1. Introduction
Phytoalexins are diverse plant secondary metabolites possessing supportive roles
against biotic and abiotic stressors, whereas they have represented potent
biological properties [1, 2]. Resveratrol (3,4,5-trihydroxy-trans-stilbene)
as a natural polyphenolic phytoalexin, can abundantly found in grapes, peanuts,
and berries, while it has recently attracted numerous attentions due to its
important biotherapy characteristics [3]. Different investigations have confirmed
that resveratrol richness in the stressed leaves [4, 5]. Asides from diverse
beneficial bioactivity effects of resveratrol, various studies have shown
significant cardiovascular protective effects in people who consume rich
resveratrol herbal products [6, 7, 8]. Resveratrol efficiently targets extracellular
ROS when administered orally, consequently acts as a great antioxidant [9].
Furthermore, this compound displays useful effects for the treatment of cancer,
hypertensive, inflammatory, Alzheimer’ and metabolic diseases [10, 11, 12, 13].
The cerium oxide (nanoceria, NCs) have demonstrated anticancer activities, due
to its interesting changes in oxidation states, while it can act similar to the
antioxidant enzymes (catalase and superoxide dismutase mimetic activities)
[14, 15, 16], subsequently, showing protective attribute as an active scavenger of
reactive oxygen/nitrogen species (ROS/RNS) with unlimited cycles that involves
changes in oxidation states of Ce/Ce [17, 18]. The strong
anticancer function of the NCs can occur via correcting homeostasis in tumor
microenvironment and angiogenesis, preventing myofibroblast formation, invasion
and tumor growth without any changes in ROS levels, enhancing the immune system,
and in some cases, some paradoxical reports explain the genotoxicity and
oxidative stress from ROS formation as the determining factors to death of cancer
cells [19, 20, 21, 22, 23, 24]. Reports suggest the bioactivity of the NCs are size-, medium-,
pH- and time-dependent [25, 26].
The combination of NCs with gold could enhance the anticancer effects of the NCs
along with the biocompatibility of the particles [27]. Due to the potential
application of gold in different nanoplatforms for the treatment of cancer, as an
active agent or a nanocarrier, it can be a good choice to form nanocomposites
with NCs [28, 29, 30]. The gold inertness and its nontoxic nature are of great use in
the design of new active nanomaterials and nanocomposites for biomedical
applications [31, 32, 33, 34]. The factors that can be crucial in determining the final
anticancer properties can be the size, shape, and ratio of the components [35, 36]. Gold nanoparticles’ toxicity is directly proportional to the size of
nanoparticles. The larger particles are less toxic, and the smaller ones have
substantial toxicity towards cancer cells [37, 38, 39, 40]. Therefore, the NC seeds’ size
influences the bioactivity of CeO@Au core-shells. The combination of NCs
and gold, and even with other metals would be an innovative approach for
advancing new nanomaterials with stronger anti-cancer properties.
Herein, the complexation of a phytochemical resveratrol and Ce was
occurred because of the oxophilic nature of lanthanide ions. For the first time,
the resveratrol and Ce complex was used as a precursor to synthesize NCs
by thermal treatment. The prepared NCs were coated with gold and the formation of
CeO@Au core-shells was fully investigated by using the conventional
characterization methods. The anticancer properties of CeO@Au core-shells
and NCs were measured compared with each other to find the effectiveness of the
gold core-shells against liver cancer cells.
2. Materials and Methods
2.1 Instruments and Materials
The following analyses were performed for characterization of the nanoparticles:
powder X-ray diffraction (PXRD), Fourier transforms infrared spectroscopy (FTIR),
transmission electron microscopy (TEM), Dynamic light scattering (DLS), and
potential. The materials were purchased from Sigma-Merck merged
chemical groups except the stated items.
2.2 Synthesis of CeO@Au Core-Shells
To prepare the nanoceria, 0.012 mol resveratrol and 0.004 mol cerium nitrate
hexahydrate (Ce(NO).6HO) were dissolved in 100 mL water
separately. The resveratrol was sonicated then stirred vigorously. The cerium ion
solution was then added dropwise to the first solution under vigorous stirring.
After the addition of cerium ion solution, a milky-like appearance of the mixture
confirmed the formation of the resveratrol-cerium complex. Then, the complex was
separated using centrifugation and put in an oven at 500 °C overnight (8
h). The prepared nanoceria was used without further purifications. Afterwards,
0.5 g of the as-synthesized nanoceria was dispersed in 2.5 mL of a concentrated
solution of Chloroauric acid (HAuCl, 0.2 M). Then, it was centrifuged and
washed one time with 10 mL of distilled water (DW) and dispersed again in 10 mL
DW. Eventually, a solution of ascorbic acid (100 mL, 1 M) was prepared and
dispersed nanoceria was added to this solution dropwise under vigorous stirring.
The CeO@Au core-shells were separated and washed several times with DW and
applied for characterization and biological tests.
2.3 Cell Culture and Toxicity Effect of Biosynthesized NCs
Liver cancer cell line (HepG2) and human foreskin fibroblasts (HFF) were
attained from Iran’s Pasteur Institute. Cell developmental was performed in RPMI
and DMEM media, supplemented with 100 g/mL penicillin plus 10%
FBS, and as a final point transferred to the incubator. In each well of a 96-well
plate, cells were seeded, incubated 24 h, and after that the viability of cells
was assessed in 24, 48, or 72 h to the biosynthesized NCs at the 0.16, 0.31,
0.63, 1.25, 2.50, 5.00 mg/mL concentrations using MTT assay.
3. Results and Discussion
3.1 Powder X-ray Diffraction (PXRD)
The PXRD confirmed the formation of gold coated nanoceria (CeO@Au
core-shells), where the peaks of CeO and Au core-shells were compatible
with the reference codes of 00-001-0800 and 00-001-1172, respectively (Fig. 1).
The crystal systems, space groups and their numbers of both nanoceria and gold
core-shells were cubic, Fm-3m and 225. The experimental 2tetha values (d-space,
intensity) appeared at 38.48 ˚ (3.34 Å, 42.6%), 44.70 ˚ (2.02, 16.0%),
64.88 ˚ (1.44 Å, 11.9%), and 77.87 ˚ (1.23 Å, 16.3%) were related
to the gold layer and the peaks observed at 28.88 ˚ (3.09 Å, 100.0%),
33.39 ˚ (2.68 Å, 36.7%), 47.79 ˚ (1.90 Å, 65.6%), 56.68 ˚
(1.62 Å, 53.3%), 59.42 ˚ (1.56 Å, 8.9%), 69.76 ˚ (1.35 Å,
10.0%), 76.99 ˚ (1.24 Å, 18.3%), and 79.43 ˚ (1.21 Å, 12.0%)
were associated with the cerium oxide core.
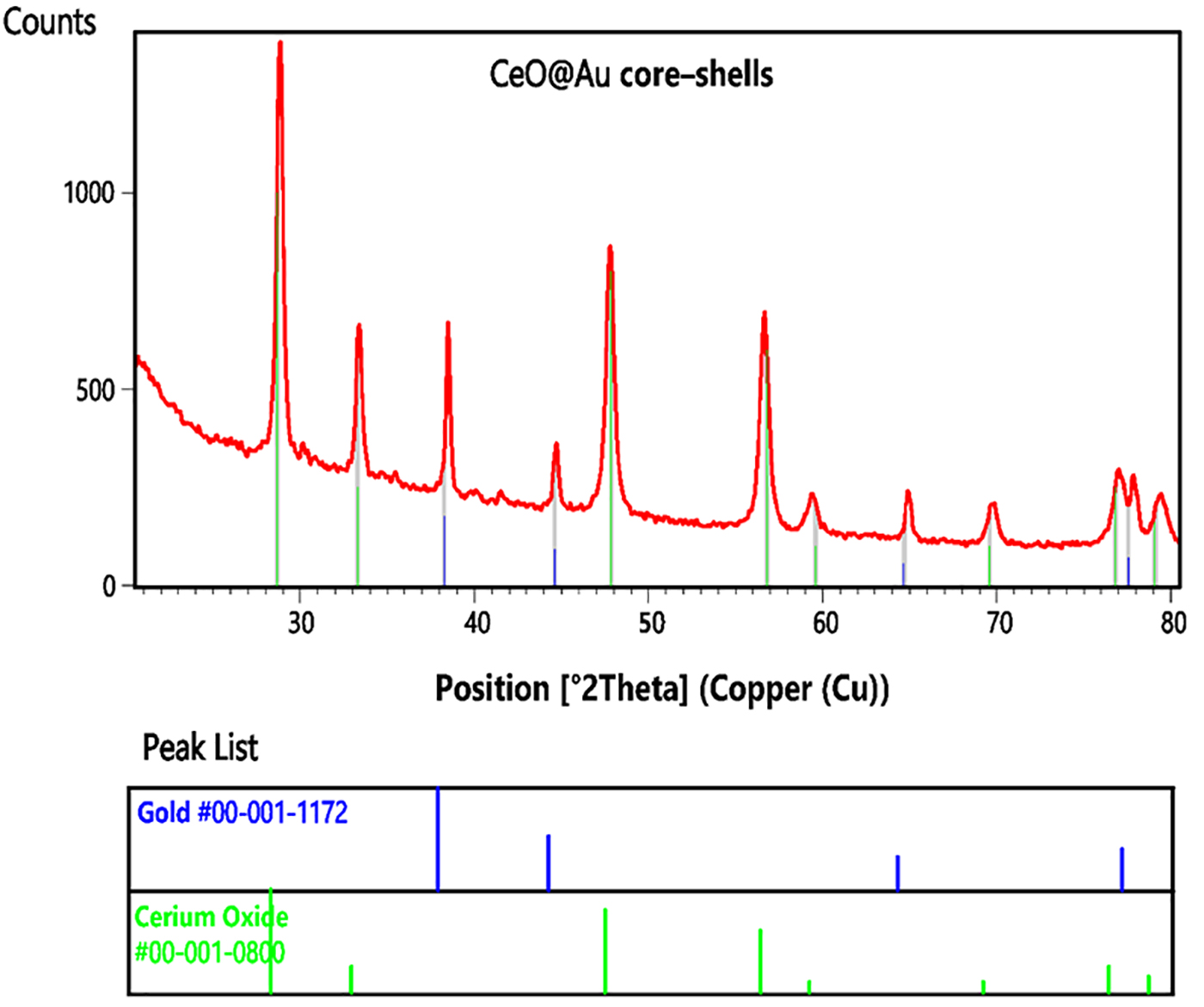 Fig. 1.
Fig. 1.
The PXRD pattern of CeO@Au core-shells. The peaks of
CeO and Au core-shells were compatible with the reference codes of
00-001-0800 and 00-001-1172.
The calculated 2theta value (d space, intensity, HKL) of cerium oxide were 28.68
˚ (3.11 Å, 100.0%, 111), 33.28 ˚ (2.69 Å, 25.0%, 200), 47.83 ˚
(1.90 Å, 80.0%, 220), 56.78 ˚ (1.62 Å, 60.0%, 311), 59.60 ˚ (1.55
Å, 10.0%, 222), 69.58 ˚ (1.35 Å, 10.0%, 400), 76.81 ˚ (1.24
Å, 25.0%, 331), and 79.08 (1.21 Å, 16.0%, 420) and the ones for gold
layer were 38.27 ˚ (2.35 Å, 42.6%, 111), 44.60 ˚ (2.03 Å, 22.6%,
200), 64.68 ˚ (1.44 Å, 14.1%, 220), and 77.55 ˚ (1.23 Å, 17.0%,
311). The comparison of the calculated and experimental data indicated the
successful synthesis of cerium oxide and the reduction of Au. According to
the Scherrer equation, the crystallite (grain) size was calculated
24.2 nm.
3.2 Fourier-Transform Infrared Spectroscopy (FTIR)
The absorption bands of FTIR between 3200–3500 cm were
associated with the hydroxyl groups of the capped ascorbic acid and possibly on
the surface of CeO (Fig. 2) [41, 42, 43]. The bands at 3100–2840 cm can
be related to the C–H stretching of ascorbic acid. Ascorbic acid usually shows
at absorption signal at 1750–1760, specifically assigned to the
carbonyl stretching of the -lactone ring in the ascorbic acid.
The band absence after reduction of gold nanoparticle and bounding to the surface
of nanoparticle could prove CeO@Au core-shells coated by ascorbic acid
[44]. The bands corresponded to C–H bending of the methyl group and carbonate
species after calcination was appeared at 1454 and 1385 cm, respectively.
The bands at 1132 and 1002 were also associated with the C–O stretching of the
–OH group of the alcohol. The band observed at 500 cm can also be
assigned to the Ce–O vibration [45].
 Fig. 2.
Fig. 2.
The FTIR spectrum of CeO@Au core-shells. The bands at
1454, 1385, 1132, 1002, and 500 cm represents biochemical groups in the
formation of NCs.
3.3 Transmission Electron Microscopy (TEM)
The TEM analysis was performed to determine the size of CeO@Au core-shells
in the solid phase. As shown in Fig. 3, the TEM images have revealed spherical,
semi-spherical, and oval morphologies with particles sizes in the range of
20–170 nm. The images revealed that the appearance of larger sizes
could possibly be due to the agglomeration of the several particles.
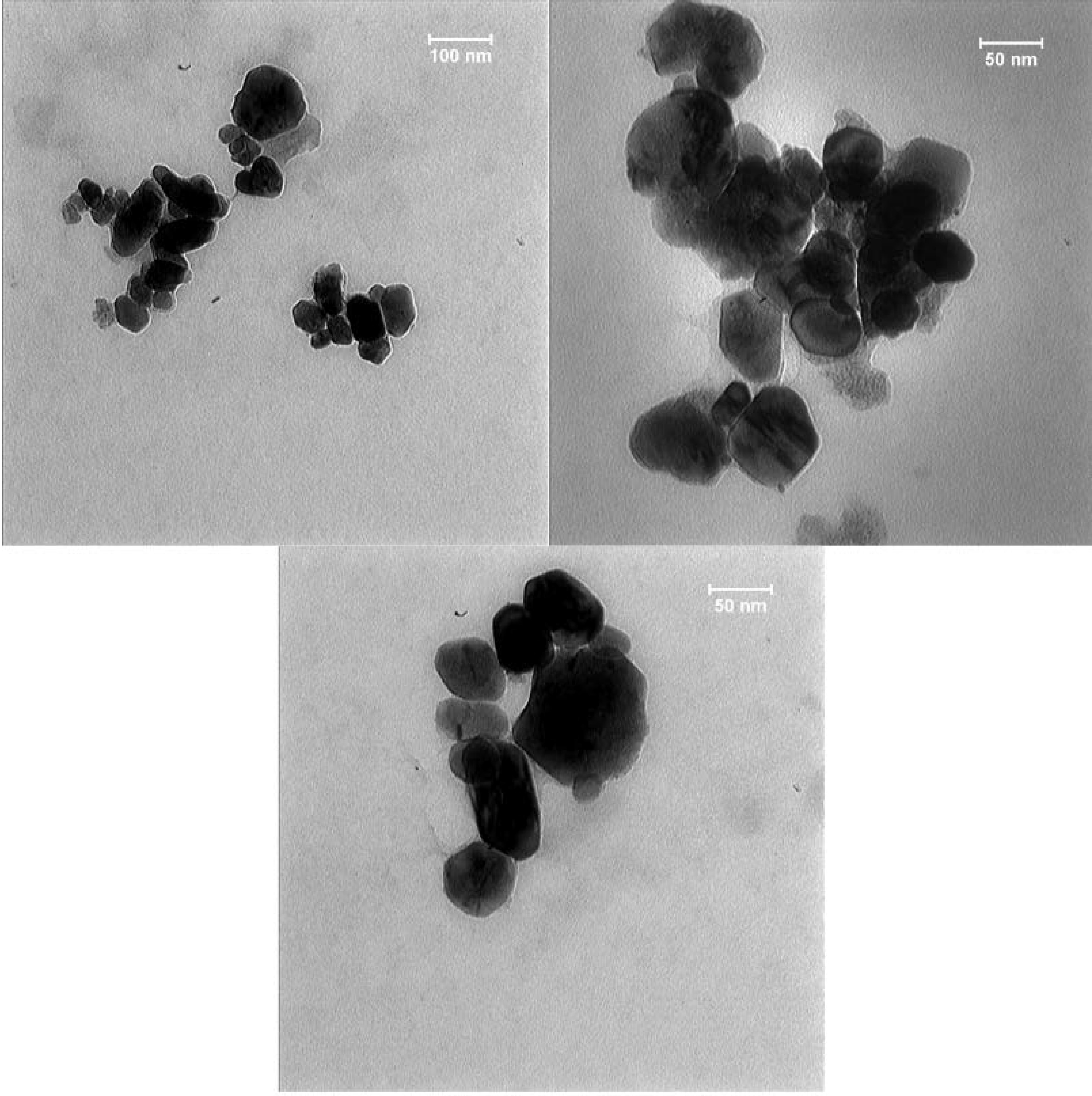 Fig. 3.
Fig. 3.
The TEM images of CeO@Au core-shells. The particles sizes
are in the range of 20–170 nm.
3.4 Dynamic Light Scattering (DLS) and Potential
The hydrodynamic size and the partial surface charge of the particles were
measured using the DLS and potential analyzer (Fig. 4). The analyses
revealed the z-average of 229 nm, where nearly 50 and 90% of the particles
showed particle sizes under 282 and 468 nm in order, respectively. The particle
size according to intensity and number of size dispersion was 262 and 115 nm,
respectively, whereas the partial surface charge was –24 mV. The analyses
demonstrated that the information obtained from solid-phase sizes and the
hydrodynamic sizes were compatible and acceptable. The PXRD and FTIR also
confirmed the synthesis of the nanoparticles. The TEM images further indicated an
aura around the particles that could be attributed to the formation of a gold
layer around the nanoceria particles.
 Fig. 4.
Fig. 4.
The hydrodynamic size of CeO@Au core-shells.
3.5 Anticancer Properties of NC
Nanotechnology-mediated delivery can be employed as a beneficial device in
increasing the bioavailability of resveratrol. Nanoscience is an emerging part of
investigation that operates at the crossways of biology, physico-chemicals,
engineering, pharmacology, and medicine [22, 46, 47, 48]. Designing of NPs (nano
particles) for an effective and controlled delivery of anticancer factors is
being considered as one of the most important applications of nanoscience
[24, 49, 50]. Few experiments have attempted to study potency of resveratrol for
the synthesis of nanoparticles. It has been shown that the solid lipid NPs loaded
with resveratrol can cross the cell membrane, while it can be enhanced via
increasing the exposure time of cells to resveratrol [51]. Another study
utilizing bovine serum albumin-bound resveratrol NPs in primary ovarian cancer of
mice showed that not only the NP-bound resveratrol was easier to work with
because of the improved solubility, it had also a greater influence on prevent of
tumor growth, compared to pure resveratrol [52]. The anti-cancer effect of gold
nanoparticles against breast, testicular, liver, and lung cancer cells has been
proven in a concentration-dependent manner [30, 51, 52, 53, 54, 55]. In another study, the
produced gold nanoparticles provided a safe and great system for the delivery of
gapmers in cancerous cells, which meaningfully down-regulated mutant p53 proteins
and changed molecular markers related to cell growth and apoptosis [56].
The anticancer activity of the produced NC was remarkable, whereas it possessed
lower toxic effect on normal cells. The results clearly exhibited the toxicity of
NC against cancer cells in a concentration and time dependent manner (Fig. 5).
Although resveratrol alone was effective against cancer cells, higher efficacy in
toxicity against cancer cell lines was observed in combination with
nanoparticles.
 Fig. 5.
Fig. 5.
Cellular toxicity effect of biosynthesized NCs and resveratrol against HepG2 as a cancerous cell lines (B and D) and HFF as a normal cell lines (A and C).
4. Conclusions
The nanoceria particles were successfully synthesized using resveratrol to form
complexes as a precursor for further calcination procedure. The synthesized
nanoceria was also coated with a gold layer to increase their anticancer
efficiency. The prepared nanoceria were fully characterized and analyzed through
conventional methods. The experiment has shown the particle size of
20–170 nm in the solid phase with the z-average hydrodynamic size of
229 nm. The present study indicated the successful synthesis of CeO@Au
core-shells. The anticancer results demonstrated that resveratrol-mediated
synthesized NCs have significant cellular toxicity properties against HepG2 and
could be utilized in hepatocarcinoma therapy. Further in vivo
investigations will significantly help to the anti-cancer and safety effects of
fabricated nanocomposites.
Author Contributions
Conceptualization, AGA, JM, FN, METY; methodology, MM, AH, AHM, SV;
investigation, AGA, JM, MSA, MM, AH, AHM, FN, MN, MQ, METY, MI; project
administration, METY, MI.
Ethics Approval and Consent to Participate
Not applicable.
Acknowledgment
The authors are warmly grateful to the Mashhad University of Medical
Sciences for support and kindly providing facilities to perform the experiments.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest. MI is serving as one of the Editorial
Board and Guest Editor of this journal. We declare that MI had no involvement in the
peer review of this article and has no access to information regarding its peer
review.Full responsibility for the editorial process for this article was delegated to GP.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5.