Academic Editor: Woo-Sung Kwon
Background: Hybrid taxa exist in nature, but their fitness can vary
greatly. Hybrids are usually thought to have lower viability and survival rate
than parental species due to the occurrence of two different genomes and
divergent evolution in each species. On the other hand, the hybrid vigour of the
F1 generation may give hybrids an advantage in mixed populations where they have
to live and compete with parental taxa. Post-zygotic selection with endogenous
genetic mechanisms may be a significant evolutionary force in hybrid formation.
Here we tested principles of post-zygotic reproductive dynamics in mixed
populations of Pelophylax water frogs that would help us understand the
origin and maintenance of such systems. Methods: Within experimental
crosses, we combined various diploid Pelophylax genotypes resulting in
211 families. Statistical analysis of progeny was used to measure fertilization
success, the rate of embryonic/tadpole mortality and the overall survival of the
progeny till the time of metamorphosis. Using Generalized Estimating Equations
models and variables defined by a mother/father included in mate pairs, we tested
which factor best explains the successful embryonal development.
Results: The development of Pelophylax offspring significantly
varied in survival rate and morphological malformations. These post-zygotic
reproductive dynamics were driven by parental combinations of species pairs. The
best values in the proportion of developing eggs, embryos, tadpoles and overall
survival showed progeny of homospecific P. lessonae crosses. Total
survival rates were relatively similar between L-E and R-E population systems but
much lower than homospecific crosses in parental taxa. However, once the early
stages passed this period, tadpoles mostly of hybrid hemiclonal origin performed
even better than pure P. ridibundus progeny. Hybrid
Hybrid taxa exist in nature, and some have developed into independent evolutionary units through homoploid hybrid speciation [1], while others remained reproductively dependent on their parental species [2]. Hybrids are usually thought to have lower viability and survival rate than parental species due to the occurrence of two different genomes and divergent evolution in each species [3]. However, hybrid fitness concerning the fitness of the parental taxa can vary greatly. Hybrid superiority caused by heterosis is the phenomenon in which hybrid traits of the F1 generation are better than the life-history traits of the parental taxa [4]. Hybrid vigour may give hybrids an advantage in mixed populations where they have to live and compete with parental taxa [2, 5, 6]. Here, post-zygotic selection may act as a significant evolutionary force in hybrid formation when pre-zygotic mechanisms that might prevent their formation in the first place are overcome [7, 8, 9].
For this post-zygotic selection, endogenous genetic mechanisms based on the occurrence of two different genomes from parents may impact early development survival rates [10]. Knowing these mechanisms would help us understand the origin and maintenance of animal systems in which hybrid asexual fish and amphibians live in sympatry with a sexual species. These animal systems are known to have dynamic reproduction in which various gametes are formed, and various genotypes originate [11, 12]. Most of them are, however, unfit. To reproduce, hybrids have to get a sexual gamete to trigger embryonic development from clonal diploid eggs (gynogenesis) or to reach a zygote through fertilization with a clonal haploid gamete referred to as hybridogenetic hemiclonal reproduction [13]. A study of larval development showed post-zygotic selection against parental genotypes to maintain all-hybrid populations of the frog Pelophylax esculentus [10]. Thus, opportunities to study the early stages of taxa forming sexual-asexual systems under controlled conditions may help us understand the possible influence of embryonic and larval variation development on the dynamics of a mixed population structure.
The European Pelophylax water frog is an optimal study system for these questions as it includes two sexual species P. lessonae (LL) and P. ridibundus (RR), and a sympatrically occurring interspecific hybrid, P. esculentus (RL). Hybrids use hybridogenesis, where a chromosomal set from one parent is eliminated from the germline while a second still complete chromosome set is transmitted clonally to gametes. To maintain a hybrid genotype in the population, male and female hybrids have to live with and mate with a parental species whose genome has been removed from the hybrid germline. The hybridogenetic mechanism maintains diploid P. esculentus in a permanent F1 hybrid constitution. Water frog populations vary in patterns of gamete production. The most common population system includes diploid hybrids of both sexes that cohabit with P. lessonae and produce clonal R gametes (the so-called L-E System). In contrast, those with P. ridibundus typically produce clonal L gametes in the R-E System [14, 15]. Detail studies of the latter in Central Europe showed that P. esculentus occurs in the male sex only, being even amphispermic. In this process, a single P. esculentus form two sperm cell types, a clonal haploid ridibundus (R sperm) and lessonae (L sperm) genomes [15, 16, 17].
In this study, we tested principles of post-zygotic reproductive dynamics in mixed populations including P. ridibundus, P. lessonae, and P. esculentus within 211 crossing experiments. In particular, we tested the following hypotheses. (1) Fertilization rates and survival rates depend on taxa included in crosses. (2) Post-zygotic developmental dynamics in mixed population systems are driven by individual parental genomic input and the sex of a parent; Pathways of reproductive mechanisms during early developmental stages may reveal how the sexual-asexual systems are maintained in nature.
Thirteen P. lessonae, 69 P. ridibundus and 83 P. esculentus males and females were collected at 23 sample sites (Supplementary Table 1). Frogs were taxon-determined by phenotypic characters [18, 19] (Fig. 1A–C). DNA was extracted from an interdigital forelegs membrane using a commercial Tissue DNA Isolation Kit (Geneaid Biotech, Taipei, Taiwan) following a manufacturer protocol.
 Fig. 1.
Fig. 1.Morphological variation (shape of the metatarsal tubercle,
dorsal/lateral/ventral colouration) in three Pelophylax taxa and
examples of early development in progeny. (A) P. ridibundus (RR). (B)
P. lessonae (LL). (C) P. esculentus (RL). (D) Stages of regular
early development in progeny from RR
The experimental procedures followed directives of the State Veterinary Administration of the Czech Republic under the Ethical Committee of the Faculty of Science, Charles University, Prague, permit number 34711/2010-30 issued by the Ministry of Agriculture of the Czech Republic. Frogs were collected under permit no. 358/2011, 278/2011 provided by the Agency for Nature Conservation and Landscape Protection of the Czech Republic. The permits for the crossing experiments were obtained from the Swiss authorities (experimental permit 119/2013: TV 5113 and TH 103).
To explore gamete production and early development in water frogs, we made 211 crosses, including 63 P. esculentus males from the R-E populations, six hybrid males and 14 females from the L-E populations, 22 P. ridibundus males and 47 females, eight P. lessonae males and five females. The schema of crossing design is given in Supplementary Fig. 1 and Supplementary Table 2.
The artificial fertilization procedure followed the methodology from Berger et al. [20] with some modifications. Males were euthanized in a buffered (pH = 7.0) 2 mg/L MS-222 solution (Sigma A-5040, St. Gallen, Switzerland); their testes were removed and stored in a Petri dish with Holtfreter’s solution (pH = 7.4) before use. Females were triggered to ovulate by an injection of salmon luteinizing-releasing hormone (LHRH, Sigma L4897, Prague, Czech Republic). For this purpose, 2 mg of the hormone were diluted in 100 mL of Holtfreter’s solution; per 10 g of body mass, 0.1 mL of this solution was injected into the abdominal cavity. After 16–18 hours, ovulation was checked by pressing the female belly carefully between the thumb and the index finger of the left hand and opening the cloaca with curved forceps. Ovulation was indicated when some eggs were released from the cloaca. Females that did not ovulate received a second hormone injection.
Testes were sliced and crushed to release sperm in a new Petri dish containing
aged tap water. Eggs from one female were gently stripped into the sperm
solutions and covered with aged tap water. The fertilization success was
indicated by egg rotation that turned the black animal pole to the top within
10–40 min after fertilization. On the second day, eggs were checked under a
microscope for the presence of the fertilization membrane, indicating successful
penetration of a sperm cell into the egg. All eggs were photographed and
transferred to 1.5 L plastic boxes (20
For the following analysis, we divided crosses into five groups that differed in
mother/father genotype combinations according to mating pairs known from natural
water frog populations: R-E (female
We analysed data in R 4.1.2 [22] using primarily Generalized Estimating Equations Models (GEEGLM) with a binomial distribution (logistic link) from the library “geepack” [23]. As a dependent variable, we used (i) presence/absence of egg fertilization, (ii) presence/absence of embryos survival till stage 25, (iii) presence/absence of tadpole survival till metamorphosis. As one cluster in the model, we defined one clutch of eggs from one crossing. For the analysis, we selected only combinations of males and females with more than six cross IDs. In order to test, which factor best explains the successful development rate of embryos, we used as possible explanatory variables: female genotype, male genotype, mitochondrial information of mother, compatibility of mitochondrial and nuclear information, population type of female, population type of male, to create set of models. We compared these models by calculation of quasi-likelihood under the independence model criterion (QIC) for GEEGLM from the library “MuMIn” [24]. We performed the stepwise selection up to the third step, including also the interaction between tested variables. Models were compared based on QIC also to a null model.
We then used GEEGLMs and a set of explanatory variables useful to test
particular hypotheses: (i) Are there differences in the proportion of fertilized
eggs/embryos survival/tadpole survival among various types of crosses (L-E
system, R-E system, primary hybridization, homospecific parental crosses)? (ii)
Which gametotype (father’s/mother’s) influence the proportion of embryos
survival among various type of crosses? (iii) Are there differences in embryonic
survival among particular progeny in interaction with mitochondrial information?
(iv) Are there differences in embryonic survival among individual mother
The type of mitochondrion may play a role in a frog sensitivity to oxygen deficiency and its survival [27, 28]. We, therefore, amplified and sequenced the mitochondrial ND2 gene following Plötner et al. [29]. PCR products were commercially Sanger-sequenced by SeqMe s.r.o. (Prague, Czech Republic). Sequence editing was performed and aligned in BioEdit v.7.0.9.0 (Bioedit Company, Raleigh, USA) [30], and variable sites in sequences were evaluated using Mega v 5.1. (Tempe, Arizona) [31].
Parentals were genotyped at ten microsatellite loci using two multiplex PCR sets. Multiplex 1: RlCA1b5 [32], Ga1a19 [33], Rrid013A [32, 34], Res14 [35]. Multiplex 2: Res22 [35], Rrid169A [36], Re1Caga10, Re2Caga3 and RlCA1b6 [33], Rrid082A [36]. PCR protocol was based on a study by Christiansen and Reyer [36]. Individual genotypes were based on species-specifity of amplified alleles described in Doležálková-Kaštánková et al. [16]. Fragment-length analyses were performed on the ABI 3730 Avant capillary sequencer (Applied Biosystems, Foster City, California, USA) with an internal size standard (GeneScan-500 LIZ, Thermo Fisher Scientific, Waltham, MA, USA); the alleles were scored with GeneMapper v. 3. 7 (Applied Biosystems, Zug, Switzerland).
To verify the preliminary phenotypic determination of the adult frogs, we run Principal Coordinate Analysis (PCoA) via covariance matrix with data standardization in GenAlEx v. 6. 41 (Canberra, Australia) [37]. PCoA was performed on multi-allelic microsatellite profiles of adult frogs used as parents in crosses.
Results of Principal Coordinate Analysis (PCoA) on microsatellite data from 143 adult individuals supported morphological taxon identification (Supplementary Fig. 2) of 81 P. esculentus (12 females, 69 males), 52 P. ridibundus (42 females, ten males) and 10 P. lessonae (four females, six males).
The cytoplasmatic background of the females was tested for the possible influence of the mito-nuclear compatibility on the type of produced gametes. Analysis of mitochondrial variation based on 55 ND2 sequences (partial length 669 bp) detected 86 variable sites (Supplementary Table 4) corresponding to three species-specific mtDNA profiles when compared with the NCBI BLAST results (GenBank numbers: MN864876, MN808439, AM749716); P. ridibundus mtDNA was found in 15 RR females; P. lessonae mtDNA contained 24 RR females, 10 RL females and four LL females; P. kurtmuelleri (KK) mtDNA was found in two RR females (Supplementary Table 3).
To begin with, we studied differences among hybrid male gonads in terms of functional development and the ability to reproduce. Pelophylax esculentus males differed in testis size and shape (Supplementary Fig. 3), with the right testis usually being smaller. Moreover, we observed atypical morphological structures with evidence of segmentation (Supplementary Fig. 3).
Experimental crosses between RR, RL and LL males and females resulted
in 211 families (Supplementary Fig. 1), with 2939 juveniles undergoing
metamorphosis (Supplementary Tables 5,6). The fertilization success (FS)
in RR
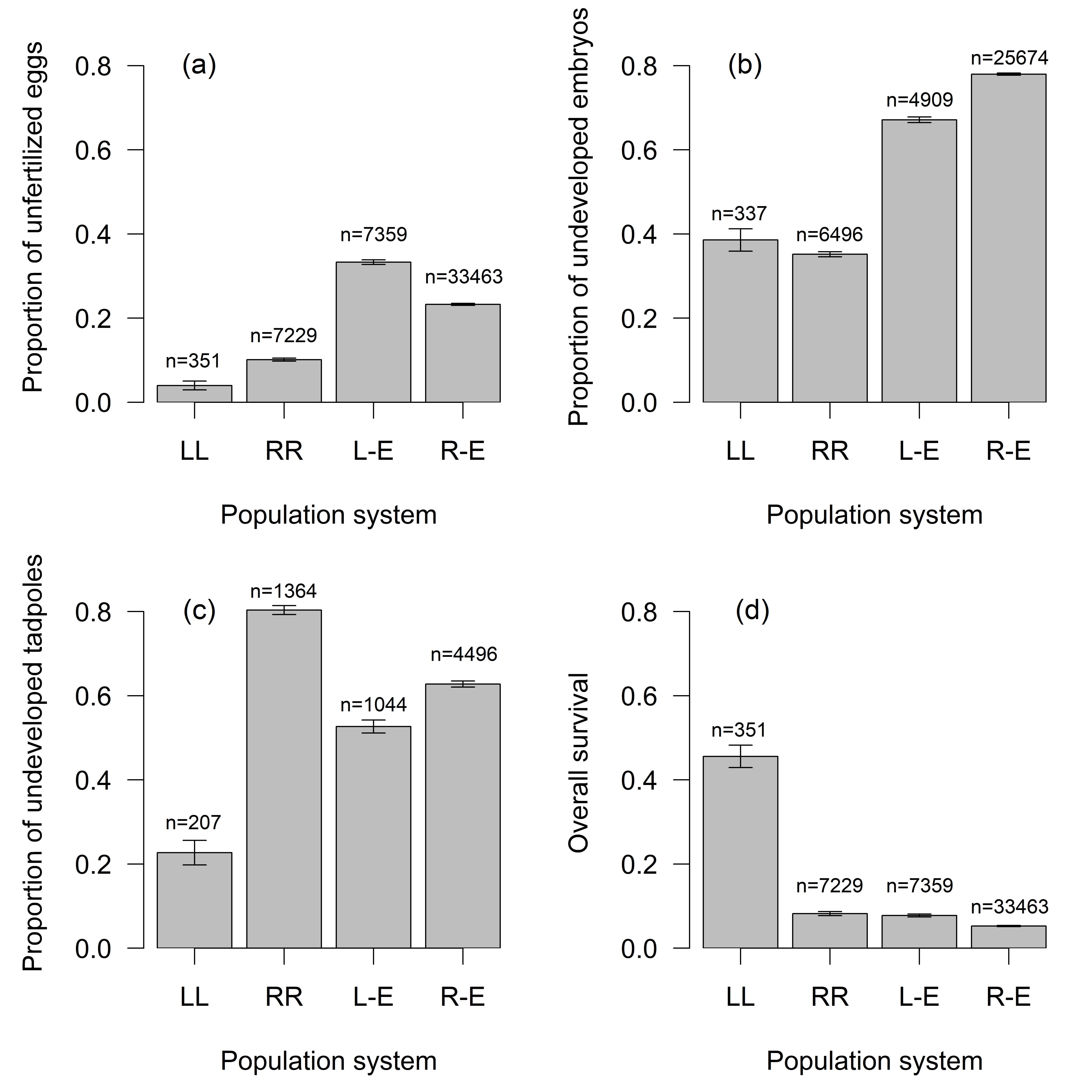 Fig. 2.
Fig. 2.Proportion of (a) unfertilized eggs, (b) undeveloped embryos,
(c) undeveloped tadpoles, (d) overall survival in relation to progeny genotype at
different population systems (mean
Female fecundity based on the quality and quantity of ovulating eggs was higher
in RR females in comparison with RL and LL females (df = 57,
For further analysis, we used families with fertile females (FS was higher than 5% and EM was lower than 90%). Within 192 families (Supplementary Table 5), there were 43028 fertilized eggs and 15417 embryos reaching stage 25, and 2939 tadpoles completing metamorphosis (Supplementary Table 6). Although most eggs cleaved, many zygotes did not get beyond the blastula stage.
R-E crosses: The number of RR
L-E crosses: Embryonic survival rates differed significantly in families with P. esculentus backcrossed to P. lessonae. Lower EM (35.4%) was observed in progeny mothered by hybrid females than in progeny fathered by hybrid males (EM 79.2%), see Supplementary Table 6, while tadpole mortality was similar (75.5/79.7%). Hybrid x hybrid crosses (resulting in RR progeny) showed extreme mortality rates (EM 99.1%, TM 100%), see Fig. 2, Supplementary Fig. 4.
Homospecific parental crosses and primary hybridizations: In parental
combinations (RR
There were significant differences among types of crosses (Fig. 2) in
proportion of fertilized eggs (n = 48402, clusters = 166, df = 3,
Proportion of fertilized eggs differed among types of crosses significantly (n =
48402, clusters = 166, df = 3,
Proportion of developed embryos (Fig. 3) depends primarily on male
gametotype (n = 41763, clusters = 188, df = 2,
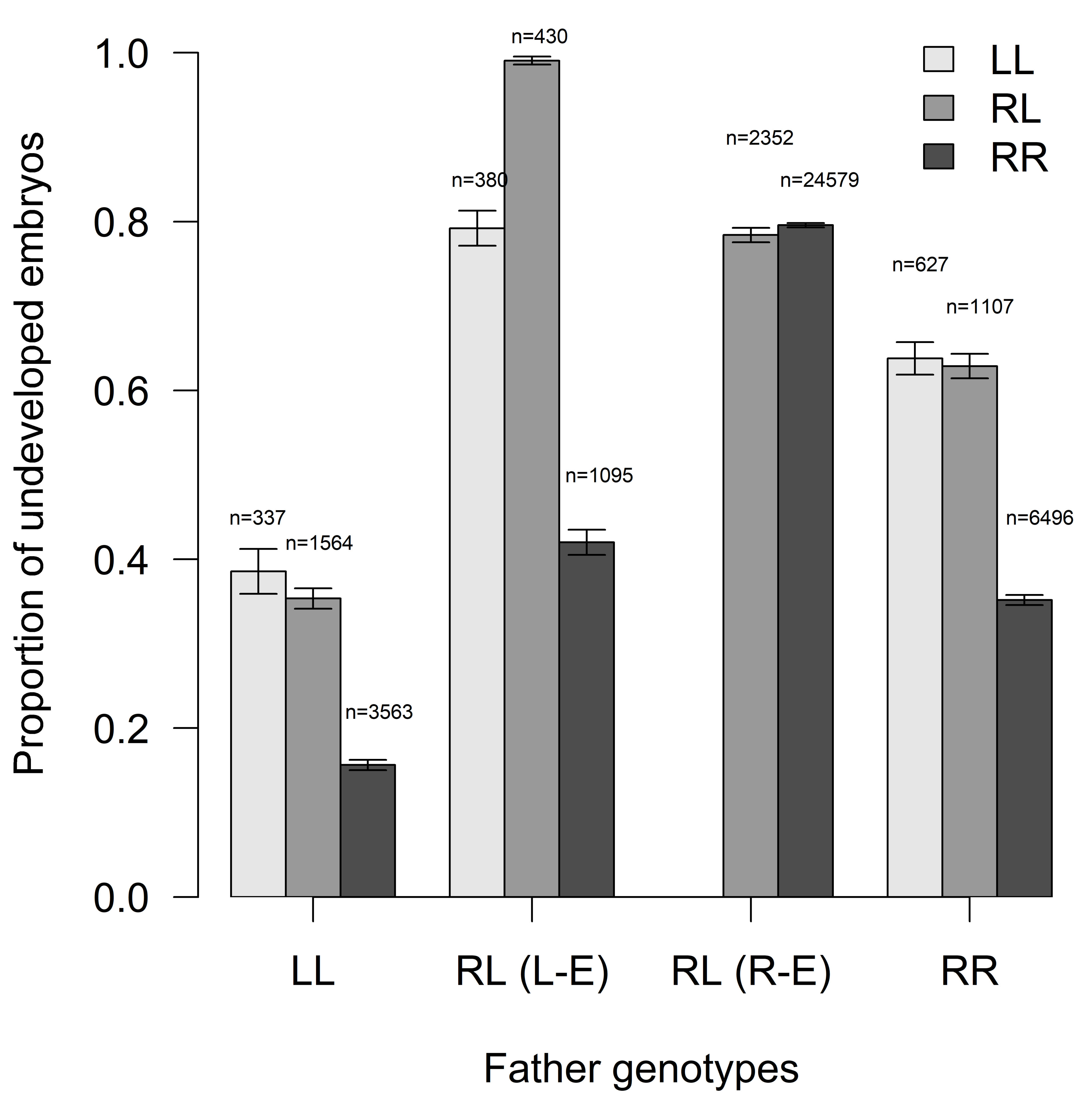 Fig. 3.
Fig. 3.Proportion of undeveloped embryos (EM) in relation to father
genotypes (mean
Proportion of developed embryos (Fig. 4) differed among expected
progeny types (n = 37644, clusters = 167, df = 3,
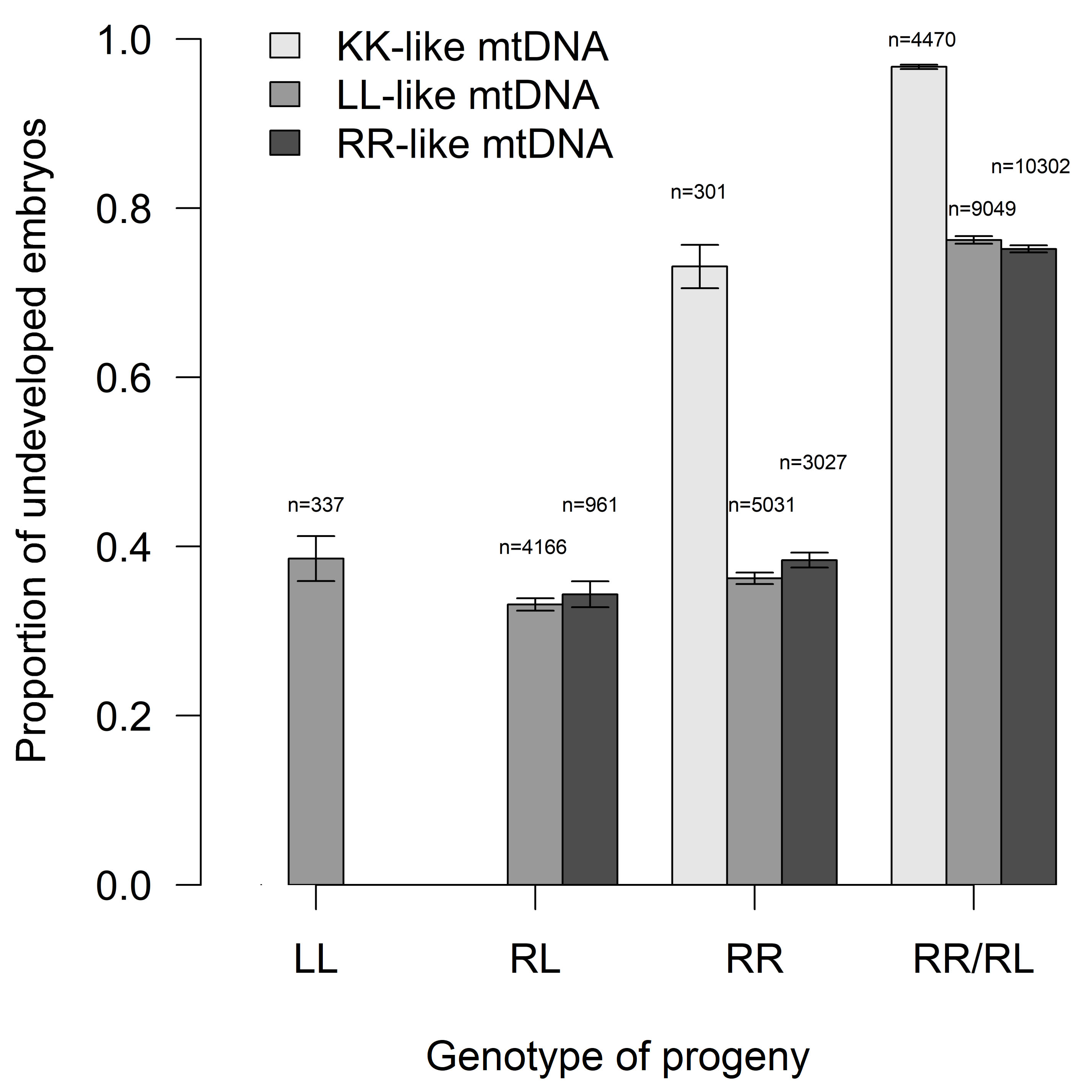 Fig. 4.
Fig. 4.Proportion of undeveloped embryos concerning their genotype and
type of inherited mitochondrion (mean
Proportion of developed embryos (Fig. 5) differed among mother
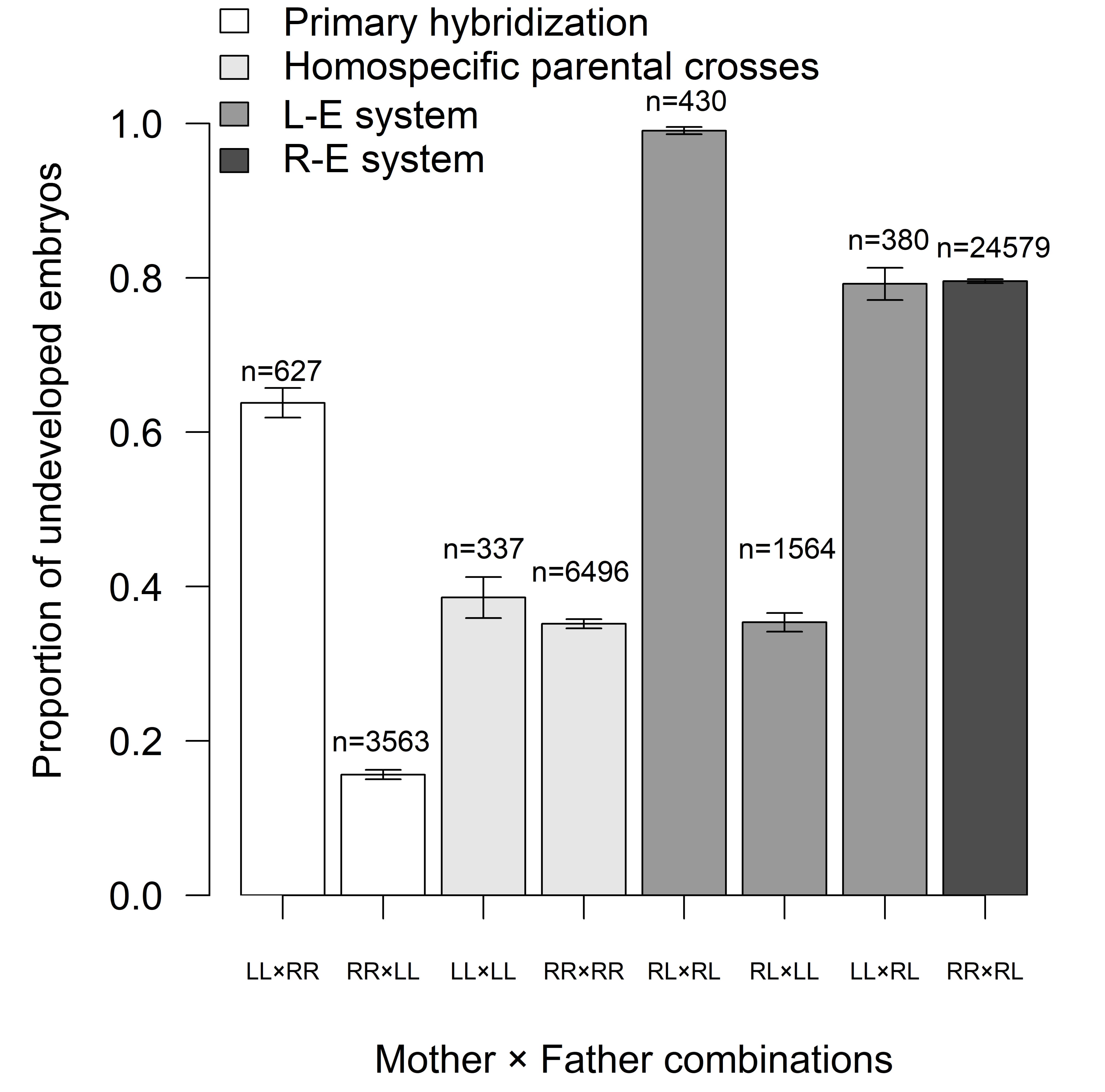 Fig. 5.
Fig. 5.Proportion of undeveloped embryos in relation to mother x father
combinations (mean
Proportion of developed embryos (Supplementary Fig. 4) did not differ
between RL males from LE and RE systems significantly (n = 28836, clusters = 135,
df = 1,
During the initial stages of embryogenesis, we captured phases of blastula cell
divisions, gastrulation and neurulation. We sorted them sequentially (Fig. 1D).
In some cases (especially RR
Our results of 211 laboratory crossbred families showed that the development of Pelophylax tadpoles varies in survival rate and morphological malformations. This post-zygotic reproductive dynamics in mixed populations is significantly driven by parental combinations of species pairs. Moreover, the father's genetic input, in particular, had a significant effect on survival rates in progeny (Figs. 3,5). Setting aside the apparent influence that the condition of the sperm has, we discuss the role of compatibility between parental haploid genomes in the survival rate of early tadpole development.
Survival rates in pure parental species pairs. Overall, the best values
in the proportion of developing eggs, embryos, tadpoles and overall survival
showed the progeny of homospecific LL
We assume that later when the embryo finishes neurulation, and the arising tadpole reaches stage 25, ecological factors may impact TM more than genetic ones. Omitting the biotic factors like infections observed in some semi-natural experiments [38, 39, 40], factors such as competition or density of tadpoles may influence the amount of successfully metamorphosed juveniles of amphibian taxa. Reading and Clarke [41] found positively correlated evidence of proportionally higher tadpole mortality in higher tadpole densities. The negative effect of density in other amphibians delayed tadpole growth due to the excretion of specific substances by tadpoles [42] and resulted in smaller sizes at the metamorphosis stage [43]. Toxic defences were observed in Bufo toads [44], Litoria [45] and other Rana tadpoles [46]. Laboratory-raised tadpoles of P. shqipericus showed high sensitivity to a temperature that strongly affected developmental and survival rates [47]. We do not know the exact causation for the low P. ridibundus tadpole performance constantly observed in our study. If environmental factors are functional players in tadpole performance, the ecological disadvantage of this species will provide positive prospects from a conservation perspective. Pelophylax ridibundus has recently been expanding and invading many areas throughout Europe [48, 49, 50, 51]. Any ecological handicap in relation to P. esculentus or P. lessonae may prevent unwanted population replacements of L-E systems by pure P. ridibundus.
Total proportions of fertilised eggs and developed embryos were relatively
similar between L-E and R-E systems but much lower than homospecific crosses in
parental taxa. However, once the early stages passed this period, tadpoles mostly
of hybrid hemiclonal origin performed even better than pure P.
ridibundus progeny. Survival rates were not affected by the hybrid genotype as
such but were defined by the parent’s gender. Greater fertility and a relatively
high proportion of developed embryos, in values comparative to LL and RR females
from homospecific crosses, were shown by hybrid females backcrossed to P.
lessonae males. Their average EM of 35% significantly differed from reciprocal
crosses between hybrid males and P. lessonae females, with an EM of
almost 80% (Fig. 5). Even higher EM (99.5%) was recorded in progeny fathered by
a hybrid male from Poland [52]. Combining two parental hybrids provided the
highest EM over mother x father pairs (Fig. 5). The low performance of embryos in
hybrid x hybrid crosses have also been previously observed [53, 54, 55]. However,
comparing mother
Low EM was not only a feature of hybrid males from L-E populations but also of conspecific males from R-E populations. The mortality of progeny fathered by such P. esculentus males was significantly higher in the initial phases of embryogenesis (EM) than during the later phases of tadpole development (TM, Supplementary Fig. 4). Our results correspond with the research of Kawamura and Nishioka [56], who presented mortality rates close to our results in two B1 families of amphispermic hybrid males from Western Germany. Some authors observed the opposite pattern i.e., a lower EM but higher TM [15, 52]. Variation in survival rates in progeny fathered by males from the R-E system may have been caused by variations in sperm production. The occurrence of different germ cells in the testes may lead to the formation of only R sperm, only L sperm, or both sperm types at once, resulting in variable progeny [16, 17, 52, 57].
One theory predicts that high embryonic mortality might be caused by the accumulation of deleterious mutations in clonally transmitted genomes [55]. A study of all-hybrid P. esculentus populations revealed post-zygotic selection against parental genotypes, P. lessonae and P. esculentus, explained by a role of low genetic diversity in clonal gametes and fixation of deleterious mutations [10]. However, this phenomenon alone cannot explain contrasting values of survival rates in early progeny between P. esculentus fathers (low values) and P. esculentus mothers (high values). We assume two alternative phenomena might have played a key role in the early development of hybridogenetic water frogs. Only maternal genes (mRNAs and ribosomes) are expressed during the initial stages of early embryonic development, whereas the expression of paternal genes starts in the late blastula [58]. The subsequent incompatibility between L and R genomes in the blastula stage may result in the interrupted or malformed development of cleaved embryos and high EM. Indeed, we detected various morphological abnormalities in hybrid offsprings such as deformed eggs, unhatched embryos, and tadpoles with irregular body shapes (Fig. 1E), correlating with earlier research [55, 59]. We, therefore, conclude that the high embryonic mortality in the hybrid offspring might have been influenced by the genomic incompatibility between the clonal genome from a father and the sexual genome from a mother. Additionally, previous authors have noted that disturbances and irregularities during hybrid spermatogenesis have led to lower fertility [15, 60]. We have no direct data on male spermatogenesis, but our observations of the resulting reproduction patterns suggest either a quality difference among the sperm produced or a generally low-fertile sperm produced by hybrid P. esculentus.
As well as laboratory-produced mate pairs typical for natural populations within
L-E and R-E systems, we crossed P. ridibundus and P. lessonae
to form new P. esculentus F1s. The successful development of newly born
F1 was also reported by Berger and Günther [52] and Hotz et al.
[61]. Here we analyzed reciprocal crosses and found significantly unequal
survival rates in progeny in which crosses of RR mothers
Finally, we observed a significant difference in embryonic survival rates depending on the type of inherited mtDNA in progeny. Due to the matrilinear heredity, assumed preferential primary hybridizations between P. ridibundus females and P. lessonae males in nature formed P. esculentus F1s with a P. ridibundus mtDNA type. However, hybrid males producing R gametes and mating with P. lessonae females may have also resulted in the transfer of P. lessonae cytoplasm to hybrid cells [66]. Indeed, not only P. esculentus but also many P. ridibundus bear P. lessonae type mtDNA. The greater effectiveness of the enzymes encoded by P. lessonae mtDNA may give some advantage to P. ridibundus and probably P. esculentus in the northern parts of their ranges [29]. Pelophylax lessonae is known for better cold tolerance reaching higher latitudes and longitudes, while P. ridibundus is a cold-sensitive species from lowlands that recolonized the deglaciated northern parts of Europe later than P. lessonae [29]. The laboratory progeny of P. ridibundus and P. esculentus showed very similar embryonic survival rates in having P. ridibundus or P. lessonae mtDNA (Fig. 4), supporting the compatibility of P. lessonae cytoplasm with the nuclear DNA background of P. ridibundus genomes collected in Central Europe. A striking contrast can be seen however, in the significantly higher numbers of undeveloped embryos in P. ridibundus and P. esculentus progeny when mtDNA were of the P. kurtmuelleri type (Fig. 4). Although Pelophylax kurtmuelleri mtDNA occurs at low levels in Central European water frogs, it may represent an opposite case to P. lessonae mtDNA, as P. kurtmuelleri is endemic to the warmer Balkans in Southern Europe. Its mtDNA may not be fully compatible with Central European, more cold-adapted, Pelophylax genomes.
Our study of early development combining taxa and sexes of three Central European water frog taxa provided some patterns on survival rates of particular progeny genotypes. The results further showed great survival rates (even compared to homospecific parental crosses) of P. esculentus F1s created de novo from parental species despite significant divergence between the nuclear and mitochondrial DNA of P. ridibundus and P. lessonae. Moreover, data indicates not only a potential variance in progeny within R-E systems due to the likely occurrence of more types of gametes produced by hybrid males but also revealed reproductive dynamics in L-E systems. Here, P. esculentus females produce highly vital progeny with P. lessonae males (with relatively unlimited sperm availability during a breeding season) and well-maintained P. esculentus in taxonomically mixed populations. In contrast, P. esculentus males seem to have only poor reproductive potential, wasting P. esculentus and P. lessonae eggs, available in limited numbers during the reproductive season in temperate Europe. Recent studies have provided alarming evidence of P. lessonae, and mixed L-E population declines over large areas in Europe [50, 51, 67]. Future research may consider some conservation measures for P. esculentus males from L-E systems, like their removal from populations threatened by declines to reduce competitive spawnings. Such actions would support mating between females of P. esculentus and P. lessonae with P. lessonae males, making natural populations stronger through the vital growth of P. esculentus and P. lessonae progeny.
MDK participated in the design of the study, collected samples, performed crossing experiments, counted eggs/embryos at different developmental phases, made photos of malformed embryos, analyzed microsatellite and mitochondrial data, and wrote the initial draft of the manuscript. PP ran the statistical analyses of the data, helped with the data interpretation and participated in draft editing. LC conceived of the study and participated in sampling, crossing experiments and manuscript writing. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All experimental protocols were approved by the Federal Commission for Animal Experiments (FCAE) and by the Standing committee on animal health under the Federal Food Safety and Veterinary Office FSVO, Switzerland, under permit nos 119/2013: TV 5113 and TH 103. We declare that all other manipulations with animals were performed in accordance with relevant guidelines and regulations (CZ02361).
We thank Jörg Plötner, Gaston-Denis Guex for technical support and help during crossing experiments. Many thanks to Mgr. Veronika Labajová, who participated in sampling and crossing procedures, and Matěj Kaštánek, who helped with eggs/tadpoles countings. We thank Christopher Murray Johnson, who proofread the manuscript, and anonymous reviewers for their helpful comments.
This research was funded by the Czech Science Foundation (grant number GA 19-24559S to M.D-K. and L.C.), and by the Academy of Sciences of the Czech Republic (grant number RVO 67985904 to M.D-K. and L.C.).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
