51,52,53,54].
The corpus callosum is the largest commissural white matter bundle in the brain [55]. The splenium of the corpus callosum receives its arterial blood supply from the vertebrobasilar system [56]. The corpus callosum may be permanently damaged in patients with serious pathological conditions, such as MS, Marchiafava-Bignami disease, tumors, ischemia, leukodystrophy, and HIV-related encephalopathy [57]. In this study, the splenium of corpus callosum was the only region which coexisted in left asymmetry and right asymmetry.
The current study has some limitations. It was not based on specific neuropsychological evaluation. In a population-based study of SVD subjects, the DTI changes, especially FA changes, might be better correlated with cognition than with T2-weighted MRI. However, only the MMSE total score of these subjects was used. The average age of subjects was close to 70 years old. Therefore, these subjects belonged to a generation that received limited education in China, thus, the total MMSE score was relatively lower than that reported in other studies for non-demented older people [58]. Future studies, must include more specific neuropsychological evaluations. In these evaluations, executive function and mental reaction speed of the elderly with SVD must specifically be determined.
In conclusion, a left-greater-than-right FA asymmetry was observed in SVD subjects. This indicates that an injured right brain was the main cause of cognitive dysfunction in in these cases. Results provided a new insight into the pathophysiology of SVD in the elderly. These asymmetry findings are important for understanding the extent of brain injury in SVD, so that they may be used as prognostic indicators for disease progression. Moreover, these findings may be correlated with responses to therapy in lesion-based neuropathology.
This work was supported by grants 2016M592452 from the China Postdoctoral Science Foundation and grants 2016JJ4090 from the Natural Science Foundation of Hunan Province and grants 2015KTSCX177 from the Department of Education major research platform foundation of Guangdong Province.
All authors declare no conflicts of interest.
