Coronary artery disease (CAD) and heart failure (HF) are major worldwide threat to health and well-being. Important progress in the treatment of CAD and HF have contributed to a decline in mortality around the world. A considerable number of epidemiological studies reported a strong independent association between elevated heart rate and major cardiovascular risk factors including atherosclerosis, ventricular arrhythmias, and left ventricular dysfunction. Ivabradine (IVA) is a pure heart rate-lowering agent with well-documented anti-anginal and anti-ischemic properties comparable to well-established anti-anginal agents, such as beta-blockers and calcium channel blockers. The heart rate reduction with IVA is beneficial in patients with CAD, chronic stable angina pectoris, and chronic HF, with an acceptable tolerance and safety profile. The pharmacodynamic and pharmacokinetic properties of this drug make it an important agent in the management of patients with CAD and HF. The aim of this short review is to explore recent results with IVA, a new medication that lowers heart rate by selectively inhibiting the If current, and to describe others future potential applications.
There is a substantial evidence base that heart rate (HR) is a powerful predictor of mortality in normal individuals (1, 2) and in patients with coronary events and chronic heart failure (CHF) (3, 4). A substantial HR reduction decreases myocardial oxygen demand and improves endocardial blood supply, and there has been considerable interest in agents that exhibit this property (5, 6).
In patients with CAD, elevated HR is an independent risk factor for major ischemic coronary events, cardiovascular mortality, and sudden cardiac death (7).
In patients with CHF, baseline HR is an independent risk factor of all-cause mortality, cardiovascular mortality, and hospitalization for CHF. HR is a major determinant of myocardial oxygen consumption and energy utilization; furthermore, an increase in HR reduces the diastolic coronary perfusion time. An increase in HR, as a consequence of increased sympathetic activity, may trigger ischemic events (8).
β-blockers are widely used in chronic stable angina (CSA) and after myocardial infarction (MI). At least part of their activity involves HR lowering. However, these agents can have undesirable negative inotropic activity and can cause a paradoxical vasoconstriction of large epicardial coronary arteries at rest and during exercise (9). Additionally, not β1 selective β-blockers may cause bronchoconstriction in patients with chronic obstructive airway disease (10) and may have negative metabolic effects, including a reduction in insulin sensitivity. A selective HR-lowering agent that does not produce these undesirable effects could thus be of therapeutic value (12). Published studies suggest that IVA exhibits an acceptable and favorable benefit-risk profile, and this drug should be considered as a viable option in patients with CSA and CHF (13).
IVA determines a dose-dependent reduction in HR, depending on the baseline HR, with a more consistent decrease in subjects with higher baseline HR. At usually recommended doses, this reduction is 10 bpm, with a plateu effect at doses > 20 mg twice a day (14).
After oral administration, peak-plasma IVA concentrations are reached in about 1 hour; its absolute oral bioavailability is approximately 40% because of first-pass elimination in the gut and in the liver.
The pharmacokinetics of IVA are linear over an oral dose from 0.5 mg to 24 mg. It is extensively metabolized in the liver and in the intestine by CYP3A4-mediated oxidation. IVA plasmatic levels decline with an effective half-life of 6 hours. About 4% of its oral doses is excreted in urine; the major metabolite N-desmethylated derivative is excreted in feces and urine (14, 15).
The safety of IVA has been estabilished in pediatric patients (age from 6 months to18 years old) and it is supported by pharmacokynetic and pharmacodynamics trials: its efficacy was displayed for treatment of tachyarrhythmias in children and young adults and also in post-operative junctional ectopic tachycardia, the most common tachyarrhythmias in the early post-operative period after open-heart surgery (16).
IVA is a specific HR-lowering agent (Figure 1), which has selective action on pacemaker activity in the sinoatrial node of the heart (Figure 2), resulting in important differences compared with non-selective HR reducing agents, such as β-blockers (11). It decreases HR and myocardial oxygen consumption at rest and during exercise (14-17). IVA is licensed for the treatment of CSA in patients with normal sinus rhythm, who have a contraindication or intolerance for β-blocker drugs, or in combination with β-blockers in patients inadequately controlled even with an optimal β-blocker dose and whose HR is > 60 bpm (18, 19). IVA is the first of a new class of HR-reducing agents without other direct cardiovascular effects (negative inotropic effect, blood pressure reduction) (20, 21). It has an excellent tolerability and safety profile, and it can be also safely combined with other currently used cardiovascular drugs, including β-blockers (22, 23).
 Figure 1
Figure 1The molecular structure of Ivabradine.
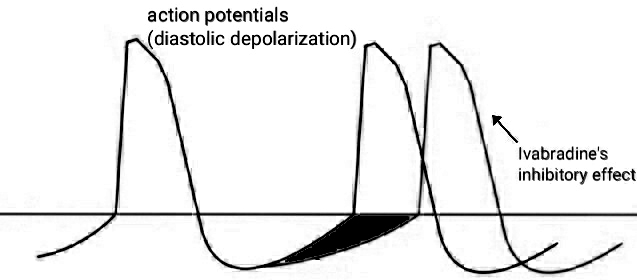 Figure 2
Figure 2Ivabradine-mediated inhibition of action potentials, with consequent slowing of distolic depolarization.
The HR-reducing effect of IVA is proportional to resting HR; extreme sinus bradycardia is uncommon. In the BEAUTIfUL (morBidity–mortality EvAlUaTion of the If inhibitor ivabradine in patients with coronary disease and left ventricULar dysfunction) study, symptomatic bradycardia was the most common adverse effect leading to discontinuation of treatment. However, it remained a rare event, even though 87% of enrolled patients were receiving β-blockers at the same time (24). Indeed, only 3% (146 patients) withdrew from the study due to symptomatic bradycardia.
As would be expected, the QT interval is prolonged with the reduction in HR. However, with appropriate correction for HR or, in studies of direct comparison of the QT interval, when the influence of the HR was controlled by atrial pacing, no significant effect of IVA was found on ventricular repolarization duration (25), QT duration, QT dispersion, or maximum and minimum QT duration (26). Consequently, IVA has no direct torsadogenic potential, though, for obvious reasons, this specific HR-reducing drug should not be administered with agents with QT-prolonging effects. Clinical trials suggest that dose-dependent reversible visual side effects reported with IVA are relatively low at treatment doses up to 10 mg bid (24, 27, 28).
IVA (5.0, 7.5, and 10.0 mg bid) has been demonstrated to be non-inferior to atenolol (50 or 100 mg/day) in terms of antianginal and anti-ischemic efficacy in 939 patients with CSA in INITIATIVE (INternatIonal TrIAl on the Treatment of angina with IVabradinE versus atenolol) (29). In this study, Tardif and coll. (29) found that the increase in exercise capacity was associated with a prolongation of exercise test duration (Table 1). Another study, ASSOCIATE (evaluation of the Antianginal efficacy and Safety of the aSsociation Of the If Current Inhibitor ivAbradine with a beTa-blockEr) explored the effect of IVA on top of atenolol 50 mg/day in 889 patients with CSA (28). In combination with atenolol, IVA induced a significant increase in total exercise duration (primary efficacy criterion) and improvement in other exercise test criteria (time to limiting angina, time to angina onset, and time to 1-mm ST-segment depression) compared with a placebo group receiving background therapy with atenolol. This study demonstrated that IVA can be added on top of β-blockers in CSA patients with insufficient HR reduction, in patients who remain symptomatic despite treatment with β-blockers (28, 30), and in patients with refractory angina (31). The results of IVA in the treatment of CSA in patients with CAD have been confirmed in a broad patient population in everyday routine practice (REDUCTION Study) (32), independently of the severity of angina and the presence of comorbidities (33).
| Daily dose | Aim | Result |
|---|---|---|
| 5-7.5 mg | To evaluate the efficacy of combination therapy with IVA in patients already treated by calcium-channel blockers and nitrates | Decreased frequency of angina attacks (8) |
| 2.5-10 mg | To study the anti-anginal and anti-ischemic effects of IVA | Dose-dependent improvement in exercise tolerance (19) |
| 7.5-10 mg | To compare the anti-anginal and anti-ischemic effects of IVA versus atenolol | IVA is as effective as atenolol in stable angina (29) |
| 7.5-15 mg | To compare the anti-arrhythmic effects of IVA versus propanolol succinate | IVA is more effective than metoprolol succinate in IST (51) |
The BEAUTIfUL study demonstrated that the treatment with IVA (5.0 or 7.5 mg bid) in 10 917 patients with stable CAD and left ventricular dysfunction (LVD) leads to a 36% reduction in relative risk for fatal and non-fatal MI. It leads also to a 30% reduction in the need for coronary revascularization and to a 22% reduction in the hospitalization for fatal and non-fatal MI or unstable angina in those with a baseline HR of 70 bpm or above (24). This study also offered a unique opportunity to evaluate prospectively for the first time the effect of HR as a prognostic factor by analyzing the effect of elevated HR on cardiovascular events in the placebo arm, in this high-risk population of patients with CAD and LVD (34). Further analysis in the 1507 patients in BEAUTIfUL who had angina at baseline demonstrated that IVA improved the primary outcome (the composite of cardiovascular death, MI and hospitalization for heart failure) by 24% and MI alone by 42%, relative to placebo (35).
The SHIfT (Systolic Heart Failure Treatment with the I(f) Inhibitor Ivabradine Trial) study was a randomized, double-blind study designed to compare IVA with placebo on outcomes in 6500 patients with symptomatic CHF (New York Heart Association (NYHA) class II-IV), left ventricular ejection fraction (LVEF) <35%, and a prior hospitalization for worsening heart failure within the previous 12 months. Randomized treatment (12-48 months) was given on top of guidelines-based therapy for CHF, including a β-blocker at optimized dose. Resting HR at baseline had to be >70 bpm. The primary endpoint was the composite of the time to first event of cardiovascular death or hospitalization for worsening heart failure. Secondary endpoints include all-cause, cardiovascular, and heart failure mortality, and hospitalization (36). The results of this study showed that IVA substantially and significantly improves outcomes in patients with CHF receiving the best possible evidence-based background treatment (37) and significantly reduced the primary composite endpoint of cardiovascular death or hospitalization for worsening heart failure by 18% (P<0.0001). The improvement in outcomes became apparent within 3 months of initiation of treatment. Benefits were maintained through the course of the trial in all pre-specified subgroups: patients below or over 65 years of age, males and females, receiving or not β-blockers at randomization, with heart failure of an ischemic or non-ischemic etiology, NYHA class II or class III/IV, with or without diabetes, and with or without hypertension. Analysis of secondary endpoints showed a strong trend toward a 10% reduction in all-cause mortality (P= 0.092), a significant reduction in death from heart failure by 26% (P=0.014), and significant reduction in hospitalization for heart failure by 26% (P< 0.0001). In this study bradycardia leaded to study withdrawal in only 1% of the overall population, which is remarkable considering that 89% were receiving β-blockers. These results support the importance of HR reduction with IVA for improvement of clinical outcomes in CHF (37, 13).
The results of the BEAUTIfUL trial (24, 34) shed new light on the role of HR control in cardiovascular disease and lead to a series of stimulating hypotheses that constitute the rationale for another trial called SIGNIfY (Study assessInG the morbidity-mortality beNefits of the If inhibitor ivabradine in patients with coronarY artery disease). This study enrolled patients with CAD and normal left ventricular function with resting HR ≥70 bpm. The primary endpoint took into consideration only CAD outcomes, i.e. cardiovascular mortality and hospitalization for MI. (38).
Patients in the study received up to 10 mg twice daily, which is higher than the currently authorized maximum daily dose of 7.5 mg twice daily. The results from the trial suggest that these high doses of IVA have rather inconsistent effects on cardiovascular outcomes: there was no significant difference between the IVA group and the placebo group in the incidence of the primary end point (6.8% and 6.4%, respectively; hazard ratio, 1.08; 95% confidence interval, 0.96 to 1.20; P=0.20). IVA was associated with a small but significant increase in the combined risk of CV death or non-fatal MI among patients with activity-limiting angina but not among those without activity-limiting angina (P=0.02 for interaction). Among patients who had stable CAD without clinical HF, lowering heart rate with IVA does not reduce the risk of CV death or non-fatal MI (39).
The impact of the data from the SIGNIFY study on the balance of benefits and risks of IVA will be now evaluated by the European Medicines Agency which will issue an opinion on whether the marketing authorization should be maintained, varied, suspended or withdrawn across the EU (40).
IVA is widely approved for use as an anti-anginal agent, with subsequent approval for use in heart failure patients. However, given its heart-rate-specific effect, IVA has been investigated in many off-label indications, as an alternative to traditional heart-rate-reducing medications.
In this respect, inappropriate sinus tachycardia (IST) is a clinical condition, characterized by an elevation in HR even in absence of a physiological stimulus. This paroxysmal tachycardia, originating in the sinus nodal area, often causes palpitations, dyspnea and exercise intolerance. It does not usually respond to current anti-arrhythmic drugs, such as β-blockers and calcium-channel antagonists.
IVA has recently shown promise in the treatment of IST, selectively inhibiting sinus node current with consequent decrease of HR in absence of haemodynamic compromise (41-50).
In a recent study the switch of the unsuccessful treatment to IVA (7.5mg twice daily) determined a significant reduction in resting HR (mean value 87.1 versus 107.3 bpm.), even greater than metoprolol succinate, with 70% patients who were free of IST-related complaints (51). Another study reported an improvement of ejection fraction and quality of life after 3 months of IVA administration in a 49-years-old man with IST and ventricular dysfunction (52). IVA seems to be effective and safe in short and medium-term treatment of IST; however, long-term follow-up studies are still necessary in order to clarify this aspect.
Another novel finding is the marked amelioration of chronic viral myocarditis after treatment with IVA in a murine model (53), which prevented the progression from coxsackievirus-induced myocarditis to dilated cardiomyopathy. This preventive and therapeutic effect of IVA is probably associated with the inhibition of the p38 MAPK pathway, the downregulation of inflammatory responses, the decreased cardiomyocytes apoptosis and the reduction of collagen expression (implicated in the genesis of fibrosis).
CAD and CHF are the most common type of heart disease a leading cause of morbidity and loss of quality of life and a major public health problem that exerts heavy economic costs. The improved survival of patients may contribute to an enlarging pool of patients who are at high risk of second MI and CHF. In these two pathologies, most ischemic episodes are triggered by an increase in HR, which induces an imbalance between myocardial oxygen delivery and consumption. Data gathered from clinical trials suggest that IVA, a selective and specific inhibitor of the If current that reduces HR without adverse hemodynamic effects, has demonstrated its efficacy in the treatment of CSA and myocardial ischemia. The indication of IVA has been extended for use in association with β-blockers in patients with CAD.
Recent results demonstrate that IVA substantially improves outcomes in patients with CHF; published data suggest that HR is an independent strong predictor of cardiovascular and all-cause mortality in men and women of all ages with and without cardiovascular disease (acute coronary events, all-cause mortality, cardiovascular mortality, sudden cardiac death, and acute coronary syndromes). Measurement of HR represents an important component of the assessment of patients with CAD and CHF: it should be viewed in the same light as other risk factors, such as high blood pressure, cigarette smoking, cardiac dysfunction, and diabetes, all of which are associated with elevated HR. A high HR has direct detrimental effects not only on myocardial ischemia, but also on the progression of atherosclerosis, ventricular arrhythmias, and left ventricular function. The risk increases at values HR >60 bpm. IVA, a drug that slows HR though an effect on the If channels, can be used neither alone (when β-blockers are contraindicated or not tolerated) or in combination with β-blockers.
IVA reduces HR without affecting sympathetic activity or cardiac contractility. IVA has anti-ischemic and antianginal efficacy equivalent to that of β-blockers and CCBs in CSA. IVA can improve cardiac outcomes in stable CAD and left ventricular systolic dysfunction, in patients who have heart rates ≥70 bpm and in patients with stable angina.
The indication of IVA has been extended for use in association with β-blockers in patients with CAD. The effects of IVA on myocardial ischemia are greater than those predicted by β-blockers-mediated HR reduction: this suggests some favorable benefits of HR reduction with IVA versus other HR-reducing therapies. These include preservation of myocardial contractility, ventricular relaxation, prolongation of diastolic perfusion time and therefore myocardial perfusion, preservation of physiological mechanisms allowing hemodynamic adaptation to exercise, and coronary vasodilatation.
The results of SHIFT study demonstrated that IVA substantially improves outcomes in patients with CHF on top of the conventional recommended medications, β-blockers and inhibitors of the renin-angiotensin-aldosterone system. Thus, IVA points toward an important approach in the treatment of patients with CAD or CHF, based upon the concept of exclusive HR reduction.
In addition, there is some evidence of its role as an inhibitor of atrioventricular node conduction. In this respect, the BRAKE-AF project is an ongoing clinical trial, whose aim is to assess IVA use for rate control in atrial fibrillation. Its results could allow inclusion of IVA within the limited arsenal of medication currently available for rate control in atrial fibrillation (49).
On the other hand, a decrease in HR improves not only cardiac but also endothelial function: HR reduction by IVA reduces oxidative stress and prevents atherosclerosis in apolipoprotein E-deficient mice. In fact it was also demonstrated to improve erectile dysfunction in parallel to decrease in atherosclerotic plaque load in ApoE-knockout mice because of this collateral antioxidant effect (54-57).
Thus, the most appropriate pharmacological approach, together with life-style change and nutritional cardiovascular prevention, could be the new frontier. In fact, bioactive dietary elements, such as polyphenols, terrestrial or marine carotenoids and PUFAs, display antioxidant effect and substantially reduce markers of oxidative stress (58-64): they can contribute to prevent several chronic disorders (including cardiovascular diseases) by reducing the inflammatory responses, improving platelet function, blood pressure/fluidity and enhancing nitric oxide bioavailability, with the final effect of reducing the economic burden of cardiovascular morbidity (65, 66).
The antioxidants such as polyphenols, especially flavonols, can cause vasodilation, modulate inflammatory markers and cardiovascular health, and possess a range of protective cardiovascular effects (67-71): the availability of these supports to the physician will increase the options in the medical management of patients with CVD.
