- Academic Editor
Background: Streptococcus mutans is a major component of
dental plaque, contributing to cariogenic biofilm formation and inducing dental
caries. Attempts have recently been made to use postbiotic mediators (PMs) to
prevent dental caries. This research evaluated the antimicrobial/antibiofilm
activity of PMs derived from Lactobacillus rhamnosus GG (LGG) and
Lactobacillus reuteri (LR) against S. mutans in vitro.
Methods: PMs were obtained from the Lactobacilli supernatants.
The minimum inhibitory concentration, minimum bactericidal concentration,
antibiofilm potential, and metabolic activity of PMs against S. mutans
were evaluated using CFU/mL, scanning electron microscopy, and XTT
(2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide)
reduction assay. The expression of gtfB gene as one of the most
important genes involved in S. mutans biofilm formation was also
measured using qRT-PCR. Results: CFU score was reduced by both PMs, but
the reduction was only significant in LGG (p = 0.02). Both PMs caused a
significant decrease in the metabolic activity of S. mutans compared
with the controls (p
Tooth decay, the most common biofilm-dependent disorder, is a major public health problem that globally affects 2.3 billion adults and 530 million children worldwide [1]. Caries is a multifactorial condition that forms from dysbiosis of host oral microbiota [2]. Dental biofilm, or plaque, constitutes a favorable environment for the growth of many bacteria, among which Streptococcus mutans (S. mutans) are known to play an important role in developing dental caries. In addition to acidic metabolites, S. mutans produces GtfB, GtfC, and GtfD, which are glucosyltransferase (GTF) enzymes that synthesize intracellular and extracellular polysaccharides [3, 4]. They are encoded by gtfB, gtfC, and gtfD genes, respectively. Water-insoluble glucans are synthesized by the action of gtfB, during the formation of extracellular polysaccharides from sucrose [5]. As a result, large amounts of insoluble glucans promote the colonization and adherence of S. mutans to tooth surfaces, which could ultimately increase dental plaque production and caries [6, 7, 8].
Due to the high cost of dental treatments, caries preventative measures are recommended to protect teeth. However, routine methods using mechanical/chemical approaches have drawbacks, such as the increased risk of fluorosis and the destruction of healthy bacteria by antimicrobial mouthwashes, which leads to the colonization of pathogens [9, 10, 11]. The potential to treat the oral environment and its various reservoirs using molecules of diverse densities and concentrations for distinct purposes offers a promising approach to modulating the host’s inflammatory response in the oral environment [12]. This could lead to significant improvements without the need for invasive therapies. Because of the rising resistance to synthetic antimicrobials and their side effects, research on alternative natural products like probiotic microorganisms and their metabolites has been recommended.
Probiotics are living microorganisms that promote host health and immunological homeostasis when administered appropriately [13]. They have been shown to decrease caries by suppressing oral pathogens and altering the microbial composition of dental plaque [14, 15, 16]. Also, among their attributes are inhibition of plaque induction by free radical scavenging and controlling mucosal permeability, in addition to destroying pathogens through secretion of lactic acid, hydrogen peroxide, and bacteriocins [17, 18]. Probiotics are generally well-tolerated, but people with compromised immune systems should not take them due to the possibility of infections [19]. Some of them, like Lactobacillus and Bifidobacterium, are acidogenic, making them unsuitable for treating dental caries [20, 21]. Postbiotics are a mixture of deactivated probiotic cells and their metabolites, which deliver some of the benefits of probiotics without the drawbacks [22]. They are suggested to suppress S. mutans biofilms [23] and oral multispecies biofilms [24] and might be used to create potent anticaries agents [25]. “Postbiotic mediators” (PMs) is a relatively new term that is gaining popularity and refers to byproducts of the metabolic activity of probiotics. PMs extracted from various microorganisms have been reported to prevent infection through attenuating pathogen growth and biofilm development [25].
Various Lactobacillus strains have shown promising effects against S. mutans in the oral cavity. But the number of studies on their PMs is relatively limited [25]. Lactobacillu rhamnosus GG (LGG) and Lactobacillu reuteri (LR) belong to the Lactobacillus genus. They have beneficial effects such as good growth capacity, strong adhesion ability, efficacy against pathogens, and production of antimicrobial and anti-colonization substances that make them attractive as probiotic strains [26, 27]. The exact effect of various lactobacilli and their byproducts and the mechanisms involved in their effectiveness against S. mutans is unclear. On the other hand, the beneficial effects of postbiotics on S. mutans have been suggested to depend upon the specific strains of the probiotics [22, 23]. The gene regulation of gtfs is crucial in S. mutans adhesion and biofilm formation and has been the focus of many investigations [28, 29, 30, 31]. Therefore, the current study aims to examine the anticaries effect of PMs derived from LGG and LR by investigating their antibiofilm effect and metabolic activity on S. mutans and assessing the expression of the gtfB gene.
Both L. rhamnosus GG (ATCC 53103) and L. reuteri (ATCC 23272)
kept in de Man, Rogosa, and Sharpe (MRS) agar (Merck, Darmstadt, Germany). Before
each test, Lactobacillus strains were cultured in MRS broth (Merck,
Darmstadt, Germany) and placed in a CO
After the incubation time of the Lactobacillus species was completed,
centrifugation (10,000
The Clinical and Laboratory Standards Institute (CLSI) guideline [32] was used
to calculate MIC and MBC. Briefly, 100
Sub-culturing broth dilutions determined the MBC of PMs at or above the MIC that inhibits the growth of S. mutans on BHI agar (Merck, Darmstadt, Germany). It was defined as the lowest concentration of an antimicrobial that caused at least 99.999% (3-log reduction) killing of the initial inoculum. The tests were repeated at least three times.
Two hundred
Scanning electron microscopy (SEM, Zeiss,
EVO 40, Jena, Germany) was used for the structural analysis of the PM-treated
biofilms. S. mutans biofilm was allowed to develop on the surface of the
sectioned teeth. Three intact permanent human premolar that was previously
extracted due to orthodontics were selected. Informed consent was obtained from
each donors. The teeth were preserved in distilled water at 37 °C until
the experiment. Before the operation, the teeth were scaled by hand and cleaned
using a fine-grain pumice-water slurry (Dental AG Ltda, São Paulo, SP,
Brazil) and Robinson bristle brushes (Labor Dental Ltda, São Paulo, SP,
Brazil) in the low-speed handpiece for the 30 s. Using a micromotor handpiece,
each tooth was cut in longitudinal sections with a diamond disc. Before
treatment, sectioned teeth were autoclaving at 121 °C, 15 lbs psi for 30
minutes, followed by treatment with 2
The XTT (2,3-(2-methoxy-4-nitro-5-sulphophenyl)-5-[(phenylamino)
carbonyl]-2H-tetrazolium hydroxide) reduction assay (XTT Kit; Roche Applied
Science, Indianapolis, IN, USA) was used to measure the metabolic activity of
S. mutans, according to the manufacturer’s instructions.
Briefly, 100
Immediately after treating S. mutans with 1/2
| Gene | Sequence (5′-3′) | Size | |
|---|---|---|---|
| gtfB | Forward | TGTTGTTACTGCTAATGAAGAA | 103 bp |
| Reverse | GCTACTGATTGTCGTTACTG | ||
| 16S rRNA | Forward | GCAGAAGGGGAGAGTGGAAT | 182 bp |
| Reverse | GGCCTAACACCTAGCACTCA |
All tests were repeated at least thrice, and data were analyzed using one-way
analysis of variance (ANOVA) followed by Tukey’s HSD (honestly significant
difference) tests. All results were presented as mean
To obtain the lowest concentration of PMs that inhibited the growth of
S. mutans, serial dilutions of PMs were utilized. No turbidity at
concentrations of more than 25% (v/v) in both PMs from LGG and LR was seen,
which showed regarded as the MIC value. Also, in both PMs, there was no growth of
S. mutans on BHI agar at concentrations above 25% (v/v), which confirms
as MBC value (MIC
CFU/mL count was used to assess the potential inhibitory effect of the PMs on
S. mutans biofilm (Fig. 1) and showed values of 3.2
 Fig. 1.
Fig. 1.Changes in S. mutans colony forming unit treated with
postbiotic metabolites. *significant difference at p
SEM images (Fig. 2) demonstrated fewer microorganisms and smaller microcolonies on the surface when PMs interacted with S. mutans, suggesting the destruction of S. mutans biofilm. In line with our CFU/mL counts, LGG PMs had a greater effect on the biofilm compared to the LR PMs.
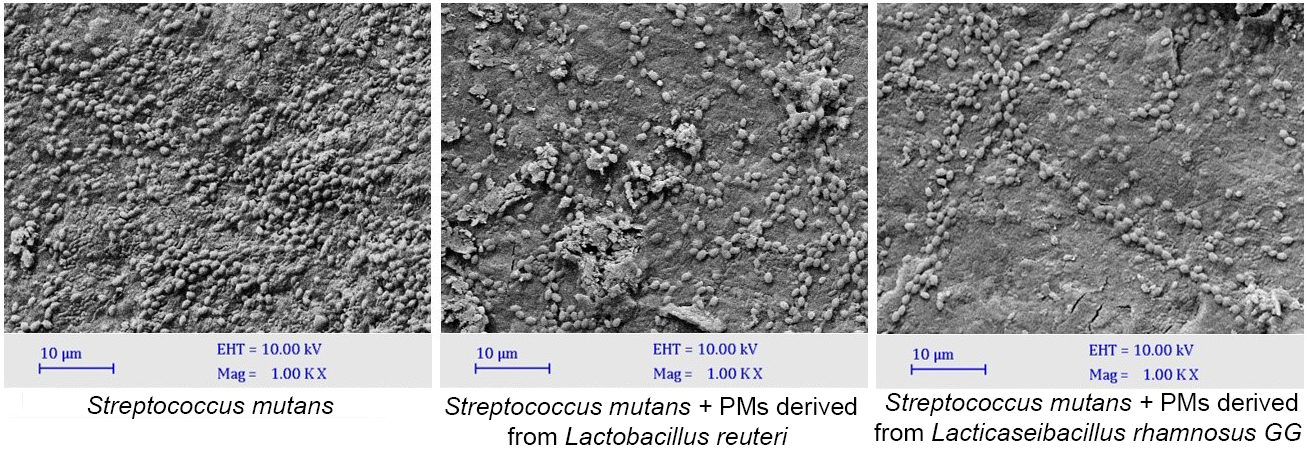 Fig. 2.
Fig. 2.
Scanning electron microscopy images of S. mutans
biofilms treated with postbiotic metabolites (Scale bar = 10
The XTT reduction assay was employed to measure the metabolic activity of S. mutans biofilms (Fig. 3). The absorbance values of the control, LGG PMs and LR PMs were 1.56, 0.72, and 0.93, respectively. The PMs from LGG and LR groups showed significant reductions in the metabolic activity of S. mutans compared to the control (p = 0.0001 and p = 0.002, respectively). These results indicate that PMs have the capacity to inhibit S. mutans metabolic activity.
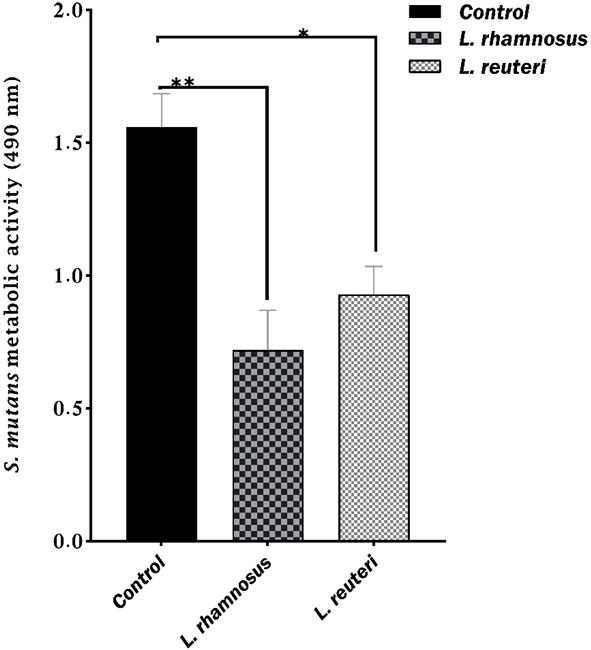 Fig. 3.
Fig. 3.The mean absorbance values of S. mutans metabolic activity based on the XTT reduction assay. *significant difference at p = 0.002; **significant difference at p = 0.0001. Error bars display standard deviations of the means.
The effect of PMs derived from LGG and LR on the expression of the
S. mutans gtfB gene was examined using qRT-PCR and presented as
fold change using the 2
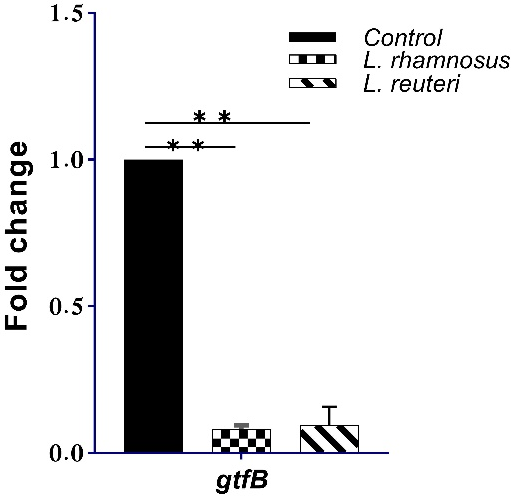 Fig. 4.
Fig. 4.The expression of gtfB gene in the control and PMs
derived from LGG and LR. **significant difference at p
Lactobacillus spp. probiotics inhibit dental caries by generating hydrogen peroxide, organic acids, or antimicrobial peptides [25, 38, 39, 40]. However, low culture viability and susceptibility to environmental conditions limit their application in functional products, hence, the increasing interest in postbiotics. PMs are substances produced or released during the metabolic activity of microorganisms and are superior to probiotics because of their clear chemical structures, safety dose parameters, and longer shelf life. They have exhibited the potential to prevent caries by inhibiting pathogens’ growth and biofilm formation [25, 41]. Applying purified byproducts or cellular components of probiotics for therapeutic investigations focusing on a certain disease would help understand the primary molecular mechanisms related to each molecule [42]. To further introduce viable options for possible use in caries control, we investigated the inhibitory activity of PMs derived from LGG and LR against S. mutans.
Our MIC and MBC results showed that the effective bactericidal concentration of PMs derived from both bacteria against S. mutans was 25% (v/v), which confirms their antibacterial potential against S.mutans. Based on the biofilm assay (Fig. 1), the CFU value in both PMs was lower than the controls, and the LGG PMs exhibited lower counts than those extracted from LR. These data indicate that PMs produced by LGG were more effective in reducing the number of S. mutans (CFU/mL) and had higher antimicrobial activity. SEM micrographs also showed a significant reduction of S. mutans biofilm in the PMs of both bacteria. Consistent with the results of the CFU counts, the LGG PMs showed a better inhibitory effect compared to the LR PMs. The biofilm-reducing activity of postbiotics is crucial for dental caries prevention.
Different factors are responsible for the various antimicrobial, antibiofilm, and anti-adherence activities of pro/postbiotics, including multiple strains of Lactobacilli and/or their PBs [25]. These factors can be byproducts or cellular components of live microorganisms or their dead/inactivated forms. Examples include biosurfactants, lipoteichoic acid, cell-free supernatant etc. [25]. According to former studies, biosurfactants significantly suppressed S. mutans biofilm [31, 43], lipoteichoic acids showed anticaries properties [23, 24], metabolites of L. fermentum TcUESC01 had anti-adherence and bactericidal activities against S. mutans planktonic cells [44], and organic acids demonstrated growth-inhibiting effects on S. mutans [25]. In agreement with our results, many studies have shown that the cell-free supernatant obtained from numerous Lactobacillus strains, including L. reuteri ATCC 23272, L. rhamnosus [30], L. rhamnosus HN001 [45], and L. reuteri AN417 [46] exhibited antibacterial activities and caused a significant decrease in the levels of S. mutans biofilms. In contrast to our findings, Chen et al. [47] reported that viable L. reuteri suppressed the cariogenic effects of multispecies biofilms, but the cell-free supernatant was not as effective. This conflict may be explained by the different biofilms used in the studies: we employed a S. mutans monospecies biofilm, while they applied S. mutans, L. rhamnosus and Actinomyces naeslundii multispecies biofilms in their research.
The XTT results obtained in the present study demonstrated that PMs from LGG and LR significantly suppressed the metabolic activity of S. mutans which decreased its ability to form biofilms (Fig. 3). The S. mutans samples treated with LGG PMs showed a higher metabolic activity reduction than LR. Our XTT findings were strongly confirmed by the CFU quantitative test results, which assess biofilms. A previous study suggested that decreases in cell metabolism may also reduce the synthesis of extracellular matrix material [48]. In line with our observations and using the XTT assay, Srivastava et al. [49] observed an 89% reduction in the activity of S. mutans following initial incubation with L. plantarum supernatant. They also reported 33% inhibition of S. mutans biofilm after 12 h treatment [49]. Similarly, L. reuteri and S. oligofermentans PMs have been suggested to decrease the survival and metabolic activity of S. mutans [50].
The glucosyltransferase gtfB gene encodes the GtfB enzymes involved in forming insoluble extracellular glucans in S. mutans, which is the major polymeric matrix of biofilms [38, 51]. This gene also regulates bacterial adhesion to the tooth surface, aggregation and coaggregation of the bacterial cells, and the integrity and stability of the biofilm structure. Therefore, its suppression may be associated with reduced biofilm/plaque formation and, ultimately, caries control [4, 5]. We employed qRT-PCR to examine the expression of gtfB in S. mutans. It showed that the coculture of this bacteria with the PMs from both LGG and LR significantly down-regulated the expression level of gtfB (Fig. 4). This was in agreement with several other coculture studies using S. mutans and multiple Lactobacilli strains, including LGG [6, 38, 52], and their PMs, including LR PMs [3, 8, 25, 29, 30, 31, 49, 53], who showed significant suppression of glucosyltransferase-encoding genes gtfB, gtfC, and/or gtfD.
To investigate the antimicrobial effects of PMs derived from two reference strains, we evaluated S. mutants, the main component of dental plaque responsible for causing tooth decay. However, the dynamic oral cavity contains many more species which could be involved in forming different microbial communities. Furthermore, there is great variability in biofilm formation and content among individuals with tooth decay. Therefore, it is suggested that future studies address this limitation and, in addition to testing other strains, conduct clinical studies to confirm the results reported in the current investigation. Also, evaluating the PMs of Lactobacilli isolated from dairy and digestive samples can offer further information on the role of postbiotics in the control of dental caries.
In conclusion, this study demonstrated the antimicrobial and antibiofilm properties of probiotic-derived PMs from LGG and LR strains against S. mutans. Our findings showed that MIC and MBC values were achieved at concentrations above 25% (v/v) for both PMs, suggesting their potential as effective antimicrobial agents. Biofilm destruction was observed in both PMs. However, destruction was significant in the LGG group. SEM analysis supported these findings, revealing fewer microorganisms and smaller microcolonies in the presence of PMs. Both PMs also significantly reduced the metabolic activity of S. mutans, as indicated by the XTT reduction assay. Moreover, treatment with LGG and LR PMs resulted in a substantial decrease in gtfB gene expression, which plays a crucial role in S. mutans biofilm formation. Overall, our results suggest that PMs from LGG and LR strains have promising potential as a natural alternative for preventing dental caries caused by S. mutans. However, further in vivo studies and clinical trials are needed to evaluate the efficacy and safety of PMs in oral environments.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
MB, MA, SE, RH, MM and MHY designed the research study. MB, MA, SE, RH, MM and MHY performed the research. RF and MP provided the methodology for the research and contributed to the writing of the draft and deep revision of the manuscript. MA analyzed the data. MB, MA, SE, RH, MP, RF, MM. and MHY wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
This study was conducted with the approval and supervision of the National Institute for Medical Research Ethics Committee (IR.NIMAD.REC.1400.148) and the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.DENTISTRY.REC.1400.191). All procedures were carried out following the relevant guidelines and regulations.
Not applicable.
This study was supported by grants from the National Institute of Medical Sciences Research, Tehran, Iran (Grant number: 4001382). The funding bodies of the study did not play any role in its design, collection, analysis, data interpretation, and manuscript writing.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
