†These authors contributed equally.
Academic Editor: Iain Hargreaves
This is an open access article under the CC BY 4.0 license.
Background: Cardiovascular disease is associated with high morbidity
and mortality. Doxorubicin (DOX) is an effective adjunct to cancer chemotherapy
but leads to cardiovascular-related side effects. Because coenzyme Q10 (CoQ10)
has been shown to protect against cardiac damage, this study was conducted to
investigate the protective effects of CoQ10 against cardiac damage in mice.
Methods: We randomly divided six-week-old male C57BL/6 mice into four
groups: control (n = 7), CoQ10 (n = 7), heart failure (HF) (n = 7), and HF+CoQ10
(n = 6) groups. HF group was induced via intraperitoneal injections with DOX (5
mg/kg) once weekly for 4 weeks. CoQ10 was solube in corn oil. The mice of CoQ10
and HF+CoQ10 group were given CoQ10 (100 mg/kg) once a day for 8 weeks. All mice
were subjected to different treatment regimens for eight weeks. Metabolic
characteristics, cardiac damage, oxidative stress markers (SIRT1, SIRT3, eNOS,
TE, P53, SIRT5, CAT, HO-1, and SOD), energy metabolism markers (PARP-1 and
PPAR-
The American Heart Association/American College of Cardiology guidelines define heart failure (HF) as “a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill or eject blood” [1]. HF is a global epidemic disease; although many treatments for HF have been developed in recent years, the mortality rate remains high [2].
The deaths of cancer survivors are typically attributable to cardiac-related factors [3]. Doxorubicin (DOX) belongs to the anthracycline class of drugs and is widely used in chemotherapy for various cancers, such as stomach cancer, breast cancer, and lung cancer [4, 5]. DOX causes cardiotoxicity as a side effect and is widely used in animal studies to induce HF [6, 7]. Oxidative stress, lipid peroxidation, apoptosis, and autophagy disorders are thought to be major factors associated with DOX-induced cardiotoxicity [7, 8, 9]. HF is an important cause of high mortality, high morbidity, and poor quality of life. However, drugs that can reduce the cardiotoxicity of DOX are lacking.
CoQ10 is a major cofactor involved in oxidative phosphorylation in mitochondria [10]. CoQ10, one of the synthetic antioxidants in the body, was the first drug used to improve mortality related to cardiac disease, reducing deaths by 50% [11]. CoQ10, which exists in oxidized ubiquinone and reduced ubiquinol forms, is produced in various tissues, most commonly the cardiac, kidney, liver, and muscle tissues [12]. CoQ , an intermediate state of CoQ oxidisation “ubisemiquinone”, is also ubiquitous and present in all tissues that have mitochondria [13]. CoQ10 is safe for treating cardiac failure and reducing major adverse cardiovascular events [14]. Furthermore, Khan et al. [15] found that CoQ10 protects the cardiac tissue of apoptotic mice by downregulating apoptosis-related genes. Additionally, CoQ10 relieves cardiac damage caused by hyperlipidemia [16]. It is consistent with the beneficial effects of other natural compounds such as quercetin and ascorbic acid and has great potential as a heart protectant in cancer patients [17]. However, the mechanism by which CoQ10 attenuates DOX-induced cardiotoxicity is unclear.
This study was conducted to determine the effect of CoQ10 on DOX-induced cardiac damage. Our findings improve the understanding of the mechanism and effect of CoQ10 in DOX-induced HF.
Six-week-old male C57BL/6 mice were purchased from Liaoning Changsheng biotechnology CO, LTD (Liaoning, China) and mice were allowed to adapt to one week. All mice were randomly divided into four groups as follows: a control group (n = 7), a HF group (n = 7), a CoQ10 group (n = 7), a HF+CoQ10 group (n = 6). Four groups mice were housed in a room under diurnal lighting conditions with a controlled temperature (24 °C). HF group was induced via intraperitoneal injections with DOX (5 mg/kg) once weekly for 4 weeks. CoQ10 was solube in corn oil. The mice of CoQ10 and HF+CoQ10 group were given CoQ10 (100 mg/kg) once a day for 8 weeks [18]. Mice in all groups were fed the appropriate diet for eight weeks. Blood samples were acquired from eyeball blood in serum tubes and stored at –80 °C until use. Heart tissues were fixed in 10% formalin and embedded in paraffin for histological evaluation. The remaining heart tissues were snap-frozen in liquid nitrogen for Real-Time Reverse Transcription Polymerase Chain Reaction (PCR) or Western blot analysis. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of Dalian Municipal Central Hospital.
Blood samples were collected, and serum was prepared by centrifugation at 2500 g for 5 min, after which the supernatant was collected and used for lactate dehydrogenase (LDH) and creatine kinase MB (CK-MB) measurements using commercially available kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Heart tissue was immersed in 1:9 mg/
Mice were anesthetized with isoflurane (1.5%) at the beginning, when hearts
were fixed by perfusion with 10% buffered formalin and used isoflurane (4%) via
a nozzle placed over the nose. The hearts were fixed overnight at room
temperature, transferred into 70% ethanol, and then embedded in paraffin.
Paraffin-embedded heart tissue slices were deparaffinized via immersion in xylene
(three times, 5 min each) rehydrated in a descending alcohol series (100%, 90%,
80%, and 70% alcohol, 5 min each), Histological changes were detected by
staining 5-
The hearts were embedded in paraffin, and serially sectioned to 5
Coronal sections of the heart tissues were fixed in 10% formalin, dehydrated in
an ascending series of ethanol, and embedded in paraffin for histological
evaluation. For immunohistochemical staining, the heart sections were
deparaffinized and rehydrated. Next, the sections were blocked with 3% H2O2 in
methanol for 15 min to inactivate endogenous peroxidases and then incubated
overnight at 4 ℃ with one of the following primary antibodies: BNP (rabbit
anti-NPPB polyclonal antibody, 1:100; Solarbio, Beijing, China); SIRT3 (Rabbit
Anti-SIRT3 Polyclonal antibody, 1:50; Solarbio); HO-1 (Rabbit Anti-HMOX-1
antibody, 1:100; Solarbio); SOD (Anti-SOD1 Polynal antibody, 1:100; Solarbio);
PARP-1 (Anti-PARP1 Rabbit mAb, 1:50; PTM BIO, Hangzhou, China); PPAR-
Proteins were extracted using radioimmunoprecipitation assay buffer (P0013B;
Beyotime, Shanghai, China). Protein samples were first separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then
transferred to polyvinylidene fluoride membranes (Immobilon, Millipore,
Billerica, MA, USA). The membranes were blocked with 5% skim milk in TBST buffer
(TBS containing 0.1% Tween-20) at room temperature for 1 h, and then incubated
with one of the following primary antibodies at 4 °C overnight: PARP-1
(Anti-PARP1 Rabbit mAb, 1:500; PTM BIO); PPAR-
Total RNA was isolated from heart tissues and complementary DNA (cDNA) was
synthesized using the TransScript One-Step gDNA Removal and cDNA Synthesis
SuperMix kit according to the manufacturer’s protocol. Gene expression was
quantitatively analyzed using qPCR and the TransStart Top Green qPCR SuperMix kit
(SuperScript VILO cDNA synthesis kit; Thermo Fisher Scientific, Inc.). The
specific gene expression levels were quantitatively analyzed by performing qPCR
using fluorescent SYBR Green technology (Light Cycler; Roche Molecular
Diagnostics).
| Gene | Primers |
| GAPDH | F: 5′-CCTCGTCCCGTAGACAAAATG-3′ |
| R: 5′-TGAGGTCAATGAAGGGGTCGT-3′ | |
| P53 | F: 5′-CCCTCTGAGCCAGGAGACATT-3′ |
| R: 5′-CCCAGGTGGAAGCCATAGTTG-3′ | |
| SIRT5 | F: 5′-GTGTTGTAGACGAAAGCCTCCTG-3′ |
| R: 5′-TCCAGTAACCTCCAGCGCCT-3′ | |
| CAT | F: 5′-CCAGCGACCAGATGAAGCAG-3′ |
| R: 5′GTGACCTCAAAGTATCCAAAAGCA-3′ |
All data are presented as mean
The metabolic characteristics of the mice subjected to the different treatments
are summarized in Fig. 1. The heart/body weight ratio did not significantly
(p
 Fig. 1.
Fig. 1.Metabolic data in different groups after treatment with CoQ10.
(A) Quantitative analysis of heart/body weight in different groups. Data are
shown as the means
Wheat-germ agglutinin and hematoxylin and eosin staining revealed cardiomyocyte hypertrophy and inflammatory cell infiltration. Compared with the control group, the HF group exhibited myocardial hypertrophy, as evidenced by an increase in the cross-sectional area of cardiomyocytes (Fig. 2B) and inflammatory cell infiltration (Fig. 2A), both of which were attenuated by CoQ10.
 Fig. 2.
Fig. 2.Cardiac tissue damage in the different groups after the
treatment of CoQ10. (A) Effect of CoQ10 on DOX-induced histopathological changes
in the cardiac tissues. Histopathological changes were evaluated using HE
staining. Arrows indicate positively stained cells. (B) WGA staining for
quantitative analysis of cardiomyocyte cross-sectional area. (C) Quantification
of positive expression. Data are shown as the means
The activity of heart biomarkers, such as SIRT1, SIRT3, eNOS, and TE, was
significantly decreased in the HF group than control group and increased in the
HF+CoQ10 group than HF group (p
 Fig. 3.
Fig. 3.Cardiac oxidative stress in different groups
after treatment with CoQ10. (A) Histological quantification of SIRT1, SIRT3, TE,
and eNOS levels. Data are shown as the means
Western blotting (Fig. 4A,B) and immunohistochemistry (Fig. 4C,D) was
performed to evaluate the levels of indicators related to energy metabolism.
Compared to in the HF group, the expression levels of PARP-1 and PPAR-
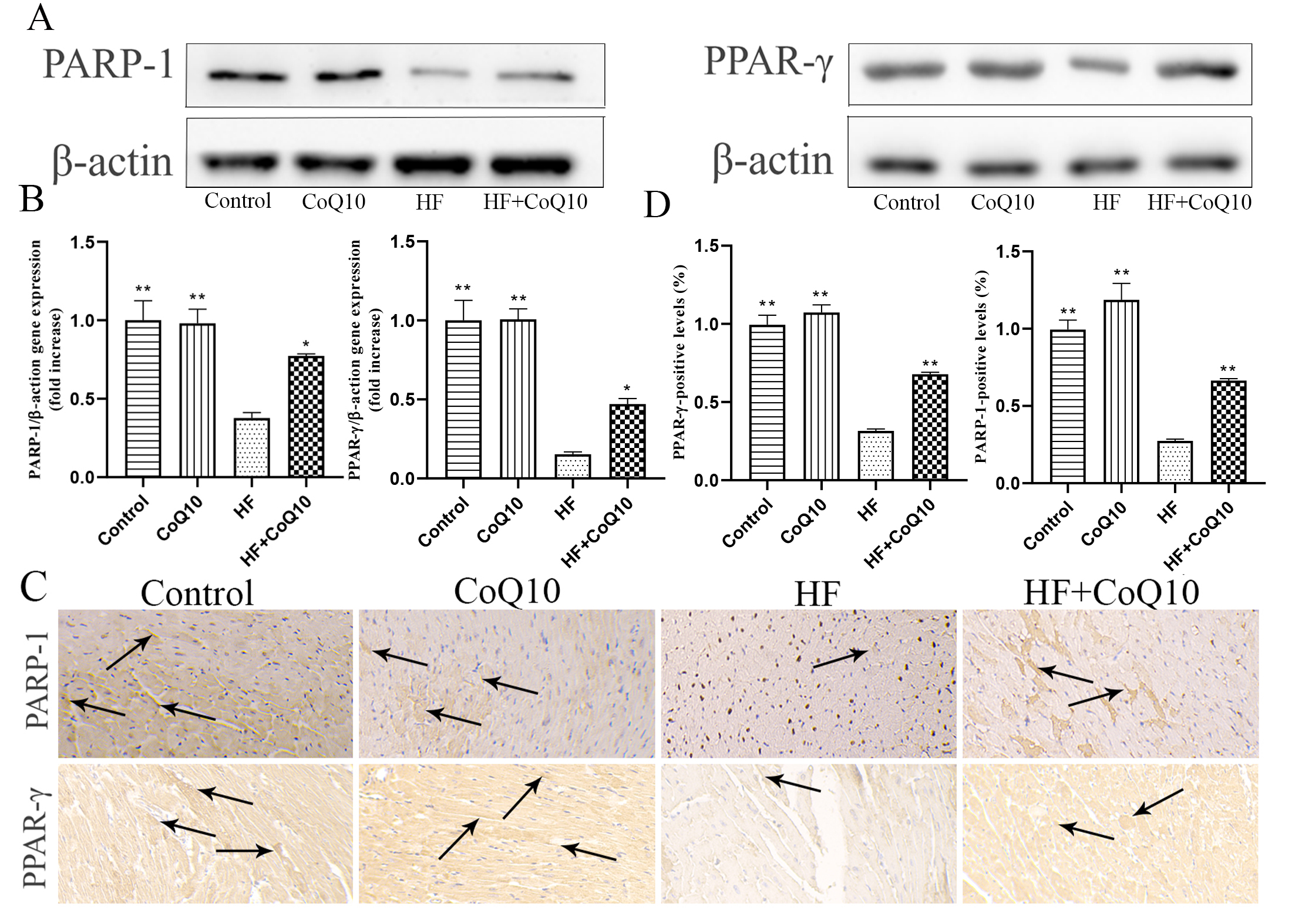 Fig. 4.
Fig. 4.Cardiac energy metabolismin different groups
after treatment with CoQ10. (A) Cardiac PARP-1 and PARP-
Immunohistochemistry (Fig. 5A,B) was performed to evaluate the levels of
indicators related to inflammation. The expression levels of IL-1
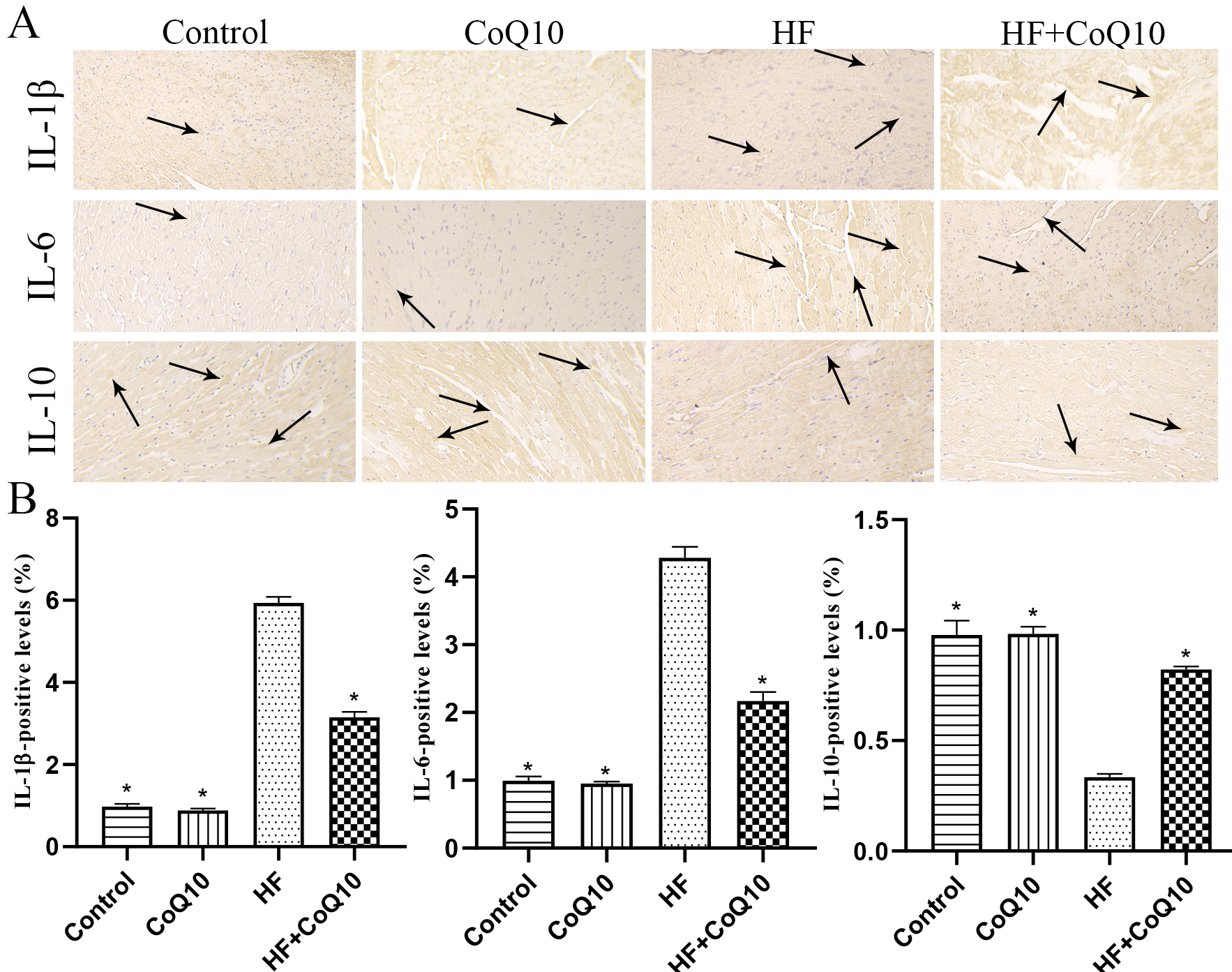 Fig. 5.
Fig. 5.CoQ10 decreased inflammation in the heart of HF mice.
(A) Representative immunohistochemistry images showing the levels of
IL-1
To examine cardiac fibrosis in the mice of different treatment groups, Masson
and IHC staining was conducted (Fig. 6). The results revealed increased collagen
deposition in the HF group compared with control group (p
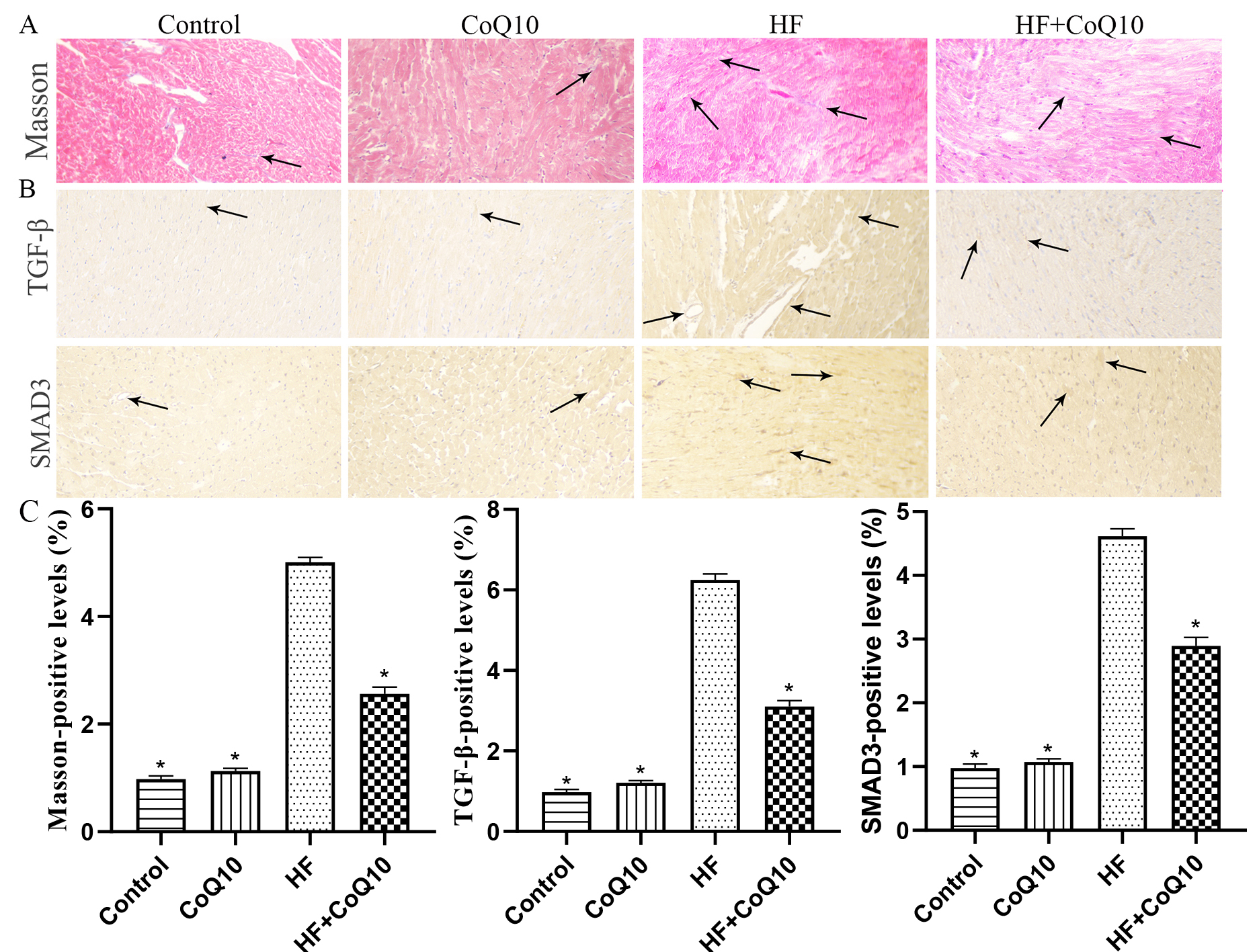 Fig. 6.
Fig. 6.CoQ10 decreases fibrosis in HF mice. (A) Masson’s trichrome
staining for collagen deposition in the heart tissue. Arrows indicate positively
stained cells. (B) Representative immunohistochemistry results for SMAD3 and
TGF-
The number of TUNEL-positive cells increased in the hearts of HF group mice
compared to in those of control group mice, whereas heart cell apoptosis was
reduced in the HF+CoQ10 group (Fig. 7A). Immunoblotting showed that expression of
the pro-apoptotic proteins BAK and caspase-8 was significantly increased and that
of BCL-XL was decreased in the HF group (p
 Fig. 7.
Fig. 7.CoQ10 decreases myocardial apoptosis in HF mice. (A) TUNEL
staining (green fluorescence) and DAPI staining (blue fluorescence)
photomicrographs to detect apoptosis in the heart tissue. (B) Representative
immunohistochemistry results for BAK, caspase-8, and BCL-XL in the heart tissues.
Arrows indicate positively stained cells. (C–D) Bar graph shows the positive
levels. Data are shown as the means
Treatment with CoQ10 reduced cardiac damage associated with DOX-induced cardiac failure. Additionally, cardiac dysfunction associated with DOX-induced HF was due to the acceleration of cardiac tissue damage, oxidative stress, myocardial fibrosis, and apoptosis. These findings are summarized in Fig. 8.
 Fig. 8.
Fig. 8.Schematic diagram showing how CoQ10 protects against cardiac damage in HF mice. CoQ10 regimen prevented cardiac dysfunction and reduced cardiac damage by reducing myocardial oxidative stress, myocardial fibrosis, and apoptosis in HF mice. Abbreviations: CoQ10, coenzyme Q10; HF, heart failure.
DOX is a chemical widely used to treat cancer but has cardiotoxic side effects, resulting in limited use [19]. The mechanism of DOX-induced cardiotoxicity is not completely understood. Furthermore, oxidative stress is considered the core mechanism of HF [20]. Recently, Tadokoro et al. [21] showed that ferroptosis is the major form of regulated cell death in DOX-induced cardiotoxicity. Recent studies also confirmed that cardiac fibrosis, apoptosis, coke death, autophagy, and acetylation are closely associated with DOX-induced cardiotoxicity [22, 23, 24].
CoQ10 is closely related to cardiovascular disease and plays an important role in protecting against these diseases, such as hypertension, hyperlipidemia, myocardial infarction, and HF [13, 25, 26, 27]. Complementary and integrative medicine (CIM), such as G. lucidum and CoQ10, have a potentially effective role in the treatment of cancer [28]. In addition, CoQ10 influences the effects of adjuvant therapy used for nervous system diseases and tumors [26, 29]. However, the mechanism of the protective effect of CoQ10 on DOX-induced HF is unclear.
The metabolic characteristics of NT-proBNP, LDH, and CK-MB showed that our animal models were successfully prepared. HF is characterized by cardiac remodeling. Our results revealed cardiac remodeling and inflammation infiltration similar to those in HF. CoQ10 treatment effectively reduced myocardial remodeling and inflammation infiltration.
Oxidative stress is important in the pathophysiology and pathogenesis of HF and is widely considered a cause of DOX-induced cardiotoxicity [30, 31]. CoQ10 is a cofactor of oxidative phosphorylation in the mitochondria, which are crucial for cellular energy production. Therefore, CoQ10 is closely associated with oxidative stress and has a high antioxidant capacity [13]. SIRT3 is a major mitochondrial deacetylase that participates in antioxidant oxidation by regulating the acetylation of antioxidant enzymes [32]. Previous studies showed that SIRT3 regulates various types of antioxidants. Moreover, a study confirmed that SIRT3 is involved in p53-mediated ferroptosis and that Sirt3 and P53 play key roles in oxidative stress [33]. The endogenous antioxidant HO-1 may be a target molecule for treating antioxidant stress injury in HF [34]. CoQ 10 improved lipid peroxidation by decreasing malondialdehyde levels in human cardiac cells [35]. In the present study, HF downregulated the expression of SIRT3 and HO-1 in myocardial tissues, whereas treatment with CoQ10 increase their expression. The levels of SIRT1, SIRT3, SIRT5, eNOS, TE, CAT, and P53 were significantly downregulated in the HF mouse heart, and these levels were restored by CoQ10.
PPAR-
Anthracyclines cause severe cellular inflammation that leads to cell death [38].
IL-1
Myocardial fibrosis is central to the pathology of HF. CoQ10 may protect against
lung fibroblast formation and alleviate fibrosis by inhibiting TGF-
Apoptosis is a form of cell death mediated by caspases. Inhibition of myocardial apoptosis can effectively protect the heart from HF [42]. BAK is an endogenous core regulator of apoptosis [43]. Salehpour et al. [44] reported that CoQ10 reduced apoptosis by regulating BAK and avoiding increased caspase activity. We found that the number of apoptotic cells was significantly increased in HF mice, whereas apoptosis was reduced in the HF+CoQ10 group.
The study has some limitations. First, although CoQ10 has a variety of therapeutic applications, it is not usually prescribed as a drug because of its low oral bioavailability, which can affect its efficacy. Secondly, it should be noted that the concentration of CoQ10 in cardiac tissue on mice was not detected before and after treatment, so complete absorption of CoQ10 was not proved in the study, which is also the focus of future studies.
CoQ10 can alleviate DOX-induced cardiotoxicity, as demonstrated by the downregulation of oxidative stress, fibrosis, inflammation, energy metabolism, and apoptosis markers. These findings suggest that CoQ10 can be used as a therapeutic intervention to reduce the side effects of DOX. We only evaluated control, HF, CoQ10, and HF+CoQ10 groups and did not examine different doses of CoQ10. In addition, we did not validate cultured cells.
SL and ZP designed the research study. YL, QY, and WY performed the research. LM and JY provided help and advice on immunofluorescence staining. SL and ZP analyzed the data. WY and LM wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of Dalian Municipal Central Hospital.
Not applicable.
This study was funded by the Beijing Jiekai Cardiovascular Health Foundation [grant number BW20220302].
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
