*Co-first author.
To study the clinical effects of scalp acupuncture plus low frequency rTMS in hemiplegic stroke patients. A total of 28 hemiplegic stroke patients were recruited and randomly assigned to the experimental group (scalp acupuncture low frequency rTMS routine rehabilitation treatment) or the control group (scalp acupuncture routine rehabilitation treatment). All patients received a diffusion tensor imaging examination on the day of admission and on the fourteenth day. Compared with pre-treatment, the upper limb motor function score and ability of daily life score increased significantly in the two groups, and motor function improvement was much greater in the experimental group. Fractional anisotropy values significantly increased in white matter tracts, such as the corticospinal tract, forceps minor, superior longitudinal fasciculus and uncinate fasciculus in the two groups. Compared with pre-treatment, the fractional anisotropy values increased and mean diffusion values decreased synchronously in the forceps minor, left inferior fronto-occipital fasciculus, left inferior longitudinal fasciculus, left superior longitudinal fasciculus and left uncinate fasciculus in the experimental group. Before and after treatment, there were no significant differences in the changes of fractional anisotropy values between the two groups, but the changes of the mean diffusion values in the experimental group were much greater than those in the control group in the left superior longitudinal fasciculus and the left uncinate fasciculus ($p<$ 0.05). Moreover, the increased fractional anisotropy values in the forceps minor in the experimental group were significantly positively correlated with the increased Fugl-Meyer assessment score. Our study concluded that based on routine rehabilitation treatment, scalp acupuncture plus low frequency rTMS can promote white matter tracts repair better than scalp acupuncture alone; the motor function improvement of the hemiplegic upper limb may be closely related to the rehabilitation of the forceps minor; the combination of scalp acupuncture and low frequency rTMS is expected to provide a more optimal rehabilitation protocol for stroke hemiplegic patients.
The incidence of stroke is increasing as the global population aging. According to the latest epidemiological survey, there are 167 million new stroke patients each year. The incidence of stroke in male patients is 1.5 times higher than that in female patients [1]. Motor dysfunction after stroke leads to a heavy financial pressure on the families. Post-stroke hemiplegic rehabilitation includes early rehabilitation treatment [2], routine treatment [3], computer aided rehabilitation training [4], secondary prevention by improving the life style [5], transcranial direct current stimulation [6], scalp acupuncture (SA) [7, 8], repetitive transcranial magnetic stimulation (rTMS) [9] and other interventions. When traditional Chi-nese treatment is combined with Western medicine rehabilitation therapy, it is more common that SA [10, 11] and rTMS are used in hemiplegia rehabilitation after stroke.
Previous studies have reported that SA can improve cerebral blood flow, promote proliferation and differentiation of endogenous and ipsilateral hippocampal stem cells, reduce excitatory amino acid expression, and weaken the cerebral vascular inflammatory reaction in post-stroke treatment [7]. rTMS, as a non-invasive electrophysiological stimulation technique, is safe, painless to patients, and simple to perform, which can also regulate cortical excitability [9]. Low frequency rTMS (LF-rTMS) can reduce cortical excitability of the contralateral cerebral hemisphere after stroke [12], decrease excessive pathological inhibition of the contralateral cortex on the ipsilateral cortex [13], enhance the ipsilateral hemisphere cortex ex-citability [14] and rebuild the balance between the bilateral cerebral hemispheres. SA or LF-rTMS can improve movement in post-stroke patients [8, 15, 16]. However, each method is often randomly used. Some valuable combinations are not effectively matched and combined, which result in a possible reduction in clinical efficacy. We hypothesize that the effect of scalp stimulation in the time window of LF-rTMS is better than that of the simple method. Han and his colleagues have synchronously using SA and LF-rTMS for patients with cerebral infarction [17], but it is unclear whether addition of the therapies can more effectively repair brain structures, especially the relationship between the remodeled white matter tracts and hemiplegic limb motor improvement.
The cell membrane, microtubules, and neurofilament of the brain nerve cell constitute the microstructure of the white matter tracts. Diffusion tensor imaging (DTI) assesses water diffusion index of the microstructural properties of white matter tracts, which can provide quantitative information about the white matter tracts microstructure and identify white matter pathological changes associated with many diseases [18]. The most commonly used DTI parameters are the anisotropic index map (fractional anisotropy, FA) and mean diffusivity map (mean diffusion, MD). The FA value reflects the proportion of the water molecules anisotropic components in the whole diffusion tensor, which indirectly reflects the white matter tracts integrity. The FA value is close to zero in the cerebrospinal fluid, and one indicates complete anisotropic diffusion. The larger FA value is the more remarkable tissue anisotropy [19]. The MD value is the characteristic average value of diffusion tensor D in the X, Y, and Z directions and reflects the whole diffusion level. An increase in the MD value indicates that cells have undergone degenerative changes such as cell membrane density reduction and an increase in water molecule diffusion degree. It was the critical idea of tract-based spatial statistics (TBSS) those white matter tracts was skeletonized [20], which can reduce redundant uncalibrated images and avoid smoothening of DTI parameters. DTI technology has been widely used to analyze the correlation between white matter changes and functional responses in thalamic stroke [21], measure corticospinal integrity after stroke [22], assess the white matter damage in stroke patients with neglect [23], predict motor function outcomes in stroke rehabilitation [24] and diagnose other white matter diseases.
The post-stroke rehabilitation of hemiplegic limb motor dysfunction is closely related to brain white matter tracts [25]. Our study applied the TBSS method to analyze the brain white matter tracts DTI parameters (FA value and MD value), to observe the changes in the white matter tracts before and after treatment in hemiplegic stroke patients, and to correlate these changes with the behavior index after the combined use of SA and LF-rTMS.
Patients were recruited from the rehabilitation unit of Shenzhen Nanshan District People's Hospital from March 2015 to January 2017. The following inclusion criteria were applied: (1). Met both diagnostic criteria under ischemic stroke of Chinese and Western medicine; (2). First-ever stroke, unilateral or ever stroke but not legacy neurological dysfunction; (3). Stable vital signs, clear consciousness; (4). Unilateral upper limb Brunnstrom evaluation: I-IV period; (5) Age from 20 to 80 years old; (6) The course of the diseases from 2 weeks to 6 months. The exclusion criteria for this study included the following: (1) A history of epilepsy; (2) Function failure in important organs such as heart, lung, liver, kidney, etc.; (3) Serious cognitive impairment and inability to cooperate; (4) Patients with a pacemaker, intracranial metal implants, or skull defects; (5) Serious cervical spine including cervical stenosis and instability of cervical spine; (6) Women during pregnancy; (7) Patients who cannot tolerate fMRI or DTI examination.
This study used a randomized, single blind, parallel controlled clinical trial design. We used the randomized number table to control the allocation scheme. The subjects were assigned to diverse groups according to the random number table; there were 14 subjects in each group. Among them, six patients were excluded in the experimental group and five patients were excluded in the control group. Eight subjects in the experimental group (SA + LF - rTMS + routine rehabilitation treatment) (1 female, 7 males; age ranged from 44 to 70 years, (52.75 $\pm$ 12.33 years)) and Nine subjects in the control group (SA + routine rehabilitation treatment) (2 females, 7 males; age ranged from 36 to 76 years (50.55 $\pm$ 13.06 years)) were included in the final statistical analysis. All study procedures were approved by the ethics committee of Shenzhen Nanshan District People's Hospital. All participants were informed about the study before the trial and signed a written informed consent.
(1) The control group: Based on secondary stroke prevention and regular rehabilitation treatment, in accordance with the SA standardized scheme, SA stimulation was applied at the middle 2/5 of the MS6 line (Dingnieqianxiexian, line from DU21 Qianding to GB6 Xuanli) in the affected hemisphere. The subjects stayed in a sitting position and the acupuncturists relayed needling according to the above acupoint areas with disposable plastic handle stainless steel acupuncture needles(Suzhou Acupuncture and Moxibustion Appliance Co. Ltd, model 0.30 mm $\times$ 25 mm, aseptic processing) after routine scalp disinfection. During the SA stimulation process, the angle between needle body and the scalp was 20 to 40 degrees. When the needle body was inserted to the sublegal and the acupuncturists felt decreasing resistance, then the acupuncturists pushed the needle 1.25 cm to 2.0 cm along to the SA acupoints line with simultaneous twirling to achieve a needling sensation. After a 40-minute SA treatment, the acupuncturists sterilized the needle with a cotton ball and compressed the pinhole for a moment. Patients underwent treatment once daily for 2 weeks with a total of fourteen treatments.
(2) The experimental group: The therapeutic process included the addition of LF-rTMS stimulation to the control treatment, LF-rTMS was used at the unaffected hemisphere and synchronously SA stimulation at the middle 2/5 of the MS6 line in the affected hemisphere. The MagstimRAPID2 transcranial magnetic therapy device (70-mm butterfly coil, maximum magnetic field strength of 2.2T) produced by the British Magstim Company was used in the trial procedure. The treatment parameters were as follows: M1 (Primary Motor Cortex) of the contralateral cerebral cortex, 70% motor threshold (Motor Threshold, MT), 1 Hz of stimulation frequency, 1200 pulse number, and 20-minute therapy once daily for 14 consecutive days.
We used a 3.0T super-conduct magnetic resonance scanner (Trio Siemens, Medical Erlangen, SKYRA) to collect brain anatomical image and DTI image data. T1 weighted sagittal scanning used a 3D-Mprage sequence: TR = 1900 ms, TE = 2.19 ms, Flip angle = 9 degrees, FOV = 256, voxel size = 1.0 mm $ \times $ 1.0 mm $\times$ 1.0 mm, Matrix = 256 $\times$ 256, Thickness = 1.0 mm, and Slices = 176. The whole brain DTI echo planar imaging (EPI) scan parameter sequence was TR = 10600 ms, TE = 95 ms, FOV = 220, voxel size = 1.7 mm $\times$ 1.7 mm $\times$ 2.0 mm, matrix = 128 $\times$ 128, Number excitation (NEX) = 1, Diffusion sensitive gradient direction = 64, b value = 1000 s/mm2, Thickness = 2 mm. Subjects remained in supine position on the scanner in a quiet and still state during the whole scanning process. DTI data were processed and analyzed with a brain image processing software Functional Software Library (FSL, Version 4.1.9, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS/UserGuide). The process steps were: (1) Data pre-processing: FDT tools provided by FSL corrected the head action and eddy current of the DTI images, and the least square method was used to fit the tensor model to obtain the FA map and MD map. (2) Registration: the nonlinear registration method with FSL was applied to register each FA image to the target template. The target template selected in this study was FSL software with the FA standard template. All FA maps of the subjects were mapped to the MNI standard space by registration. (3) Skeleton extraction: first, the mean FA template was produced by using all the FA image registries; second, the mean FA template skeleton was extracted; then each FA was mapped onto an mean FA template skeleton, resulting in a cerebral FA white matter tract skeleton for each participant. (4) The nonlinear registration in step (2) was applied to the MD maps, then we projected subjects' warped MD maps onto the mean FA skeleton (using the FA data to find the projection vectors), resulting in a cerebral MD white matter tract skeleton for each participant. (5) The statistical analysis matrix was designed, and then, paired t-test analysis was performed in both groups for pre- and post-treatment. A p-value of < 0.05 was used to determine the statistical significance, and the statistical results were added to the mean FA image (white) and mean FA skeleton (green).
The Fugl-Meyer Assessment (FMA) was applied to evaluate the motor function of the hemiplegic upper limb[26]. The assessment consists of a total of 10 items (this assessment is on an ordinal scale, in which 0 means unable to perform, 1 is a partial performance, and 2 is complete performance). The FMA has a total score of 100, 66 points to the upper limb and 34 points to the lower limb. The upper limb part of the FMA includes the reflex activity; extensor flexor synergy movement; cooperative motion activities; cooperative movement activities; no hyperreflexia; wrist, elbow and straight shoulder flexion stability of 90 degrees; finger movement and coordination ability; and speed. The Modified Barthel Index (MBI) evaluates the activity of daily living[27]. It consists of a total of 10 items; full marks indicate a score of 100, including excretion, grooming, using the toilet, eating, transformation (bed to chair), walking activities (walking in or around the ward, not including a long walk), dressing, bathing, walking up and down the stairs.
Stata12.0 software (StataCorp, Texas, USA) was used for statistical analysis; Quantitative data are presented as mean standard deviation. If the test data satisfied the normality and homogeneity variances, paired t-test was used to assess for differences between preand post-treatment within each group. Independent sample t-test was used to evaluate differences of FMA score, MBI score and the two index changes between groups, and a two-tailed p-value of < 0.05 was used to determine the statistical significance. If the data could not meet the above mentioned two criteria, the Wilcoxon test was used. If the significant increased FA image or significant decreased MD image of the white matter tracts were demonstrated by FSL software within group after treatment. We would extract the quantitative FA value and MD value of the special white matter tracts, a one-tailed p-value of < 0.05 was used to determine the statistical significance within the group before and after treatment. Pearson's correlation coefficient test was used to assess the correlation between the image data and behavioral results, and a two-tailed p-value of < 0.05 was used to determine the statistical significance.
All patients’ lesions had good consistency (Fig. 1). There was no significant difference between the two groups in age, gender, months since stroke and pre-treatment behavioral score (p > 0.05). After treatment, the FMA and MBI scores increased in both groups, and the differences were statistically significant (FMA: the experimental group: p < 0.0001, the control group: p < 0.001; MBI: the experimental group: p < 0.001, the control group: p < 0.001). The changes in the FMA scores in the experimental group were much greater than those in the control group, the differences were statistically significant (FMA: p < 0.05) (Table 1). The changes of the MBI scores were not statistica significant between the two groups (MBI: p > 0.05) (Table 1).
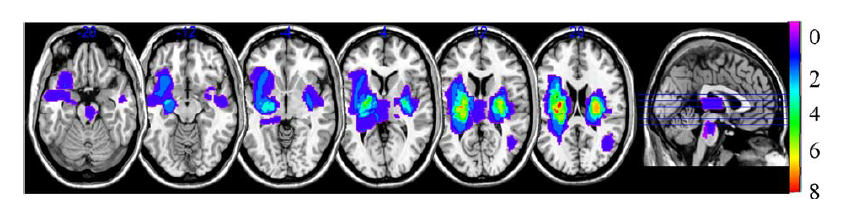 Fig. 1.
Fig. 1.Lesion distribution in all stroke subjects. We constructed a lesion overlap image for all participants, and then manually traced the outline of the lesion on individual 3D T1 images using MRICRON (http://www.mccauslandcenter.sc.edu/mricro/mricron/), thereby creating a lesion mask for each patient. After the spatial normalization process, all the individual lesion masks were used to construct a group lesion mask for the patients [28].
| Group | Number | Age (year) | Duration | Gender | Lesion | MBI (score) | FMA (score) | ||
|---|---|---|---|---|---|---|---|---|---|
| (month) | Pre | Post | Pre | Post | |||||
| EG | 1 | 60 | 0.5 | Male | Right basal ganglia | 60 | 80 | 21 | 35 |
| 2 | 70 | 0.7 | Male | Left basal ganglias lateral ventricles | 32 | 52 | 2 | 10 | |
| 3 | 54 | 0.63 | Male | Right basal ganglia | 72 | 85 | 15 | 35 | |
| 4 | 57 | 2 | Female | pons valorie | 38 | 53 | 4 | 15 | |
| 5 | 49 | 0.87 | Male | Left basal ganglias insula | 62 | 85 | 31 | 47 | |
| 6 | 48 | 2.66 | Male | Right basal | 85 | 90 | 28 | 40 | |
| ganglia-corona | |||||||||
| radiata | |||||||||
| 7 | 44 | 1 | Male | Left basal ganglia | 61 | 80 | 44 | 55 | |
| 8 | 59 | 0.7 | Male | Left thalamus-corona radiata | 42 | 52 | 24 | 37 | |
| 52.75 ± 12.33 | 1.13 ± 0.77 | 7:01 | 56.50 ± 18.00 | 72.13 ± 16.69* | 21.13 ± 14.00 | 34.25 ± 15.09*1 | |||
| CG | 9 | 36 | 1.27 | Male | Right basal ganglia | 57 | 64 | 14 | 25 |
| 10 | 52 | 2.73 | Male | Left fronto parietal lobe | 75 | 80 | 23 | 30 | |
| 11 | 52 | 1.67 | Male | Left basal ganglias occipital lobe-parietal lobe | 77 | 90 | 40 | 44 | |
| 12 | 45 | 2 | Male | Left basal ganglia | 42 | 56 | 10 | 25 | |
| 13 | 63 | 2.33 | Male | Right basal ganglia | 42 | 56 | 6 | 12 | |
| 14 | 45 | 0.57 | Male | Right brain stem and centrum ovale | 36 | 44 | 5 | 12 | |
| 15 | 76 | 2.6 | Female | Right basal ganglia | 40 | 50 | 15 | 19 | |
| 16 | 34 | 0.7 | Male | Left basal ganglia | 10 | 26 | 6 | 9 | |
| 17 | 47 | 1 | Female | Right basal ganglia-frontal lobe | 29 | 42 | 6 | 9 | |
| 50.55 ± 13.06 | 1.65 ± 0.81 | 7:02 | 45.33 ± 21.42 | 56.44 ± 19.59* | 13.89 ± 11.44 | 20.55 ± 11.70* | |||
EG: the experimental group, CG: the control group. The difference of the age, duration, gender, pre-MBI score, pre-FMA score in the experimental group and the control group were not statistically significant, which had homogeneous comparability; Compared with pre-treatment, *p < 0.05; Comparison of the changes between groups, *p < 0.05.
Based on routine rehabilitation therapy, the control group received 14-days SA stimulation, and the paired t-test results showed that the FA value increased in the bilateral anterior thalamic radiation, left corticospinal tract, left cingulate fasciculus (cingulate gyrus) and right cingulate fasciculus (hippocampus), forceps minor, left inferior fronto-occipital fasciculus, left inferior longitudinal fasciculus, and left superior longitudinal fasciculus (p < 0.05, uncorrected) (Fig. 2, Table 2). The decrease in the MD value was not statistically signifi- cant (p > 0.05, uncorrected). In the experimental group, the subjects received SA plus LF-rTMS for 14 days of continuous treatment, and the paired t-test demonstrated that the FA value increased in the right anterior thalamic radiation, right cingulum (cingulate gyrus), left cingulum (hippocampus), forceps major, forceps minor, left inferior fronto-occipital fasciculus, left inferior longitudinal fasciculus, bilateral superior longitudinal fasciculus and left uncinate fasciculus (Fig. 3A, Table 3). The MD value decreased in the bilateral anterior thalamic radiation, left corticospinal tract, left cingulum (cingulate gyrus), forceps minor, left inferior fronto-occipital fasciculus, left inferior longitudinal fasciculus, left superior longitudinal fasciculus and left uncinate fasciculus (Fig. 3B, Table 4).
 Fig. 2.
Fig. 2.FA changes of the white matter tracts in the control group significantly increased after treatment compared to before treatment. DTI images in the control group is presented by using FSL software (version4.1.9, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS/UserGuide). Red shows the special white matter tracts with increased FA value (tbssfill was used here which thicken the TBSS results). Green indicates the mean FA skeleton in the control group. White represents the mean FA image in the control group.

|
 Fig. 3.
Fig. 3.In the experimental group, FA changes of the white matter tracts significantly increased while MD changes significantly decreased after treatment compared to before treatment. DTI images in the experimental group is presented by using FSL software (version4.1.9, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS/UserGuide). Red shows the special white matter tracts with significantly increased FA value in figure 3A (tbssfill was used here which thicken the TBSS results). Blue denotes the special white matter tracts with significantly decreased MD value in figure 3B (tbss fill was also used here which thicken the TBSSresults). Green indicates the mean FA skeleton in the experimental group. White represents the mean FA image in the experimental group.
| MNI (coordinate) | Voxel number | Hemisphere | White matter tracts | FA value | p value | |||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | pre(Mean ± SD) | post (Mean ± SD) | ||||
| 12 | 7 | 2 | 329 | Right | Anterior thalamic radiation | 0.212 ± 0.018 | 0.217 ± 0.017 | 0.036 |
| 10 | -33 | 32 | 89 | Right | Cingulum (cingulate gyrus) | 0.130 ± 0.125 | 0.135 ± 0.123 | 0.009 |
| -25 | -17 | -29 | 75 | Left | Cingulum (hippocampus) | 0.150 ± 0.014 | 0.164 ± 0.024 | 0.024 |
| 0 | -37 | 13 | 294 | — | Forceps major | 0.232 ± 0.013 | 0.236 ± 0.013 | 0.012 |
| -3 | 28 | 7 | 210 | — | Forceps minor | 0.220 ± 0.013 | 0.224 ± 0.013 | 0.011 |
| -23 | 21 | 8 | 565 | Left | Inferior fronto-occipital fasciculus | 0.106 ± 0.017 | 0.109 ± 0.019 | 0.011 |
| -46 | -16 | -19 | 929 | Left | Inferior longitudinal fasciculus | 0.131 ± 0.008 | 0.134 ± 0.008 | 0.006 |
| -37 | -40 | 25 | 125 | Left | Superior longitudinal fasciculus | 0.148 ± 0.023 | 0.152 ± 0.026 | 0.018 |
| 38 | -49 | 23 | 243 | Right | Superior longitudinal fasciculus | 0.169 ± 0.015 | 0.171 ± 0.013 | 0.047 |
| -32 | 4 | -9 | 328 | Left | Uncinate fasciculus | 0.175 ± 0.037 | 0.179 ± 0.037 | 0.001 |
| MNI (coordinate) | Voxel number | Hemisphere | White matter tracts | MD value (x 10-3 mm2/s) | p value | |||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | pre (Mean ± SD) | post (Mean ± SD) | ||||
| -15 | -1 | -17 | 323 | Left | Anterior thalamic radiation | 0.281 ± 0.152 | 0.275 ± 0.134 | 0.03 |
| 13 | -5 | 9 | 271 | Right | Anterior thalamic radiation | 0.364 ± 0.081 | 0.338 ± 0.059 | 0.038 |
| -26 | -23 | 19 | 226 | Left | Corticospinal tract | 0.227 ± 0.025 | 0.218 ± 0.032 | 0.03 |
| 25 | 20 | 93 | Left | Cingulum (cingulate gyrus) | 0.124 ± 0.061 | 0.120 ± 0.056 | 0.044 | |
| -19 | 50 | 5 | 121 | — | Forceps minor | 0.408 ± 0.025 | 0.403 ± 0.023 | 0.012 |
| -25 | 31 | 8 | 234 | Left | Inferior fronto-occipital fasciculus | 0.513 ± 0.038 | 0.508 ± 0.044 | 0.047 |
| -41 | 4 | -26 | 487 | Left | Inferior longitudinal fasciculus | 0.282 ± 0.018 | 0.277 ± 0.018 | 0.031 |
| -37 | -14 | 32 | 2157 | Left | Superior longitudinal fasciculus | 0.272 ± 0.015 | 0.265 ± 0.014 | 0.009 |
| -41 | 5 | -26 | 147 | Left | Uncinate fasciculus | 0.299 ± 0.028 | 0.291 ± 0.023 | 0.047 |
The FA value increased and the MD value decreased synchronously in the forceps minor, left inferior fronto-occipital fasciculus, left inferior longitudinal fasciculus, left superior longitudinal fasciculus and left uncinate fasciculus in the experimental group (p < 0.05, uncorrected) (Table 5). It is worth mentioning that the FA value increased in the experimental group compared to the control group after treatment in the same white matter tracts, and the difference was not statistically significant (p > 0.05). The MD value improvements in the left superior longitudinal fasciculus and the left uncinate fasciculus in the experimental group after treatment were much more noticeable than in the control group, and the difference was statistically significant (p < 0.05) (Table 6).

|
Our correlation analyses revealed the FA change of the forceps minor in the experimental group after treatment was significantly positively correlated with the hemiplegic upper limb FMA change (Pearson’s r = 0.851, p = 0.007) (Fig. 4). However, no significant correlations between the changes of FA or MD and the changes of FMA or MBI scores were found in other white matter tracts. The control group showed no significant correlation between the improvement of white matter tracts and the change of motor function.
 Fig. 4.
Fig. 4.Correlation between the FA changes of white matter tracts with improved FMA scores of hemiplegic upper limb in the experimental group (Pearson’s r = 0.851, p = 0.007).
So far, magnetic resonance imaging (MRI) studies have indicated that SA or rTMS can activate functionally related brain areas [29, 30], delay brain gray matter injury process [31, 32], and promote cerebralwhite matter tracts reconstruction [33, 34]. In our study, based on routine rehabilitation therapy, two courses of SA stimulation including acupuncture acupoints located at the middle 2/5 of the ipsilateralMS6 line (correspond to the M1 projection area of the precentral gyrus) combined with 1 Hz LF-rTMSin the contralateral cerebral cortex M1 area for 14 consecutive days. TBSS [19] evaluates the changesin the brain white matter structure after combined use of the two methods, and FMA [26] and MBI [28] were used to evaluate the behavioral index. Our study showed that based on routine rehabilitation therapy, SA alone or SA plus LF-rTMS can both improve hemiplegic patients’ upper limb motor function and the activity of daily living (ADL). SA plus LF-rTMS had a better clinical effect in improving FMA. In TBSS analysis, we also found that significant differences in the DTI parameters of motorrelated brain white matter tracts, including the anterior thalamic radiation, corticospinal tract, cingulate fasciculus (cingulate and hippocampus), forceps major, forceps minor, inferior front-occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, and uncinate fasciculus in the two groups. The FA changes in the forceps minor were positively correlated with motor function changes in the FMA score in the experimental group. FMA of the upper limb (arm and hand) represents the degree of affected limb movement function recovery [26]. A significant positive correlation showed that the FA value increased more, the motor function recovered better. We currently found there was no significant relationship between the FA value changes in the white matter tracts and motor function improvements in the control group and that the MD value in the white matter tracts did not noticeably decline. These results suggested that combined use of the two methods could preferentially promote white matter tracts remodeling. The motor function improvement of the hemiplegic upper limb may be closely related to forceps minor rebuilding.
The function of the corpus callosum is to coordinate the bilateral cerebral hemispheres, and it is the main channel that connects bilateral motor ataxia, bilateral brain perception and cortex cognitive[35].
The forceps minor is a part of the corpus callosum genu, and is composed of radial fibers in the bilateral cerebral hemisphere[36]; it consists of large white matter tracts that can connect the bilateral prefrontal cortex in the brain and plays a very important role in motion control as well as maintaining brain connective integrity[37]. Kopitzki and his team had found that damage to the forceps minor may be related to the weakened motor function[38]. The FA value in the forceps minor can predict the rehabilitation outcome of an exercise control[39]. Our study strategy was to place a TMS coil on the contralateral cerebral cortex area M1 and provide SA stimulation on the 2/5 of the ipsilateral MS6 line. M1 is the primary motor cortex, and MS6 is the main projection area of M1 located at the precentral frontal gyrus in the scalp[8}. M1 and MS6 probably have equipotent effects. Two different interventions enhanced the ipsilateral cortex excitability by different mechanisms[40,41]. The forceps minor is the major and most important white matter tracts which connect the bilateral prefrontal cortex[42]. In our study, FA value increasing in the forceps minor may probably effectively strengthen the physical connection of the prefrontal cortex in the bilateral cerebral hemisphere[43], which can contribute the positive significance for the precentral gyrus located in the prefrontal cortex corresponding to the somatic motor function improvement (including the motor function of the arm and hand). We were able to arrive at this conclusion by using TBSS analysis in conjunction with behavior index, but the specific neurobiological mechanism needs to be verified with animal experiments.
In addition to the forceps minor, a previous study found that FA value of the motor-related brain white matter tracts declined, such as anterior thalamic radiation, corticospinal tract, superior longitudinal fasciculus, inferior longitudinal fasciculus, cingulate and uncinate fasciculus in stroke patients[44]; these white matter tracts play an important role in voluntary movement and motion control. The cingulate fasciculus (hippocampus) is very important in cognitive processing associated with exercise[45]. We found increased FA value and synchronously decreased MD value in white matter tracts such as the forceps minor, left inferior fronto-occipital fasciculus, left superior longitudinal fasciculus, left inferior longitudinal fasciculus and left uncinate fasciculus. In the experimental group, the MD value improvement in the left superior longitudinal fasciculus and left uncinate fasciculus was significantly greater than that in the control group. Based on our study results and the DTI parameter changes of white matter tracts after stroke in past study[44], we speculated that SA plus LF-rTMS may not only improve the forceps minor structure which connect to the bilateral prefrontal cortex, but also play an important role in restructuring other highly motor-related white matter tracts (although the latter had no significant correlation with FMA, possibly because the sample size was small, the injury lesion was not completely consistent or the observed duration was relatively short), but rebuilding of these white matter tracts is still not ignored in our study and in post-stroke rehabilitation.
In our study, the combination of the two methods probably presented better additive effect in the white matter tracts and behavioral analyses. These results could be related to individual clinical applications and mechanisms. Recent studies have indicated that with LF-rTMS (1 Hz) stimulation of the contralateral cerebral M1 area, the vicinal cortical activity signal decreased, inhibiting the stimulated cerebral cortex activation, and this stimulated effect can last for 20 minutes after treatment [46]. Some experimental studies have shown that 1 Hz LF-rTMS can increase action potential and excitatory postsynaptic current in hippocampal CA1 neurons, which inhibit cortical excitability [47]. Tan et al. [48] reported that LF-rTMS can regulate parasympathetic nervous system excitability. Our study had equivalent results compared to the above-mentioned research. The SA plus LF-rTMS can promote motion-related brain white matter tracts FA value increasing and MD value decreasing, especially in the forceps minor. This may relate to the inhibitory effect of LF-rTMS on the contralateral M1 area and weaken the excessive inhibition of the contralateral motor cortex, which is pathological to the ipsilateral motor cortex after stroke to rebuild interhemispheric balance [49]. Based on the previous treatment, we administered two cycles of SA therapy on the ipsilateral MS6, which was equal to M1 in the prefrontal precentral gyrus (covering the ipsilateral M1 area). Two courses of SA direct stimulation further promoted ipsilateral motor cortex restructuring and white matter tracts remodeling, and corrected interhemispheric imbalance. In addition, SA can activate the contralateral somatosensory association cortex, the postcentral gyrus, and the parietal lobe [41], which differs from LF-rTMS [50]. Furthermore, SA can sustain influence over the brain, even after cessation of acupuncture stimulation [40].
Based on the routine rehabilitative therapy, SA combined with LF-rTMS or SA alone can both promote white matter tracts reconstruction in stroke hemiplegic patients, enhance the hemiplegic upper limb motor function and improve ADL. SA plus LF-rTMS can significantly facilitate white matter tracts recovery compared to SA alone. Limb motor function improvement may be closely related to forceps minor repair. The combined use of SA and LF-rTMS is expected to lead to more optimized hemiplegic rehabilitation programs with hemiplegic stroke patients.We used TBSS to analyze the rehabilitative effect of the white matter tracts in hemiplegic patients with post-stroke by using the combination of SA and LF-rTMS. We discovered a noticeably positive correlation between motion processing related to brain white matter tract (forceps minor) and behavioral changes. Our study results suggested that the combination of different mechanism rehabilitative therapies can optimize the rehabilitative prescription of stroke patients, and provide novel clinical solutions to precise post-stroke rehabilitation in the future.
This work was funded by the Science and Technology Planning Project of Guangdong Province (#2014A020212046), Guangdong Provincial Administration of Traditional Chinese Medicine (#20151082), Shenzhen Governmental Basic Research Grant (#JCYJ 20150402152130181, #JCYJ20140411094009911) and Shenzhen Nanshan District Education (Health) Project ( #2014041).
All authors declare no conflicts of interest.

