Academic Editor: Neven Zarkovic
We aimed to compare the predictive value of different inflammatory markers in renal cell carcinoma (RCC). Four hundred ninety-five patients treated with nephrectomy for primary localized or locally advanced RCC between 2010 and 2018 were included in the retrospective analysis. The median follow-up for the entire cohort was 48 months. Based on the preoperative laboratory measurements, patients with higher neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), systemic inflammatory response index (SIRI), systemic immune-inflammation index (SII), neutrophil/erythrocyte ratio (NER), derived neutrophil/lymphocyte ratio (dNLR), and lower lymphocyte/monocyte ratio (LMR) and hemoglobin/platelet ratio (HPR) had worse cancer-specific survival (CSS). In the multivariate analysis tumour stage, grade, age and high SIRI constituted independent factors predicting CSS. The model including SIRI values achieved C-index 0.903 (alternative multivariate models with SII and NLR 0.902 and 0.890, respectively). Age, tumour grade and high NER (or high SIRI/ SII in alternative models) were prognostic for overall survival. Markers of systemic inflammation might provide additional prognostic information (especially SIRI, SII, NLR and NER) and further increase the predictive accuracy of available models in localized and locally advanced renal cell carcinoma. For the first time, we show the prognostic value of neutrophil-to-erythrocyte ratio, which constitutes an independent risk factor of overall survival.
Localized or locally advanced renal cell carcinoma (RCC) remains the disease that is treated surgically with curative intent [1]. The main concern, though, is connected with a significant rate of late recurrence, reaching in some publications up to 12% [2]. Specific markers are under investigation to be used in prognostic models, as staging and grading appear to be insufficient, and one observes various outcomes in cases of the indistinguishable clinicopathological characters [3]. The preoperative, early assessment may enforce the optimization of personalized therapy and better risk stratification of patients treated surgically [4]. Then, the appropriate grouping of patients is important for the sake of future management techniques (e.g., surgery versus active surveillance), an adequate follow-up scheme implementation, and selection of optimal candidates for potential adjuvant treatment inside clinical trials [5].
As a great variety of studies focused on hematological markers, they are used more and more frequently for prognostic purposes [6, 7]. The idea of their clinical implementation comes from the observations on specific interactions within the tumor microenvironment and immune system effector cells [8]. Peripheral blood neutrophils, lymphocytes, monocytes and platelets that are involved in systemic inflammatory reactions are available from routine preoperative blood tests [9]. Within the tumor microenvironment, certain differences in the subpopulations of leukocytes lie at the root of host immunity and reflect tumor aggressiveness [8]. As a consequence, respective peripheral blood count-derived parameters are under investigation for their use in oncology as potential markers, among them most recognizable are the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) [6]. Other researchers estimated the role of combinations of the peripheral blood counts’ values in a variety of scores, such as the systemic inflammatory response index (SIRI) [4], or the systemic immune-inflammation index (SII) [10]. The hope for their future incorporation into everyday practice is based on the fact that the combination of three parameters (e.g., neutrophils, lymphocytes, platelets) better reflects the prognosis and may result in greater accuracy of the respective predictive model [4]. However, the potential utility of the systemic inflammatory markers in the prediction of RCC treatment outcomes is still undetermined. Not to mention the fact that different markers were not simultaneously studied and sufficiently compared in terms of their predictive accuracy. Additionally, the optimal cutoff values of several markers are still a matter of analyses [6]. Worth emphasizing is the fact that these markers are easily obtainable from routine laboratory tests performed in everyday practice, and hence provide an inexpensive and widely available tool for clinicians [9]. The additional use of hemoglobin-to-platelet-ratio (HPR) and neutrophil-to-erythrocyte ratio (NER) represent the novel idea of focusing on red blood cell lineage within anti-cancer response based on the experience coming, e.g., from the oncological outcomes of the bladder cancer management described in other papers [11], and in our previous studies [12].
The usefulness of the prognostic models to categorize the cancer progression risk is significantly increased, when the combination of pre-treatment data is engaged [9], while some of the inflammatory markers were found to act as independent prognostic factors also in non-metastatic RCC [7]. Lastly, if novel therapeutics that are targeted to inflammatory pathways are engaged, preoperative values of biomarkers may serve as critical inclusion criteria and become accessory prognostic factors for patients [13].
In the present paper, we aimed to re-evaluate the prognostic significance of clinicopathologic features and compare the predictive value of different inflammatory markers in patients with localized or locally advanced RCC, who received radical or partial nephrectomy.
This is a retrospective single-tertiary-center study. Medical records of patients treated with partial or radical nephrectomy between 2010 and 2018 were reviewed. Among 778 nephrectomies, 654 were performed because of renal cell carcinoma. Fifty-four patients with distant metastases at the time of surgery and 5 patients with surgery for RCC recurrence (and no data on primary RCC) were excluded from further analysis. For further 6 patients the data were missing and for 90 patients there was no follow-up. Finally, 495 patients with primary localized and locally advanced RCC, who underwent nephrectomy in our center, were included in the analysis.
Information about demographic, clinical and pathological features were collected. None of the patients received additional treatment before radical surgery. Data on the modality of surgery, type of nephrectomy, perioperative complications and the length of hospitalization were gathered, as well. Data extracted from the pathology report included tumour stage, grade and histological subtypes. Tumours were graded according to the WHO/ ISUP or Fuhrman classification when adequate. Preoperative laboratory findings were also collected.
Ratio between the respective preoperative blood cell counts were calculated to
produce several inflammatory markers (neutrophil/lymphocyte ratio (NLR),
platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR), derived
neutrophil/lymphocyte ratio (dNLR), hemoglobin/platelet ratio (HPR) and
neutrophil/erythrocyte ratio (NER)). The systemic immune-inflammation index (SII)
was calculated according to the following formula: SII = neutrophil
Statistical analysis was performed in SAS software version 9.4 (SAS Institute,
Warsaw, Poland). Results are presented as the number and percentage for
categorical variables and medians, accompanied by the interquartile range (IQR)
for continuous variables. Differences between groups were tested with Fisher’s
test for categorized variables and Mann-Whitney U test for continuous variables.
The differences in the time to the event were analyzed using the Kaplan–Meier
method and log-rank test. In univariable analyses Cox proportional hazards were
used to identify factors associated with RFS, OS and CSS. Continuous values of
inflammatory markers were categorized based on the cutoff determined with
receiver operating curve (ROC) and Youden’s index analysis. Correlations between
variables were checked using the Spearman test. Univariable analyses provided
variables for multivariable analyses performed with Cox proportional hazard.
Hazard ratio (HR) supplemented with 95% confidence interval (95% CI) were
generated. Several multivariable models were constructed using alternative
inflammatory markers, which demonstrated significance in univariable analyses.
Inflammatory markers showing strong correlations between each other were not
incorporated into a single model. To assess the predictive accuracy for each
model the Harell’s C-index was calculated. For all statistical analyses, a
two-sided p-value
There were 495 consecutive patients enrolled in the study, predominantly male (60%) (Table 1). The majority of tumors were T1a stage (66%) and grade 2 (61%). Pathological examination revealed clear-cell carcinoma in nearly 80% of cases. Patients were treated surgically with radical intent and, in the majority of cases, nephron-sparing surgeries were performed (68%). During the median follow-up of 48 months (IQR 30–58) cancer-specific survival reached 95.35%, overall survival—91.31%, while recurrence-free survival—89.29%. Baseline patients’ characteristics are presented in Table 1.
| Characteristics | No./Mean | %/IQR | |
| Gender | female | 195 | 39.4 |
| male | 300 | 60.6 | |
| Age | 63 | 54–70 | |
| BMI | kg/m |
24.7 | 27.8–31.3 |
| Stage | T1a | 327 | 66.06 |
| T1b | 100 | 20.20 | |
| T2a | 21 | 4.24 | |
| T2b | 9 | 1.82 | |
| T3 | 36 | 7.27 | |
| T4 | 2 | 0.40 | |
| Grade | 1 | 136 | 27.47 |
| 2 | 305 | 61.62 | |
| 3 | 41 | 8.28 | |
| 4 | 13 | 2.63 | |
| Tumour diamater | cm | 3.7 | 2.5–5.0 |
| Histology type | clear cell | 395 | 79.80 |
| papillary type 1 | 58 | 11.72 | |
| papillary type 2 | 18 | 3.64 | |
| chromofobe | 24 | 4.85 | |
| Surgical treatment | Radical nephrectomy | 156 | 31.52 |
| NSS | 339 | 68.48 | |
| Outcomes | |||
| Recurrence | No | 442 | 89.29 |
| Yes | 53 | 10.71 | |
| Death | No | 452 | 91.31 |
| Yes | 43 | 8.69 | |
| Cancer Death | No | 472 | 95.35 |
| Yes | 23 | 4.65 | |
| Follow-up | months | 48.2 | 30.1–58.1 |
| Laboratory parameters | |||
| Hemoglobin | 139 | 127–148 | |
| Neutrophils | 4.42 | 3.37–5.73 | |
| Lymphocytes | 1.85 | 1.48–2.38 | |
| Platelets | 225 | 191–280 | |
| Red blood cells | 4.69 | 4.34–4.98 | |
| SIRI | 1.87 | 1.26–2.84 | |
| SII | 506 | 346–807 |
The associations between the values of selected markers and clinicopathologic features were analyzed. Elevated SIRI, SII and NLR were related to advanced staging, larger tumor size, and performance of radical nephrectomy, whereas high SIRI was additionally associated with male gender and tumour grade. The associations in the subgroups with low and high values of inflammatory markers (NLR, SII, SIRI) are summarized in Table 2.
| NLR |
NLR |
p | SIRI |
SIRI |
p | SII |
SII |
p | ||
| No. (%)/Median (IQR) | No. (%)/Median (IQR) | No. (%)/Median (IQR) | No. (%)/Median (IQR) | No. (%)/Median (IQR) | No. (%)/Median (IQR) | |||||
| Gender | Male | 189 (59) | 111 (64) | 0.25 | 173 (55) | 127 (69) | 0.002 | 194 (63) | 106 (56) | 0.11 |
| Female | 133 (41) | 62 (36) | 139 (45) | 56 (31) | 112 (37) | 83 (44) | ||||
| Age | 63 (54–69) | 63 (55–71) | 0.23 | 63 (54–69) | 62 (55–71) | 0.38 | 63 (55–70) | 62 (52–69) | 0.37 | |
| BMI | kg/m |
27.8 (24.8–31.2) | 27.7 (24.6–31.8) | 0.89 | 27.6 (24.7–31.2) | 28 (25.2–31.8) | 0.58 | 27.8 (25.2–31.2) | 27.3 (24.2–31.8) | 0.56 |
| Stage | T1 | 287 (89) | 139 (80) | 0.016 | 283 (91) | 143 (78) | 0.001 | 280 (92) | 146 (77) | |
| T2 | 15 (5) | 16 (9) | 13 (4) | 18 (10) | 11 (4) | 20 (11) | ||||
| T3 | 20 (6) | 16 (9) | 16 (5) | 20 (11) | 15 (5) | 21 (11) | ||||
| T4 | 0 (0) | 2 (1) | 0 (0) | 2 (1) | 0 (0) | 2 (1) | ||||
| Grade | 1 | 91 (28) | 45 (26) | 0.245 | 91 (29) | 45 (25) | 0.22 | 94 (31) | 42 (22) | 0.032 |
| 2 | 200 (62) | 105 (61) | 18 (6) | 116 (63) | 184 (60) | 121 (64) | ||||
| 3 | 26 (8) | 15 (9) | 27 (9) | 14 (8) | 24 (8) | 17 (9) | ||||
| 4 | 5 (2) | 8 (5) | 5 (2) | 8 (4) | 4 (1) | 9 (5) | ||||
| Tumour diamater | cm | 3.4 (2.5–5.0) | 4 (2.5–6.0) | 0.071 | 3.45 (2.4–5.0) | 4 (2.5–6.5) | 0.026 | 3.4 (2.1–4.5) | 4 (3–7) | |
| Histology type | clear cell | 257 (80) | 138 (80) | 0.127 | 248 (79) | 147 | 0.9 | 245 (80) | 150 (79) | 0.9 |
| other | 65 (20) | 35 (20) | 64 (21) | 36 (20) | 61 (20) | 39 (21) | ||||
| Surgery | RN | 91 (28) | 65 (38) | 0.042 | 85 (27) | 71 (39) | 0.009 | 77 (25) | 79 (42) | 0.0001 |
| NSS | 231 (72) | 108 (62) | 227 (73) | 112 (61) | 229 (75) | 110 (58) | ||||
| Outcomes | ||||||||||
| Recurrence | No | 290 (90) | 152 (88) | 0.45 | 285 (91) | 157 (86) | 0.07 | 280 (92) | 162 (86) | 0.052 |
| Yes | 32 (10) | 21 (12) | 27 (9) | 26 (14) | 26 (8) | 27 (14) | ||||
| Death | No | 299 (93) | 153 (88) | 0.13 | 292 (94) | 160 (87) | 0.021 | 286 (93) | 166 (88) | 0.034 |
| Yes | 23 (7) | 20 (12) | 20 (6) | 23 (13) | 20 (7) | 23 (12) | ||||
| Cancer death | No | 314 (98) | 158 (91) | 0.003 | 305 (98) | 167 (91) | 0.002 | 300 (98) | 172 (91) | 0.0006 |
| Yes | 8 (2) | 15 (9) | 7 (2) | 16 (9) | 6 (2) | 17 (9) | ||||
| Note: Number of patients (no.) with percentage (%) is shown for categorized variables and median with interquartile range (IQR) for continuous variables. Differences were tested with Fisher’s test for categorized variables and Mann-Whitney U test for continuous variables. | ||||||||||
Cut-off values for SIRI, SII, NLR, PLR, LMR, dNLR, HPR and NER were calculated to predict cancer-specific survival using ROC. For comparison different ROCs with respective areas under the curve (AUC) were demonstrated (Fig. 1).
 Fig. 1.
Fig. 1.Receiver operating curves (ROC) for prediction of cancer-specific survival (A), overall survival (B) and recurrence-free survival (C) for hematological markers (NLR, dNLR, SIRI, PLR, LMR, HPR, NER) in the whole cohort (with respective AUC) in long-term analysis (42 months). Abbreviations: NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; SIRI, systemic inflammatory response index; SII, systemic immune-inflammation index; NER, neutrophil/erythrocyte ratio; dNLR, derived neutrophil/lymphocyte ratio; LMR, lower lymphocyte/monocyte ratio; HPR, hemoglobin/platelet ratio.
When analyzing the preoperative systemic inflammatory markers, the CSS was worse
in patients presenting with elevated NLR (
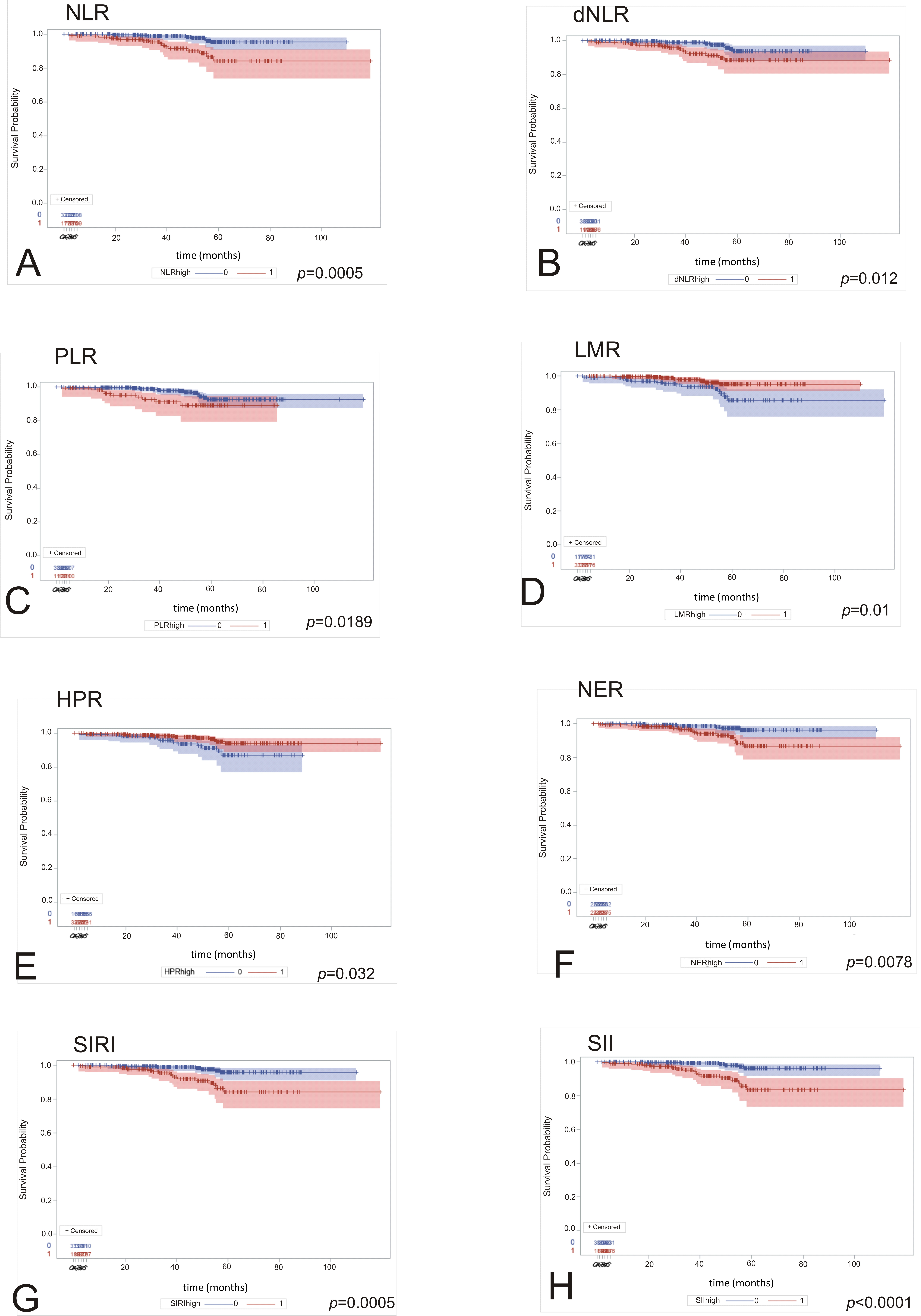 Fig. 2.
Fig. 2.The Kaplan–Meier curves for cancer-specific survival (CSS) in patients stratified by low (blue line) vs. high (red line) values of the following biomarkers: NLR (A), dNLR (B), PLR (C), LMR (D), HPR (E), NER (F), SIRI (G) and SII (H). Abbreviations: NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; SIRI, systemic inflammatory response index; SII, systemic immune-inflammation index; NER, neutrophil/erythrocyte ratio; dNLR, derived neutrophil/lymphocyte ratio; LMR, lower lymphocyte/monocyte ratio; HPR, hemoglobin/platelet ratio.
Univariate analysis of the associations between CSS and clinicopathologic
factors revealed that age (p = 0.009), tumour grade (p
| Univariate analysis - whole cohort - CSS | |||||
| Clinicopathological features | HR | Lower 95% CI | Upper 95% CI | p | |
| Age | (years) | 1.05 | 1.01 | 1.09 | 0.009 |
| Gender | male vs. female | 0.67 | 0.30 | 1.52 | 0.33 |
| Grade | ref = Grade 1 | 1 | |||
| Grade 2 | 3.121 | 0.698 | 13.889 | 0.13 | |
| Grade 3 | 6.389 | 1.065 | 38.462 | 0.042 | |
| Grade 4 | 90.31 | 17.544 | 500 | ||
| Staging | ref = stage T1a | 1 | - | - | |
| stage T1b | 6.79 | 1.7 | 27.03 | 0.007 | |
| stage T2a | 16.83 | 3.4 | 83.33 | 0.001 | |
| stage T2b | 23.71 | 3.97 | 142.86 | 0.001 | |
| stage T3 | 21.52 | 5.56 | 83.33 | ||
| stage T4 | 345.13 | 55.56 | - | ||
| Tumour diameter | 1.27 | 1.16 | 1.40 | ||
| Type of RCC | clear cell vs. other | 1.45 | 0.43 | 4.89 | 0.55 |
| Hematological markers | |||||
| SIRI | high vs. low | 4.24 | 1.75 | 10.32 | 0.001 |
| SII | high vs. low | 5.33 | 2.10 | 13.54 | 0.0004 |
| NLR | high vs. low | 4.11 | 1.74 | 9.70 | 0.001 |
| NER | high vs. low | 3.33 | 1.30 | 8.38 | 0.01 |
| PLR | high vs. low | 2.63 | 1.14 | 6.08 | 0.02 |
| HPR | low vs. high | 2.382 | 1.050 | 5.40 | 0.04 |
| dNLR | high vs. low | 2.79 | 1.21 | 6.46 | 0.02 |
| LMR | low vs. high | 2.84 | 1.23 | 6.56 | 0.015 |
In multivariate analysis, staging (p
| Multivariate analysis- whole cohort- CSS | |||||
| Predictive factor | HR | Lower 95% CI | Upper 95% CI | p | |
| Staging | ref = stage T1a | 1 | |||
| stage T1b | 5.16 | 1.28 | 20.83 | 0.02 | |
| stage T2a | 13.18 | 2.43 | 71.43 | 0.003 | |
| stage T2b | 4.30 | 0.53 | 35.71 | 0.2 | |
| stage T3 | 6.53 | 1.43 | 30.3 | 0.02 | |
| stage T4 | 17.09 | 1.51 | 200 | 0.02 | |
| Grade | ref = Grade 1 | 1 | 0.001 | ||
| Grade 2 | 1.630 | 0.347 | 7.634 | 0.53 | |
| Grade 3 | 1.542 | 0.218 | 10.87 | 0.66 | |
| Grade 4 | 25.837 | 3.367 | 200 | 0.002 | |
| Age | (years) | 3.512 | 1.272 | 9.701 | 0.02 |
| SIRI* | high vs. low | 3.154 | 1.174 | 8.472 | 0.013 |
| SII** | high vs. low | 3.30 | 1.21 | 8.99 | 0.02 |
| NLR*** | high vs. low | 2.616 | 1.006 | 6.802 | 0.043 |
| Note: hematologic markers were included in separate models, three models were developed with the following accuracy- C-index: *0.903, **0.902, ***0.890. Model without markers achieved C-index = 0.877. | |||||
Overall survival was significantly shorter in individuals with high NLR
(
 Fig. 3.
Fig. 3.The Kaplan–Meier curves for overall survival (OS) in patients stratified by low (blue line) vs. high (red line) values of the following biomarkers: NLR (A), dNLR (B), PLR (C), LMR (D), HPR (E), NER (F), SIRI (G) and SII (H). Abbreviations: NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; SIRI, systemic inflammatory response index; SII, systemic immune-inflammation index; NER, neutrophil/erythrocyte ratio; dNLR, derived neutrophil/lymphocyte ratio; LMR, lower lymphocyte/monocyte ratio; HPR, hemoglobin/platelet ratio.
Associations between OS and selected clinicopathological variables or laboratory
parameters are shown in Tables 5,6. in the univariate analysis age (p =
0.004), tumour grade (p
| Univariate analysis - whole cohort - OS | |||||
| Clinicopathological features | HR | Lower 95% CI | Upper 95% CI | p | |
| Age | (years) | 1.040 | 1.013 | 1.067 | 0.004 |
| Gender | male vs. female | 1.404 | 0.732 | 2.693 | 0.30 |
| Grade | ref = Grade 1 | 1 | |||
| Grade 2 | 2.818 | 1.085 | 7.299 | 0.03 | |
| Grade 3 | 4.293 | 1.241 | 14.925 | 0.02 | |
| Grade 4 | 36.813 | 10.989 | 125 | ||
| Staging | ref = stage T1a | 1 | |||
| stage T1b | 1.61 | 0.73 | 3.55 | 0.24 | |
| stage T2a | 3.61 | 1.23 | 10.64 | 0.02 | |
| stage T2b | 3.73 | 0.87 | 16.13 | 0.076 | |
| stage T3 | 3.42 | 1.44 | 8.13 | 0.005 | |
| stage T4 | 62.5 | 13.89 | 250 | ||
| Tumour diameter | 1.149 | 1.055 | 1.250 | 0.001 | |
| Type of RCC | clear cell vs. other | 2.099 | 0.750 | 5.876 | 0.15 |
| Hematological markers | |||||
| SIRI | high vs. low | 2.161 | 1.186 | 3.936 | 0.01 |
| SII | high vs. low | 2.206 | 1.210 | 4.022 | 0.009 |
| NLR | high vs. low | 1.946 | 1.067 | 3.548 | 0.03 |
| NER | high vs. low | 2.735 | 1.426 | 5.247 | 0.0025 |
| PLR | high vs. low | 2.027 | 1.070 | 3.839 | 0.03 |
| HPR | low vs. high | 1.761 | 0.964 | 3.217 | 0.06 |
| dNLR | high vs. low | 1.587 | 0.870 | 2.892 | 0.13 |
| LMR | low vs. high | 1.915 | 1.053 | 3.482 | 0.03 |
| Multivariate analysis - whole cohort - OS | |||||
| Predictive factor | HR | Lower 95% CI | Upper 95% CI | p | |
| Grade | ref = Grade 1 | 1 | |||
| Grade 2 | 2.733 | 1.05 | 7.09 | 0.04 | |
| Grade 3 | 4.012 | 1.155 | 13.89 | 0.03 | |
| Grade 4 | 34.011 | 10 | 111.11 | ||
| Age | (years) | 1.04 | 1.01 | 1.07 | 0.002 |
| NER* | high vs. low | 2.395 | 1.236 | 4.643 | 0.01 |
| SIRI** | high vs. low | 1.87 | 1.02 | 3.43 | 0.04 |
| SII*** | high vs. low | 1.873 | 1.016 | 3.455 | 0.04 |
| Note: hematologic markers were included in separate models, three models were developed with the following accuracy- C-index: *0.750, **0.747, ***0.745. Model without markers achieved C-index = 0.725. | |||||
In multivariate analysis, tumour grade (p
The RFS was shorter in individuals with elevated PLR (
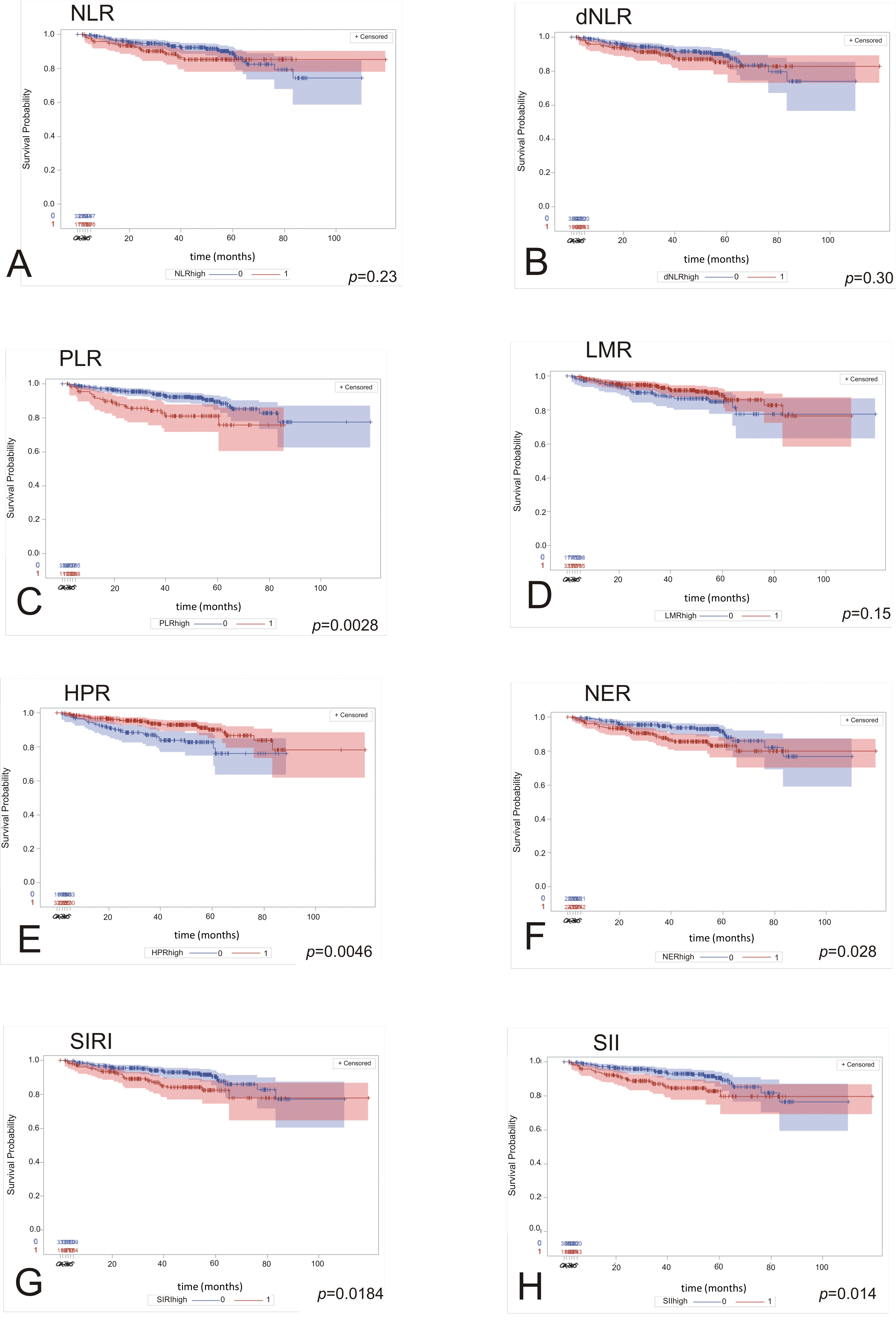 Fig. 4.
Fig. 4.The Kaplan–Meier curves for recurrence-free survival (RFS) in patients stratified by low (blue line) vs. high (red line) values of the following biomarkers: (a), dNLR (B), PLR (C), LMR (D), HPR (E), NER (F), SIRI (G) and SII (H). Abbreviations: NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; SIRI, systemic inflammatory response index; SII, systemic immune-inflammation index; NER, neutrophil/erythrocyte ratio; dNLR, derived neutrophil/lymphocyte ratio; LMR, lower lymphocyte/monocyte ratio; HPR, hemoglobin/platelet ratio.
In univariate analysis, the associations between RFS and pathologic factors,
tumour grade (p
In multivariate analysis, only tumour grade (p
| Univariate analysis- whole cohort-RFS | |||||
| Clinicopathological features | HR | Lower 95% CI | Upper 95% CI | p | |
| Age | (years) | 1.008 | 0.986 | 1.030 | 0.45 |
| Gender | male vs. female | 0.705 | 0.411 | 1.210 | 0.20 |
| Grade | ref = Grade 1 | ||||
| Grade 2 | 2.132 | 0.934 | 4.878 | 0.07 | |
| Grade 3 | 5.593 | 2.079 | 15.152 | 0.001 | |
| Grade 4 | 30.950 | 10.989 | 90.909 | ||
| Staging | ref = stage T1a | 1 | |||
| stage T1b | 1.86 | 3.88 | 0.89 | ||
| stage T2a | 6.41 | 15.15 | 2.7 | ||
| stage T2b | 6.41 | 21.74 | 1.9 | ||
| stage T3 | 5.62 | 11.76 | 2.69 | ||
| stage T4 | 19.23 | 142.86 | 2.55 | ||
| Tumour diameter | 1.215 | 1.133 | 1.304 | ||
| Type of RCC | clear cell vs. other | 1.517 | 0.684 | 3.361 | 0.30 |
| Hematological markers | |||||
| SIRI | high vs. low | 1.895 | 1.104 | 3.254 | 0.02 |
| SII | high vs. low | 1.946 | 1.133 | 3.343 | 0.02 |
| NLR | high vs. low | 1.392 | 0.801 | 2.419 | 0.24 |
| NER | high vs. low | 1.843 | 1.059 | 3.206 | 0.03 |
| PLR | high vs. low | 2.309 | 1.313 | 4.059 | 0.004 |
| HPR | low vs. high | 2.147 | 1.250 | 3.689 | 0.006 |
| dNLR | high vs. low | 1.328 | 0.771 | 2.288 | 0.30 |
| LMR | low vs. high | 1.484 | 0.862 | 2.556 | 0.15 |
| Multivariate analysis- whole cohort-RFS | |||||
| Predictive factor | HR | Lower 95% CI | Upper 95% CI | p | |
| Grade | ref = Grade 1 | 1 | |||
| Grade 2 | 1.847 | 0.80 | 4.26 | 0.15 | |
| Grade 3 | 4.701 | 1.12 | 9.80 | 0.03 | |
| Grade 4 | 23.92 | 5.46 | 52.63 | ||
| Tumour diameter | (cm) | 1.129 | 1.03 | 1.23 | 0.007 |
In this study, we evaluated the prognostic value of several systemic inflammatory markers in patients with localized or locally advanced renal cell carcinoma. We focused on the role of the markers of the well-established usefulness (NLR, dNLR, PLR, LMR) in the survival analysis, together with the new tools that are easily calculated from peripheral blood count (SII and SIRI), and, finally, the implementation of red blood cells lineage derivatives (HPR and NER).
There is a growing number of studies focusing on the involvement of inflammatory markers in the clinical practice of individuals that developed RCC [4, 6, 7, 9, 14]. The available papers, however, emphasize the role of particular parameters (e.g., NLR, PLR, LMR) derived from peripheral blood counts, but direct comparisons between different markers are lacking. Recently, novel inflammatory indices such as systemic SIRI and SII were also reported as prognostic for RCC oncological outcomes [4, 15]. Furthermore, hematological parameters have a well-documented prognostic role only in the setting of metastatic disease, in which IMDC and MSKCC models are mainly used [16, 17].
In the present paper, we found that patients treated surgically for localized or locally advanced RCC, who presented preoperatively with higher NLR, PLR, SIRI, SII, NER, dNLR, and lower LMR and HPR, had a significantly worse CSS. Multivariate analysis showed that tumour stage, grade, age and inflammatory markers constituted independent factors predicting CSS. As the markers of systemic inflammatory response demonstrate a strong correlation between each other, we decided to create three alternative models using a single systemic inflammatory marker for each multivariate model. The model based on clinicopathologic features, which did not include any inflammatory markers was characterized by already high accuracy (C-index 0.877). Further addition of inflammatory indices provided more accurate prognostic information. The CSS predictive model with the highest accuracy included SIRI values and achieved a C-index of 0.903, while alternative multivariate models included SII and NLR and were characterized by C-index 0.902 and 0.890, respectively. Therefore, different inflammatory markers seem to provide the same prognostic information, as they show a strong correlation with each other (SIRI, SII and NLR) and their incorporation into the model results in similar accuracy increment.
Cut-off values for the categorization of particular markers into elevated or
decreased largely differ between studies. In the paper by Elghiaty et
al. [3] patients with non-metastatic RCC up to 7 cm in diameter, who presented
with higher MLR (cut-off value of 0.21), were at greater risk of recurrence and
had worse CSS. Then, Hu et al. [6] evaluated the prognostic significance
of NLR (cut-off 2.78), dNLR (cut-off 2.05) and PLR (cut-off 185) in RCC cases
treated with nephrectomy and found the association between poorer OS and
increased values of NLR, dNLR and PLR. On the contrary, Lucca et al.
[18] enrolled localized RCC cases and described MLR as potentially the best
prognostic tool (when compared to NLR and PLR) that reflects the systemic
inflammatory response. Conversely, another study proposed PLR as the most
reliable marker for overall and cancer-specific survival prediction in patients
with non-metastatic clear cell RCC, who underwent nephrectomy [19]. To sum up,
conflicting reports on the superiority of different inflammatory markers exist
and none have been clearly proven as of highest prognostic value yet. Here, based
on the predefined threshold values, Kaplan–Meier curves revealed that higher NLR
(
There is still limited evidence on the prognostic value of the novel markers of systemic inflammation such as systemic inflammatory response index and systemic immune-inflammation index [4, 15, 20]. Interestingly, in our study the novel markers of systemic inflammation i.e., higher SIRI and SII, occurred to be associated with worse CSS and represented independent predictors of CSS, comparably to NLR. CSS prognostic models including SIRI and SII outperformed the model that based on staging, grading and age alone. Moreover, in the multivariate analysis these inflammatory markers were also significant predictors of OS but did not prove to be an independent tool for RFS estimations. This observation complies with the paper by Chen et al. [4], in which the authors reported worse CSS and OS in individuals with elevated SIRI. Worth emphasizing is the fact that AUC for SIRI was far greater, when compared to NLR, PLR and MLR [4]. In our study SII exhibited superior AUC compared to other markers (including SIRI) for all investigated outcomes, as well. Another retrospective study reported that elevated SII is associated with OS and CSS in non-metastatic RCC even after propensity score matching but on the contrary to our results the authors did not provide data on other inflammatory markers that could be obtained simultaneously [20].
In the current study, we also included hematological biomarkers derived from red
blood cell lineage, i.e., HPR and NER. Among the potential mechanisms involved in
the association between red blood cells and RCC, other authors listed e.g.,
anemia, systemic inflammation leading to iron deficiency or decrease in renal
erythropoietin production [21]. Here, elevated NER was related to shorter
recurrence-free, cancer-specific and overall survival in univariate analysis.
Moreover, NER proved to be an independent risk factor for shorter overall
survival, together with age and tumour grade in multivariate analysis. Decreased
HPR was associated with poorer OS and CSS in univariate analysis, as well.
Furthermore, the prognostic values of HPR and NER in RCC were hardly studied, and
only few studies were performed that were focused on red blood cell derivatives
[22, 23]. Tang et al. [11] described that low HPR (
None of the studied inflammatory markers were independently associated with recurrence-free survival. Although higher SIRI, SII, NER, PLR and lower HPR were related to shorter time to reccurence, only tumour diameter and grade served as independent predictors for RFS in the multivariable model. This corresponds with the majority of prognostic scores, which rely solely on histopathologic (staging, grading, necrosis, tumour diameter) and clinical features (age, symptoms) [25]. On the other hand, one of the meta-analysis reported the predictive value of elevated NLR for RFS/PFS in RCC (HR = 2.18) [26]. Some authors pinpoint the phenomena of retrospective study bias as the clue for the incoherence of the published data [25]. Furthermore, worth mentioning is the fact that our cohort consisted of non-metastatic cases. Other authors found the correlation between predefined threshold of NLR and PFS in the metastatic setting [27, 28]. Further studies investigating the role of inflammatory markers on the recurrence risk are warranted.
We are conscious of several limitations of our study that are listed below. It was a single-center retrospective analysis and selection bias could not be excluded. Some clinical data were not collected, and some other biomarkers easily obtained from peripheral blood count were not included e.g., lymphocyte to red blood cell ratio (LRR) [24]. Furthermore, no data on the postoperative values to be used in the follow-up were collected. Local and distant RCC recurrences were not analyzed separately. Due to the retrospective nature of the study, the follow-up was inconsistent between patients. Nevertheless, we managed to analyze in the relatively large contemporary cohort the impact of several markers of the inflammatory response, easy to adopt in the patients with RCC for clinical purposes.
In our study, we compared the prognostic value provided by different inflammatory markers in patients with localized or locally advanced RCC. We have found that markers of systemic inflammation might provide additional prognostic value (especially SIRI, SII, NLR and NER), and further increase the predictive accuracy of available models. Clinicopathological features (tumour stage, grade and patient’s age), however, remain the most important independent prognostic factors for oncological outcomes in RCC patients treated with nephrectomy. For the first time, we show the prognostic value of neutrophil-to-erythrocyte ratio, which constitutes an independent risk factor for overall survival
AUC, area under the curve; CI, confidence interval; CSS, cancer-specific survival; dNLR, derived neutrophil/lymphocyte ratio; HPR, hemoglobin/platelet ratio; HR, hazard ratio; IQR, interquartile range; LMR, lymphocyte/monocyte ratio; LRR, lymphocyte to red blood cell ratio; MLR, monocyte/lymphocyte ratio; NER, neutrophil/erythrocyte ratio; NLR, neutrophil/lymphocyte ratio; OS, overall survival; PLR, platelet/lymphocyte ratio; RCC, renal cell carcinoma; RFS, recurrence-free survival; ROC, receiver operating curve; SII, systemic immune-inflammation index; SIRI, systemic inflammatory response index.
Conceptualization, ŁZ and AŚ; methodology, ŁZ, and AŚ; software, AŚ; validation, ŁZ, AŚ, and PZ; formal analysis, ŁZ, and AŚ; investigation, ŁZ, AŚ; resources, ŁZ, AŚ, KG, ŁM, CŚ, PZ; data curation, ŁZ, and AŚ; writing, ŁZ; writing and re-editing, ŁZ, AŚ; visualization, ŁZ, AŚ; supervision, AW, PR; project administration, ŁZ, and AŚ; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study was conducted under the Ethics committee vote AKBE/72/2021 of the Medical University of Warsaw. Informed consent was obtained from all subjects involved in the study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
