Oocyte quality influences early embryonic survival, establishment and maintenance of pregnancy, fetal development and adult diseases. The developmental competence of oocytes is acquired gradually and increases with follicular development. The ability of an oocyte to develop into an embryo depends on, having enough specific information in the form of mRNA or proteins. If this information is insufficient, defects in nuclear or cytoplasmic maturation, or in both processes, may arise and thus affect the in vitro development of fertilized oocytes. The greater developmental competence of oocytes aspirated from larger follicles is reported as compared with smaller follicles. Oocyte developmental competence is greatly correlated with the morphology of the cumulus oocyte complexes (COCs). Apart from morphological or biochemical markers, molecular markers have also been investigated. Until now, no specific markers of oocyte developmental competence could be described for the oocyte developmental competence. To, utilize female germplasm to its maximum, there is a need to enhance developmental competence of lesser competent oocytes derived from the follicles which are not fully grown. The oocyte pre-maturation and maturation conditions affect gene expression not only in the oocyte but till the blastocyst stages too. Strategies have been discussed in this review would be useful to enhance the developmental competence of oocytes.
In recent past, in vitro embryo production (IVEP) technique has gained significant impetus in relation to genetic improvement and reproductive management of domestic animals as well as in enhancing our understandings about the regulation of embryo development. Even after substantial improvements in IVEP technique with improved protocols, only 30–40% of such oocytes reach the blastocyst stage, for future uses, either to be transferred to a recipient or frozen. Most of the embryos are arrested at 8-16 cell stage of embryonic development; it becomes obvious that post-fertilization embryo culture is the most critical period of the process in terms of determining the blastocyst yield. However, it is not only the reason of poor developmental competence in fact outcome of IVEP is rather dependent on the quality and origin of the oocytes (1) therefore, within certain limits culture conditions may not have a major influence on the capacity of the immature oocyte to form a blastocyst. Oocytes retrieved from bovine ovaries collected from slaughterhouse or ovum-pick-up are used as a major source for IVEP. However, these oocytes are extremely heterogeneous in developmental competence and ultimately reduce the efficiency of nuclear transfer, blastocyst yield, embryo transfer and the pregnancy outcomes. The poor developmental competence of in vitro matured oocytes has been proposed due to failure of the timely onset of embryonic genome activation resulting from incomplete cytoplasmic maturation of these oocytes (2). Once the oocyte is removed from the follicle its ability to develop to the blastocyst stage is more or less determined. Thus, the oocyte developmental competence has become a crucial concern in assisted reproductive approaches both in human and livestock species.
Oocyte developmental competence is defined as the ability of the oocyte to complete maturation, undergo successful fertilization and reach the blastocyst stage. The in vitro meiotic and developmental competence of oocytes is related to follicle size, estrous cycle stage and the level of atresia influenced by other follicles, mainly the dominant follicle. Larger follicles yielded significantly more oocytes (3-5) with many layers of granulosa cells and a higher proportion of in vitro produced blastocysts, suggesting that larger follicles may contain several growth factors enhancing morphological and functional status of the COCs and embryo yield (6). To improve developmental potential in vitro, oocytes which have received enough follicular instructions before they are collected and matured should be used. Oocytes with high degree of competence capable of undergoing nuclear and cytoplasmic maturation is an essential feature for high blastocyst yield (7). Temporarily inhibiting resumption of meiosis to allow cytoplasmic maturation to proceed in vitro, thereby improving development, or modifications of maturation media, blastocyst yields in vitro using oocytes recovered from slaughtered heifers and cows rarely exceed 40% on a consistent basis. In order to improve IVEP outcomes there is still a need to understand molecular mechanisms taking place during follicular growth, develop assays for screening of competent oocytes, and methods to enhance the cytoplasmic maturation.
During growth, oocyte accumulates several molecules in it, which later on contribute for its maturation, fertilization and early embryo development. These key molecules govern several important events occurring both in the nucleus as well as in cytoplasm during oocyte growth and maturation. The storage of pool of these molecules is referred as molecular maturation (8) or cytoplasmic maturation (9). During this process oocyte accumulates sufficient amount of mRNAs, ribosomes, proteins, and cytoplasmic organelles (like mitochondria, microtubules etc.) to be used later post fertilization (10). During the process volume of oocyte increases about 300 fold. In mammals, a direct and positive correlation has been reported between oocyte diameter and its developmental competence (11-13). This may be the fact why only a few oocytes amongst the morphologically looking similar oocytes develop into viable blastocysts. Further, it could also be the reason why in vivo developed oocytes have better competence than oocytes derived from in vitro cultured preantral follicles (14). The transcriptional activity of oocyte is increased significantly during growth of the bovine oocyte and decreases around the beginning of the antral stage when the oocytes achieve a diameter of about 110 μm (15, 16) and oocyte is able to store these mRNAs for longer time without degradation. Translational activity of bovine oocyte is maximum at its germinal breakdown (GVBD) stage and decreasing again at metaphaseII (MII) stage (17). Extensive remodeling and repositioning of intracellular organelles takes place at GVBD, throughout the transitions to metaphase I (MI), polar body extrusion (PBE) and MII, including movements of vesicles, mitochondria, Golgi and endoplasmic reticulum (18) (Figure 1) and this could impact on the cryosurvivability of oocytes derived from different meiotic stages (19-22). Other proteins are also accumulated around the GVBD such as ribosomal and mitochondrial proteins, histones, the zona pellucid (ZP) glycoproteins, and kinases (23, 24). Other aspect associated with the cytoplasmic maturation is the mitochondrialgenesis and their distribution between endoplasmic reticulum and the oolema (25) as no new mitochondria are generated in the embryo till blastocyst stage. Additionally, the Golgi apparatus is associated with lipid vesicles and moves to the subcortical region of the oocyte where it forms cortical granules required at the time of zona reaction.
 Figure 1
Figure 1Schematic representation of oocyte growth and attainment of developmental competence. With the growth of follicle, oocyte also grows in size reaching about 100 μm at GV stage and 110 μ m at GVBD. Simultaneously, numbers of mitochondria also increase in number and upon GVBD mitochondria move towards periphery. At GV stage chromosomes are condensed and at GVBD, chromosomes decondense and chromosomes divide, first PB extruded and chromosomes are aligned at metaphase plate. Golgi body at GV stage is dispersed throughout the cytoplasm in form of continuous filament and upon GVBD it gets fragmented and make cortical granules which are dispersed in the periphery of the ooplasm. GV= germinal vesicle, GVBD= germinal vesicle breakdown, PB = polar body.
Understanding the molecular mechanisms (signaling pathways) playing role between cumulus cells and oocyte is the ultimate key to explore the mechanism of acquisition of oocyte developmental competence and may be helpful in enhancing developmental competence during different in vitro or in vivo treatments. The appropriate interplay between oocyte and follicular cells is indispensable for proper oocyte development, folliculogenesis, and progression to ovulation (26, 27). This bidirectional communication in cumulus–oocyte complexes (COCs) is mediated through gap junctions (27, 28) and is crucial for the promotion of cell growth (29), cell survival (30), suppression of luteinization (31) and maintenance of cumulus cells (CCs) metabolism (27, 32). Oocyte secreted factors (OSFs) are amongst the important mediators of bidirectional communication, which regulates gene expression in CCs that are associated with oocyte maturation and subsequent embryo development. Major OSFs that regulate oocyte developmental competence are growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) (33, 34). It is possible that BMP15 and GDF9, in combination, activate SMAD1/5/8 and SMAD2/3 pathways and enhance the expression of hyaluronan synthase 2 (HSA2), gremlin 1(GREM1), tumor necrosis factor-induced protein 6 (TNFAIP6), epidermal growth factor receptor (EGFR) and prostaglandin-endoperoxide synthase 2 (PTGS2) etc in CCs which through paracrine action act on oocyte and further enhance the oocyte competence (Figure 2). This vicious cyclic mechanism goes on and helps in the acquisition of oocyte developmental competence (13). Activation of these pathways is important for oocyte development in mono-ovulatory large animals (34). BMP15/GDF9 supplementation during in vitro maturation (IVM) is likely to promote the uniform distribution of active mitochondria, thereby improving functional competence (31) and in vitro embryo development (4).
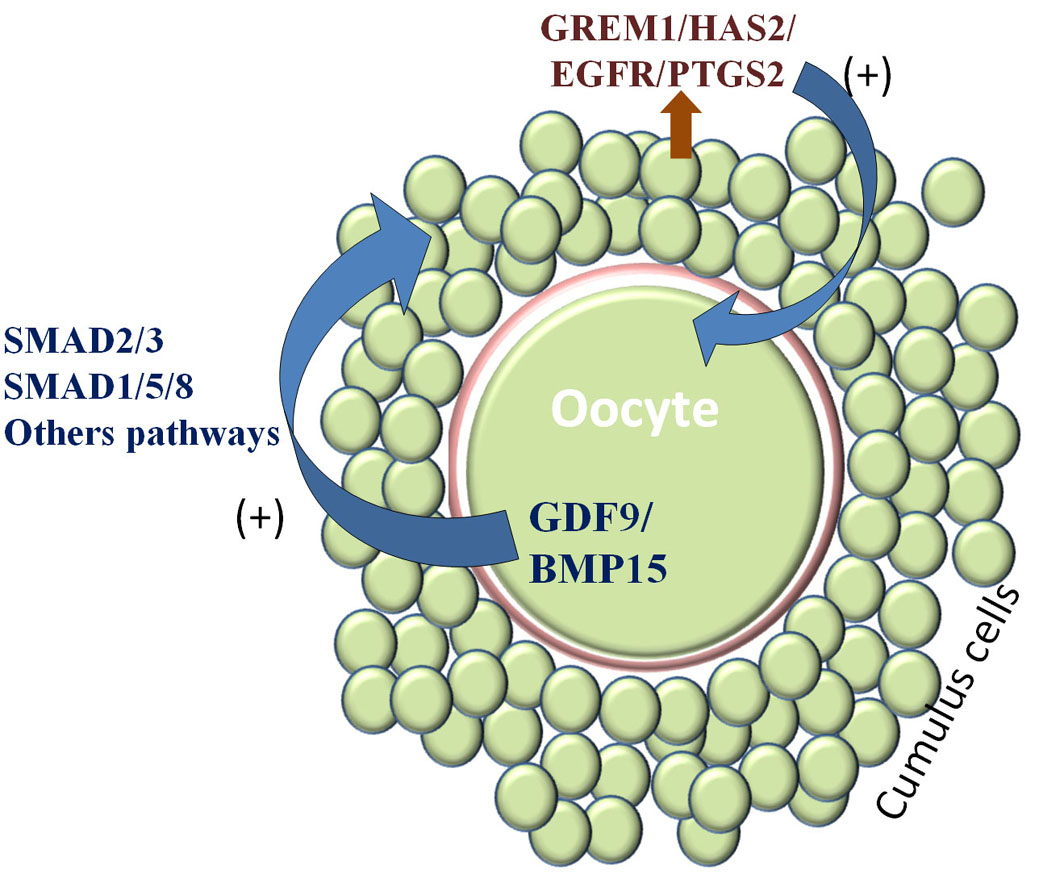 Figure 2
Figure 2Acquisition of oocyte developmental competence. Developing oocyte secretes some factors GDF9/BMP15/etc (oocyte quality regulators) which act on surrounding cumulus cells through SMAD2/3, SMAD1/5/8, and others pathways and enhance the gene expression of GREM1/HAS2/EGFR/PTGS2 etc (oocyte quality predictors) and in turn these molecules act on the oocyte to enhance the expression of GDF9/BMP15. This cycle continues and oocyte gets competence.
Morphological assessment is based on number of layers and compactness of cumulus, homogeneity of the ooplasm, and extrusion of first polar body (35, 36). However, these morphological evaluations are not reliable enough to act as the sole criteria for the evaluation of oocyte competence; therefore, other alternative selection criteria are needed for selecting the most viable oocytes for IVEP. Positive correlation between follicle size while oocyte retrieval and blastocyst rate has been observed in sheep, depicting the oocyte diameter as a determining factor in acquiring meiotic competence in various species, including buffalo (4, 5). Despite this morphological similarity, there was a transcriptomic difference in the cumulus cells from small and large follicles. In particular, the cumulus cells from large follicles were enriched in transcripts that regulate metabolism, cell differentiation and adhesion (37).
During the growth phase, oocytes synthesize a variety of proteins, including glucose-6-phosphate dehydrogenase (G6PDH), more G6PDH activity is reported in growing oocytes (38), and its activity decreases in oocytes having completed growth phase and are likely to have achieved developmental competence (39). Brilliant Cresyl Blue (BCB) staining is known to be a noninvasive method, as it allows the selection of competent oocytes among a heterogeneous pool. BCB screening is based on the ability of G6PDH to reduce the BCB stain present in oocyte. Growing oocytes, having higher G6PDH activity reduce BCB relatively in a quicker mode and become colorless, whereas fully grown oocytes with lesser G6PDH activity remain blue (40). BCB+ COCs have been reported to have significantly better developmental competence than BCB- COCs (5, 13).
Morphological assessment based on thickness and compactness of cumulus, homogeneity of the ooplasm and extrusion of 1st polar body has been used as convenient ways of evaluating oocyte quality (35). However, these morphological evaluations are not reliable enough to act as the sole criteria for the evaluation of oocyte competence, therefore, the selection criteria are needed for selecting the most viable oocytes for embryo production. Studies on oocyte gene expression have revealed the specific molecular markers to characterize successful oocyte maturation, a good number of genes have been identified as potential predictors of oocyte competence both in cattle and buffaloes (1, 13, 41). Since these transcripts are indicators of the oocyte cytoplasmic maturation status, identification of the same may provide new knowledge and powerful tool/s to assess oocyte competence individually by analyzing its cumulus component.
Several transcripts were found associated with cumulus cells but only few of them have been found to influence oocyte competence (42). The cumulus genes involved in oocyte competence reported were HAS2, inhibin A (INHBA), EGFR, GREM1, betacellulin (BTC), cluster of differentiation (CD44), TNFAIP6, and PTGS2 etc (13, 42). These differentially expressed genes may be important markers of the oocyte’s ability to reach the blastocyst stage. In human, interleukin 7 (IL7) has been identified as a maturation-specific OSF, may be a potential candidate for the development of a screening test of egg quality (43).
Zona pellucida (ZP) birefringence is another morphological criterion to select high-quality oocytes. For an optimal competence of oocyte, cytoplasmic oocyte maturation must undergo in synchrony with nuclear maturation. The zona pellucida is a unique extracellular coat surrounding the oocyte and its thickness increases with oocyte growth and it plays very important role during ovulation, fertilization and early embryonic development (44). The ZP is composed of filaments, which are forming a three-dimensional network structure that shows a high birefringence (45, 46). ZP birefringence measures ZP thickness and uniformity. Observation of ZP birefringence intensity with a polarized microscope has been reported to identify oocyte developmental competence and thus to improve subsequent IVEP outcomes. A high ZP birefringence is associated with better embryo quality and implantation and pregnancy rates (47-49). A high ZP birefringence reflects a healthy oocyte with full nuclear and cytoplasmic maturation (47). This quality indicator may be used to reinforce the selection parameters already in use.
Selecting developmentally competent oocytes and zygotes based on their morphology are more often influenced by personal judgments and lack universal standards. Di-electrophoresis (DEP), the motion of neutral particles due to the application of an external nonuniform electric field, has been a useful non-invasive technique for the extensive manipulation of living cells and DNA (50). Recently, di-electrophoretic approach was investigated as a potential, noninvasive method of competence prediction that measures the speed of oocyte/zygote migration in an electric field (14 peak-to-peak volts; frequency of 4 MHz). The faster oocytes were developmentally more competent than the slower ones (51). Oocytes and zygotes with different cytoplasmic contents can possess different tendencies of polarization and speed of migration in the electric field. Di-electrophoretically separated oocytes and zygotes showed difference in the rate of blastocyst development accompanied by difference in transcriptional abundances (51). Based on the same principle a device has been developed for the faster separation of competent oocytes/ embryos (52).
The above mentioned methods of oocyte developmental competence assessment in vitro has been summarized in Table 1.
| S.No. | Methods | Description | References |
|---|---|---|---|
| 1 | Morphological basis of assessment | based on number of layers and compactness of cumulus, homogeneity of the ooplasm, and extrusion of first polar body | 35, 36 |
| 2 | Biochemical assay | BCB staining: Based on G6PDH activity, BCB+ COCs significantly more competence than BCB- COCs. | 5, 13, 40 |
| 3 | Molecular markers | Cumulus cells expressed genes: HAS2, EGFR, GREM1, PTGS2, BTC, INHBA etc. | 1, 13, 41, 42 |
| 4 | Zona birefringence | ZP birefringence measures ZP thickness and uniformity; a high ZP birefringence is associated with better embryo quality and implantation and pregnancy rates. | 47-49 |
| 5 | Di-electrophoretic migration | Measures the speed of oocyte/zygote migration in an electric field; faster oocytes are developmentally more competent. | 51, 52 |
Coasting refers to the arrest of gonadotropin support in presence of endogenous luteinizing hormone (LH) to stimulate follicular differentiation and oocyte competence (53). Introduction of follicle-stimulating hormone (FSH) coasting – FSH withdrawal – introduced a few years ago in cow (53). Maximal oocyte competence acquisition occurs in large animals, including cows, between the FSH surge and the pre-ovulation LH surge (8). Gonadotropin starvation exerts a selective pressure that eliminates the smaller follicles and increases the proportion of medium-to-large follicles (53, 54). Moreover, this coasting may induce slight atresia in the cumulus outer layers, which is favourable to grade 3 COCs formation and therefore improved developmental competence (55). These findings were confirmed by the high rates of cleavage (90%) and blastocyst (80%) following 48h of coasting in the cow (53). Accelerated coasting in case of human ovarian hyperstimulation syndrome (OHSS) through treatment with GnRH-antagonist after pituitary suppression with GnRH agonist offered a novel approach to reduce estradiol level, avoid cycle cancellation, and maintain excellent oocyte maturation rate and thus high pregnancy rate with prevention of OHSS (56). Nivet et al. (57) demonstrated that the optimal period between FSH surge and transvaginal aspiration is 54±7 h and well-defined competence window is crucial to obtain optimal oocyte quality in ovarian stimulated milking cows. The coasting strategy following superovulation improves oocyte competence, blastocyst yield and pregnancy outcome when used in ovum pickup-in vitro production programs (58); therefore, it is a useful tool to improve oocyte competence through the contribution in the increase of the potential pool of oocytes to be selected.
Morphological appearance of oocytes, which is generally used as marker for the selection of oocytes for IVEP, is not sufficient to accurately predict the competence of oocytes. Defined molecular signatures of oocyte competence acquired during maturation by the surrounding somatic cells would be useful to predict the developmental potential of oocytes. Brilliant Cresyl Blue (BCB) staining is known to be a noninvasive method, as it allows the selection of competent oocytes among a heterogeneous pool. BCB screening test could be effectively used for selecting competent oocytes (13, 59) (Figure 2). BCB screening is based on the ability of G6PDH to reduce the BCB stain present in oocyte. Growing oocytes, having higher G6PDH activity reduce BCB relatively in a quicker mode and become colorless, whereas fully grown oocytes with lesser G6PDH activity remain blue (13, 40). BCB+ oocytes are significantly larger in diameter compared with BCB- oocytes as reported in various species i.e. cattle (59), buffalo (13), goats (60), and pigs (61). It has been reported that significantly more number of BCB+ oocytes were obtained when oocytes were retrieved from large follicles compared to small follicles in buffalo (5). In addition, BCB+ oocytes contain significantly higher levels of mtDNA copy number than BCB− oocytes (62, 63). BCB+ oocytes expressed significantly higher level of oocyte competence markers (13). A significantly higher blastocyst rate is recorded in BCB+ oocytes than BCB- oocytes (13, 60).
The acquisition of developmental competence is a sequential process which occurs along with the follicular growth in ruminants. This developmental process includes both nuclear and cytoplasmic maturation (64). Thus, the fully matured oocytes having completed follicular information in the form of messenger RNA (mRNA) or proteins must be collected for improvement in bubaline in vitro culture (1). If oocytes are collected before the acquisition of adequate information, developmental potential of embryo decreases (65). There are some important events occurring in the oocyte during the late follicular growth, which are essential to achieve full developmental competence (66). This has already been proved as cumulus-oocyte complexes (COCs) derived from large follicle (LF >6 mm) have better cytoplasmic maturation and show higher developmental competence, whereas small follicle (SF <6 mm)-derived oocytes are less competent due to inadequate cytoplasmic maturation (5, 67). Hence, blastocyst rate can be improved either by selecting more competent LF oocytes or by ensuring cytoplasmic maturation of SF oocytes.
In most of the mammals, oocytes are maintained at germinal vesicle (GV) stage until pre-ovulatory luteinizing hormone (LH) surge. During this period of meiotic arrest, oocytes undergo morphological and biochemical changes to achieve developmental competence. However, when oocytes are removed from follicle, they spontaneously resume nuclear maturation with impaired oocyte capacitation and result in lower rate of embryo development. The proportion of bovine oocytes exhibiting developmental competence greatly increases in oocyte derived from follicles >8.0 mm (large antral). Some oocytes derived from 3.0 mm follicles (small antral) have acquired a degree of developmental competence, but require additional “pre-maturation” in vivo prior to final maturation by the surge in gonadotrophin levels to induce competence (68).
Pre-maturation in vivo can be driven by advancing follicular growth with FSH administration; the oocytes derived from cows subjected to FSH treatment prior to ovum pick up (OPU) have higher developmental competence than those derived from untreated cows (69, 70). Competence of SF-derived oocytes in vitro may be enhanced by providing sufficient pre-maturation incubation for a specific period of time (71) in the presence of meiotic inhibitors such as roscovitine, cycloheximide, 6-dimethylaminopurine and butyrolactone. Amongst all meiotic inhibitors, roscovitine is an effective and reversible inhibitor of cyclin-dependent kinase 2 (72), capable of arresting the cells in late G1 and G2/M cell cycle transition. It has less detrimental effects on oocyte developmental competence than other inhibitors and has been used effectively to reversibly block the nuclear maturation of oocytes for certain time period in bovine (71, 73), equine (65), ovine (74), porcine (75) and bubaline (9). Incubation of COCs before actual maturation period for 24 h with roscovitine (roscovitine prematuration treatment) showed more BCB+ oocytes than control (9). Also the mRNA expression of oocyte competence molecular markers i.e. BMP15 and GDF9 (in oocytes) and GREM1, PTGS2 and EGFR (in cumulus cells) was significantly higher in roscovitine prematuration treatment group than control (9). Roscovitine prematuration treatment significantly improved the blastocysts rate in small follicle derived oocytes than the control (9) (Figure 2). Sanchez et al. reported that prematuration of small follicle derived oocytes in polycystic ovary syndrome patients with C-Type Natriuretic Peptide (CNP) for 24 h improved oocyte developmental competence and embryo yield. CNP secreted by mural granulosa cells is presently considered as a natural inhibitor for oocyte maturation (76). It binds with the Natriuretic Peptide Receptor 2 (NPR2), expressed in the cumulus cells and induces the production of cGMP. Cyclic GMP enters the oocyte via gap-junctional communication and regulates the levels of cAMP by competing for the hydrolyzing activity of oocyte specific phosphodiesterase 3A (PDE3A) (77); thus, maintaining oocytes under meiotic arrest.
Despite the significant improvements made towards the efficiency of IVP protocols in livestock, the rate of embryos developing normally remains lower than that seen in in vivo-produced embryos (78). In mammals, oocytes are arrested at GV stage till LH surge in vivo but when oocyte are removed from follicle (cumulus oocyte complexes, COCs), its meiosis is resumed and oocyte again arrested at MII stage and the in vitro maturation time varies species wise (79, 80). During this maturation time though oocytes get nuclear maturation completed but due to incomplete mimicking environment as par with in vivo, its cytoplasmic maturation is not proper, which results in the lower competence of such oocytes.
In vitro maturation of COCs over the granulosa cell monolayer improves embryo development (81, 82). Supplementation of gonadotropins and follicular fluid has been found to improve in vitro maturation by improving the expression of germ cell markers (83, 84). As the oocytes and embryos express receptors for different growth factors (85, 86), supplementation of insulin like growth factor-1, (IGF-1), platelet derived growth factor (PDGF), epidermal growth factor (EGF) etc. have been found to improve maturation and embryo development (87). Recently, mesenchymal stem cells conditioned media has been reported to contain several important growth factors and improved the competence of oocytes and embryos (88) (Figure 3). Similarly, leptin receptor has also been reported in oocytes and supplementation of leptin in maturation media improved the competence of buffalo oocytes and embryos (89). Post thaw survivability is another issue in case of in vitro produced embryos especially in buffalo and large amount of cytoplasmic lipid droplets in in vitro produced buffalo embryos has been suggested as a major cause of reduced post thaw survival (36, 90). L-carnitine has been found to reduce cytoplasmic lipid droplets in embryos by modulation its metabolism as well as acting as antiapoptotic agent; thus, improved post thaw survivability (36).
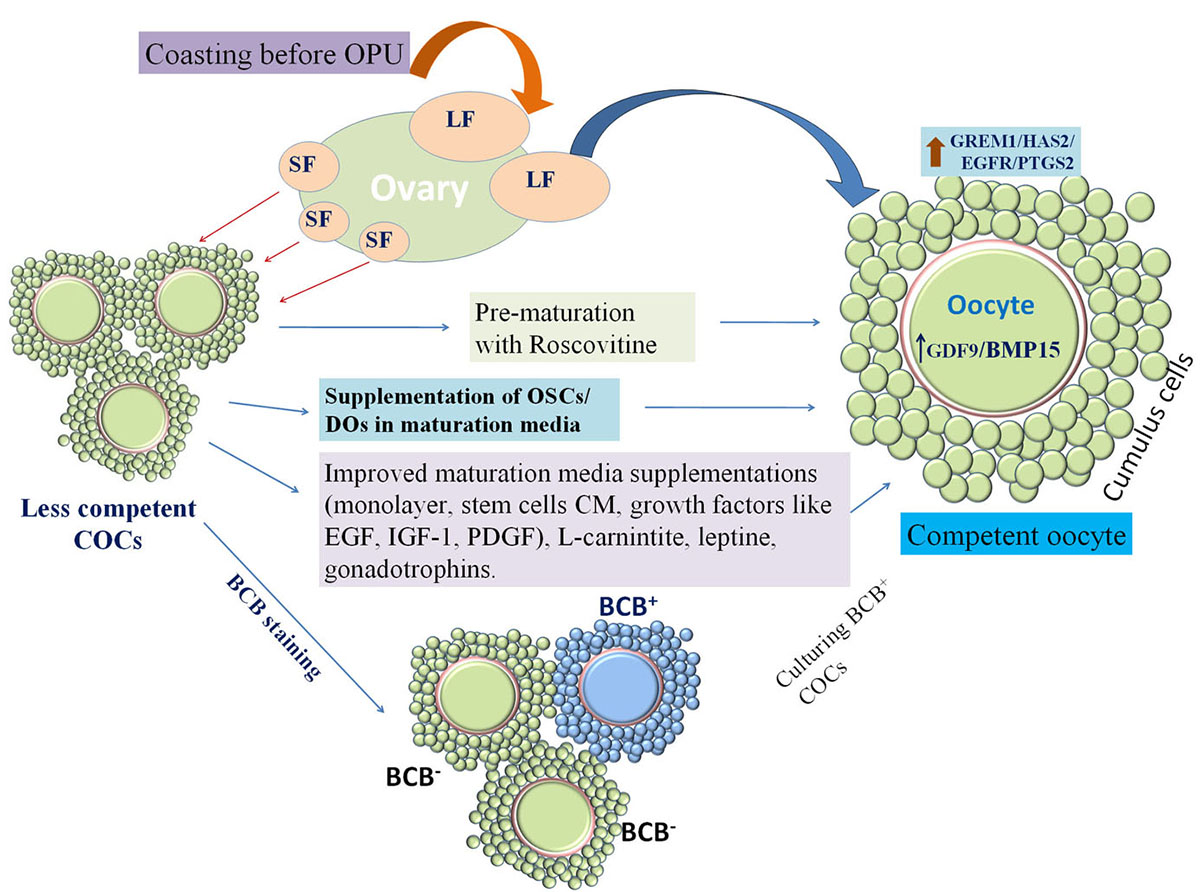 Figure 3
Figure 3Schematic summary of important strategies to improve in vitro embryo production (IVEP). Large follicle (LF) derived cumulus oocyte complexes (COCs) have more developmental competence than small follicle (SF) derived COCs. Coasting before ultrasound guided ovum-pick up (OPU) increases development of more competent medium size follicles which can be retrieved through OPU and further used for IVEP, while this coasting technique cannot be utilized in case of slaughterhouse derived COCs. In that case competent COCs can be screened by Brilliant Cresyl Blue (BCB) staining and BCB+ COCs are found to be more competent. Further, to utilize female germplasm to the maximum SF derived COCs can be prematured with nuclear inhibitors like roscovitine before actual oocyte maturation to enhance the developmental competence of SF derived COCs. While in vitro oocyte maturation medium can be enriched by oocyte secretory factors (OSFs) or supplementation of denuded oocytes (DOs) as they secrete OSFs. Moreover, COCs may be matured over cumulus monolayer and maturation medium may be supplemented with growth factors (like; EGF (epidermal growth factor), IGF-1(insulin-like growth factor), PDGF (Platelet derived growth factor), LIF (leukemia inhibitory factor) etc.), stem cell conditioned medium (CM), as it contains several growth factors), L-carnitine, leptine (antioxidant), gonadotrophins (FSH, LH), steroids (estradiol) etc.
Oocyte secretory factors (OSFs) are amongst the important mediators of bidirectional communication, which regulates gene expression in CCs that are associated with oocyte maturation and subsequent embryo development. Major OSFs that regulate oocyte developmental competence are growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15). Positive effect of OSFs on oocyte developmental competence has been reported in buffalo (4). They found enhanced developmental competence of buffalo oocytes when in vitro maturation media was supplemented with GDF9 (175 ng/ml) or BMP15 (100 ng/ml) alone or in combination, though the additive effect was more. As these factors are secreted from oocytes, supplementation of denuded oocytes (DOs) in ratio of 2:1 in the maturation media significantly improved blastocysts rate (Figure.2). This effect was more pronounced when OSFs were supplemented in small follicle derived oocytes than large follicle derived oocytes (4). Similar findings have also been reported in mouse (91), pig (92), cattle (30, 93) and goat (94, 95). Hussein et al. (30) depicted qualitative temporal changes in oocyte paracrine factor production during maturation and Li et al. (96) found that GDF9 and BMP15 mRNA expression levels were closely associated with oocyte maturation, fertilization, embryo quality, and pregnancy outcomes. OSFs exert synergistic beneficial effects on nuclear and cytoplasmic maturation, rapid utilization of energy and management of oxidative stress (94). Competent oocytes express higher levels of CCs transcripts, such as HAS2, GREM1, EGFR and TNFAIP6, which are responsible for cumulus expansion (13). A significant increase in the expression of these genes was observed in both the DOs co-cultured and in the GDF9–BMP15 combination group in LF oocytes (4).
Mitochondria are energy supplying organelles, whose functional integrality is essential for cellular survival and development. Mitochondria in the oocyte can provide adenosine triphosphate (ATP) for fertilization and preimplantation embryo development (97) and they can act as stores of intracellular calcium and proapoptotic factors as well. Mitochondria have their own genetic materials, mitochondrial DNA (mtDNA) that is derived from maternal mtDNA exclusively, for the paternal mitochondria are not retained in the fertilized oocyte at the four cell stage (98). There are approximately 1,00,000 to 2,00,000 mtDNA copies in a mammalian oocyte (99), which are divided among all daughter cells during the developmental progress of embryos. Because there is no mtDNA replication until post implantation, this renders oocytes more susceptible to any kind of mitochondrial dysfunction (100). A mitochondrial pre-fertilization threshold need to be ensured, as mitochondria are diluted out during post-fertilization cleavage, there should be sufficient copies of mtDNA per blastomere to allow transmission of mtDNA to each cell of the postimplantation embryo after the initiation of mtDNA replication during the early postimplantation stages (101). Recent studies have shown that mitochondrial dysfunctions, such as the structural, spatial and genetic abnormalities in the oocyte, may influence normal embryo development, so mitochondrial characteristics and other mitochondrion-related changes can serve as signs of oocyte quality. The importance of ATP levels during in vitro maturation has been demonstrated in bovine oocytes. Higher quality oocytes, assessed by morphology, contained significantly higher ATP levels and produced significantly higher blastocyst rates after fertilization (102). Mitochondrial DNA encodes 13 of the subunits of the electron transfer chain (ETC) complexes, associated with the process of oxidative phosphorylation, along with 22 tRNAs and 2 rRNAs that are necessary for mRNA expression (103). Expression of these mitochondrial genes is vital for cellular function, especially as the ETC is the cell’s major generator of ATP (104) whilst mutation or deletion can result in severe cellular impairment (105). In ovine, Cotterill et al. (106) observed increase in the mtDNA copy number across oogenesis reflects the changing ATP demands needed to orchestrate cytoskeletal and cytoplasmic reorganization during oocyte growth and maturation and the need to fuel the resumption of meiosis in mature oocytes following the pre-ovulatory gonadotrophin surge (106). Supplementation of mitochondrial DNA to mitochondria deficient oocytes increased development to blastocyst, the final stage of preimplantation development, and promoted mitochondrial DNA replication prior to embryonic genome activation in mitochondrial DNA deficient oocytes (107). Thus, supplementing oocytes with mitochondria may be used as a strategy to overcome mtDNA deficiency and enhance oocyte developmental competence.
The above mentioned strategies for the enhancement of oocyte developmental competence in vitro has been summarized in Table 2.
| S.No. | Methods | Description | References |
|---|---|---|---|
| 1 | Coasting to induce competence in large mammals | Arrest of gonadotropin support in presence of endogenous LH to stimulate follicular differentiation and oocyte competence, high rates of cleavage (90%) and blastocyst (80%) achieved in the cow | 53, 54, 58 |
| 2 | Screening and culture of competent oocytes | Significantly higher blastocyst rate is recorded in BCB+ oocytes than BCB- oocyte | 13, 59, 60 |
| 3 | Pre-maturation culture of oocytes | Prematurartion inculabation with nuclear inhibitors like roscovitine, butyrolactone etc. improved developmental competence of SF derived COCs | 9, 65,71, 73, 74, 75, |
| 4 | Interventions while in vitro maturation of oocytes | ||
| 4.1. | In vitro maturation over granulosa cell monolayer | Improved embryo development | 81, 82, 83, 84 |
| 4.2. | Supplementation of growth factors (IGF-1, EGF, PDGF) in maturation media | Improved embryo development | 85, 86, 87 |
| 4.3. | Supplementaion of mesenchymal stem cells conditioned media | Improved embryo development | 88 |
| 4.4. | Supplementation of leptin | Improved embryo development | 89 |
| 4.5. | Supplementation of L-carnitine | Reduce cytoplasmic lipid droplets in embryos, cryosurvivability, improved embryo development | 36 |
| 5 | Supplementation of oocyte secretory factors | Endogenous OSFs (supplementation of denuded oocytes) or exogenous OSFs (GDF9/BMP15) improved embryo development. | 4, 91, 92, 93, 94, 95 |
| 6 | Supplementation of mitochondria | Increased development to blastocyst, preimplantation development, mitochondrial DNA replication | 107 |
COCs
cumulus oocyte complexes
in vitro embryo production
Germinal Vesicle
Germinal Vesicle Break Down
Metaphase –II
Metaphase-I
Polar Body Extrusion
Zona Pellucida
cumulus cells
Growth Differentiation Factor 9
bone morphogenetic protein 15
Hyaluronan Synthase 2
Inhibin βA; EGFR epidermal growth factor receptor
Gremlin 1
Betacellulin
Cluster of Differentiation 44
Tumor Necrosis Factor-Induced Protein 6
Prostaglandin-endoperoxide synthase 2
interleukin 7
Glucose-6-Phosphate Dehydrogenase
Brilliant Cresyl Blue
Oocyte Secretory Factors
Mega Hertz
Luteinizing Hormone
Follicle-Stimulating Hormone
Ovarian Hyperstimulation Syndrome
Gonadotrophin Releasing Hormone
Mitochondrial DNA
Large Follicle
Small Follicle
Ovum Pick Up
C-Type Natriuretic Peptide
Natriuretic Peptide Receptor 2
Cyclic Guanosine Mono Phosphate
Phosphodiesterase 3A
Insulin-Like Growth Factor-1
Platelet Derived Growth Factor
Epidermal Growth Factor
Denuded Oocytes
Adenosine Triphosphate
Electron Transfer Chain.
