†These authors contributed equally.
Academic Editors: Viviana di Giacomo and Silvia Sancilio
Background: Ovarian cancer (OC) is the most deadly tumor in gynecology and there is no effective biomarker for diagnosis and treatment. The role of Transmembrane Protein 98 (TMEM98) in ovarian cancer is still unclear. Methods: The expression and prognostic effect of TMEM98 in OC were analyzed using the public database. Cell Counting Kit-8 proliferation experiment, scratch experiment, Transwell invasion experiment, flow cytometry, TUNEL staining, and in vivo and vitro experiment were used. Results: TMEM98 was significantly downregulated in OC tissues and cell lines compared to the normal ovarian tissue and cells lines. In addition, patients with lower TMEM98 levels exhibited inferior survival. Low expression of the TMEM98 promoted proliferation, migration, invasion, vasculogenic mimicry, and inhibited apoptosis in OC cells. The expression of Caspase-3 was significantly downregulated and the expression of Bcl-2 was significantly increased in the silencing-TMEM98 group. Moreover, low expression of TMEM98 promotes OC development in vivo. Bioinformatics analysis showed that TMEM98 expression was negatively correlated with poly ADP-ribose polymerase expression. Conclusions: This study demonstrates that TMEM98 is low expressed in OC and impacts the prognosis of OC patients. TMEM98 inhibits proliferation and promotes apoptosis and finally exerts a certain tumor-suppressor effect on OC.
Ovarian cancer (OC) is a gynecological tumor with the highest mortality rate among women in the world [1]. Early-stage OC has a favorable prognosis, while the 5-year survival rate of advanced-stage OC is only 20–30% [2]. Due to the insidious onset of OC, approximately 85% of the OC cases were diagnosed as advanced stage (stage III–IV disease) [3]. Therefore, OC has become a ‘silent killer’, threatening the life and health of women. Although effective treatment strategies have made progress in recent years, the survival rate has hardly improved [4]. In the current clinical practice, CA125 and HE4 are used to diagnose OC. However, the sensitivity of CA125 and HE in the diagnosis of early-stage OC is lower and the above markers can be also increased in some conditions [5]. Therefore, it is critical to investigate more specific biomarkers to improve the diagnostic accuracy and therapeutic efficacy for OC.
Human transmembrane proteins (TMEMs) are widely expressed in the membrane structure of human tissues and organs [6, 7]. Moreover, TMEMs are involved in regulating several physiological processes, including apoptosis, autophagy, adhesion, ion transmembrane transport, mediating cell chemotaxis, and signal transduction [8, 9, 10]. However, few studies are reported regarding TMEM98, which has the highest RNA expression in the human normal ovary compared to other normal tissues or organs [11, 12].
In the previous study, TMEM98 can promote endothelial cell adhesion by inducing
the expression of ICAM-1/VCAM-1 and trigger vascular smooth muscle cell
proliferation and migration through activation of ERK and AKT/GSK3
In this study, we investigated the role of TMEM98 in regulating OC development and sought to identify its potential biological function.
The clinical sample data sets of OC were downloaded from The Cancer Genome Atlas (TCGA), GEO and GTEx database to analyze TMEM98 mRNA expression and RNA SEQ data. GEPIA website (http://gepia.cancer-pku.cn/) was used to analyze the difference of TMEM98 mRNA expression between OC and normal tissues in the TCGA and GTEx. TNMplot database (https://tnmplot.com/analysis/) was used to analyze the difference of TMEM98 mRNA expression between tumor and normal tissue in various tumors. TCGA database was used to analyze the expression of TMEM98 and clinical stage of OC patients. KM-plotter (http://kmplot.com/analysis/) was used to analyze the relationship between TMEM98 and progression-free survival (PFS). GSEA software was used for the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis to explore the biological function of TMEM98 in the progression of OC. The correlation between TMEM98 and other genes was analyzed online by the cbioportal website (https://www.cbioportal.org/).
Two human cell lines, OC SKOV3 cells and ovarian epithelial IOSE80 cells were
obtained from American Type Culture Collection (ATCC, USA). Cells were cultured
in DMEM with 10% fetal bovine serum (FBS, Cellmax) at 37 °C in a
humidified incubator filled with 5% CO
To establish cell models with low-expressing/over-expressing TMEM98, the siRNA/Plasmid targeting TMEM98 with chemical modification and 10ul negative control products were transfected into 30%–50% confluence SKOV3 and IOSE80 cells, which were cultured in the 6-well plate by reagent system in the light of the product specifications. After incubated for 48 h, the expression of TMEM98 was detected by western blot (WB) and then the relative experiments were performed to check the influence of TMEM98 on biological behaviors.
Total proteins extracted from all cells were resolved on SDS-PAGE gels. And
then, proteins on the gel were transferred to PVDF membranes (Millipore,
Billerica, MA, USA) which were blocked with 5% BSA in TBST for 1 hour and
incubated with TMEM98 antibody (1:1000, Proteintech) or
Cell growth was detected via Cell Counting Kit-8 (CCK-8, Dalian Meilun, Dalian,
China) assay. Transfected cells were seeded into 96-well plates at a density of
3000 cells per well. CCK-8 experiment was performed according to the
manufacturer’s instructions. OD values were determined after 24, 48, 72, and 96
hours to evaluate cell growth. After adding 10
Cell Cycle and Apoptosis Analysis Kit (Yeasen, Shanghai, China) was used for analyzing the cell cycle. Transfected cells were harvested and fixed with 70% ethanol overnight at 4 °C. Then the cells were treated with RNase A and stained with propidium iodide for 30 minutes at 37 °C. Flow cytometry was used to calculate the percentages of cells in the G0/G1, S, and G2/M phases to analyze the distribution of the cell cycle.
Annexin-VFITC Apoptosis Detection Kit I (BD Biosciences) was used for analyzing
apoptosis. Cells were resuspended by cold PBS at a density of 1
Transfected cells were seeded into six-well plates with 10% FBS. When 80% of
the well was covered with cells, a 100
Female BALB/c nude mice aged 4 to 8 weeks old were used. Mice were divided into three groups and subcutaneously injected with silencing-TMEM98 (si-TMEM98) or overexpression-TMEM98 (oe-TMEM98) SKOV3 cells, respectively.
UltrasensitiveTM SP (Mouse/Rabbit) IHC Kit (Mai-xin Biotechnology Co., Fuzhou,
China) was used to perform immunohistochemistry. The tumor tissue was made into 5
Data are presented as mean
By analyzing the data from TCGA, GSE9891, and GTEX databases, the TMEM98 gene
has a higher expression in the normal ovary tissue compared to other human organs
(p
 Fig. 1.
Fig. 1.The expression of TMEM98 in OC and normal ovary. (A,B) The
expression of TMEM98 between OC tissue and normal ovarian tissue via the GEPIA
online website (*p
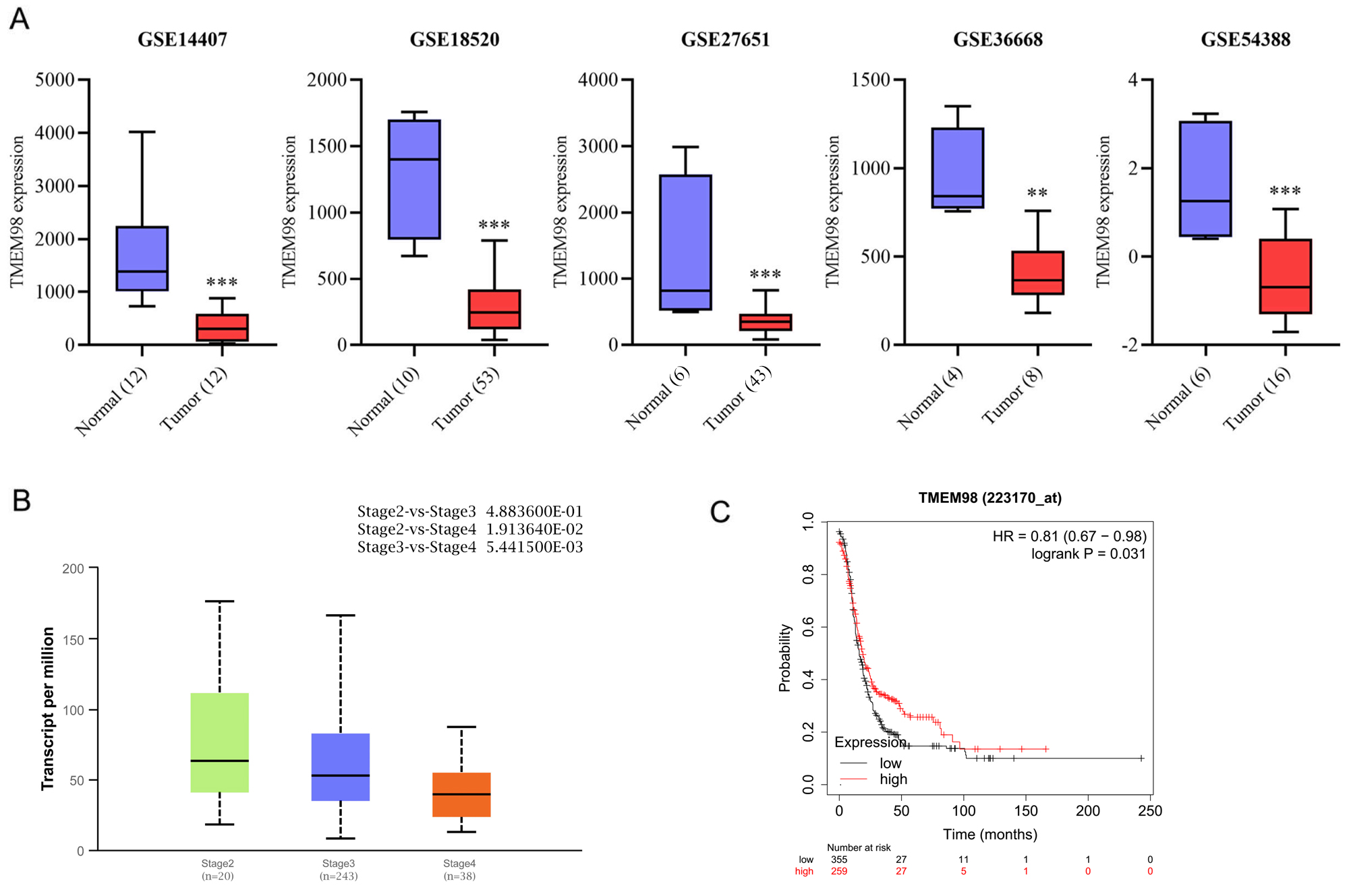 Fig. 2.
Fig. 2.Association of TMEM98 expression with clinicopathological
features and survival. (A) Validation of TMEM98 expression levels in OC
tissue compared with normal tissue from GEO datasets (**p
In the TCGA database, 301 clinical samples of different stages of OC were
downloaded, including 20, 243, and 38 patients who had stage II, III, and IV
diseases, respectively. The expression of TMEM98 according to the clinical stages
was analyzed online using the Ualcan website (http://ualcan.path.uab.edu/). The
results showed that TMEM98 expression was negatively correlated with the clinical
stage. The expression of TMEM98 decreased as the clinical stages progressed (Fig. 2B). The expression of TMEM98 was significantly lower in stage III (p
| Variables | n | TEME98 expression | p | ||
| Low (%) | High (%) | ||||
| Age (n = 376) | |||||
| 245 | 127 (67.6) | 118 (62.8) | 0.330 | ||
| 131 | 61 (32.4) | 70 (37.2) | |||
| Stage (n = 373) | |||||
| I–II | 23 | 12 (6.4) | 11 (5.9) | 0.840 | |
| III–IV | 350 | 175 (93.6) | 175 (94.1) | ||
| Grade (n = 366) | |||||
| 1–2 | 43 | 15 (8.2) | 28 (15.3) | 0.035 | |
| 3–4 | 323 | 168 (91.8) | 155 (84.7) | ||
| Lymphatic invasion (n = 148) | |||||
| No | 48 | 23 (30.3) | 25 (34.7) | 0.562 | |
| Yes | 100 | 53 (69.7) | 47 (65.3) | ||
| Vascular_invasion (n = 103) | |||||
| No | 40 | 17 (34.7) | 23 (42.6) | 0.411 | |
| Yes | 63 | 32 (65.3) | 31 (57.4) | ||
Since TMEM98 is correlated with the clinical stage of OC, the relationship between TMEM98 and PFS was further explored using the KM-plotter from the TCGA database. The results showed that PFS was significantly longer in patients with high mRNA levels of TMEM98 compared to those with low mRNA levels of TMEM98 (p = 0.031). The finding suggested that TMEM98 had a prognostic effect on OC (Fig. 2C).
To further explore the effect of TMEM98 on the malignant behavior of OC, SKOV3
and IOSE80 cell lines were selected for comparison experiments. In this
experiment, small interfering RNA (siRNA) was used to knock down the TMEM98 gene
and TMEM98 expression level was detected using WB. The results showed that
compared with the normal control (NC) group, after siRNA-TMEM98 knockdown in
SKOV3 cells and IOSE80 cells, the TMEM98 protein expression in the si-TMEM98
group was significantly reduced in SKOV3 cells and IOSE80 cells
(Supplementary Fig. 1A, **p
We downloaded the RNA SEQ data of OC clinical samples from the TCGA database. According to the expression of TMEM98, we used GESA software for KEGG enrichment analysis. The results showed that TMEM98 was closely related to apoptosis, cytoskeleton, and cell adhesion (Fig. 3).
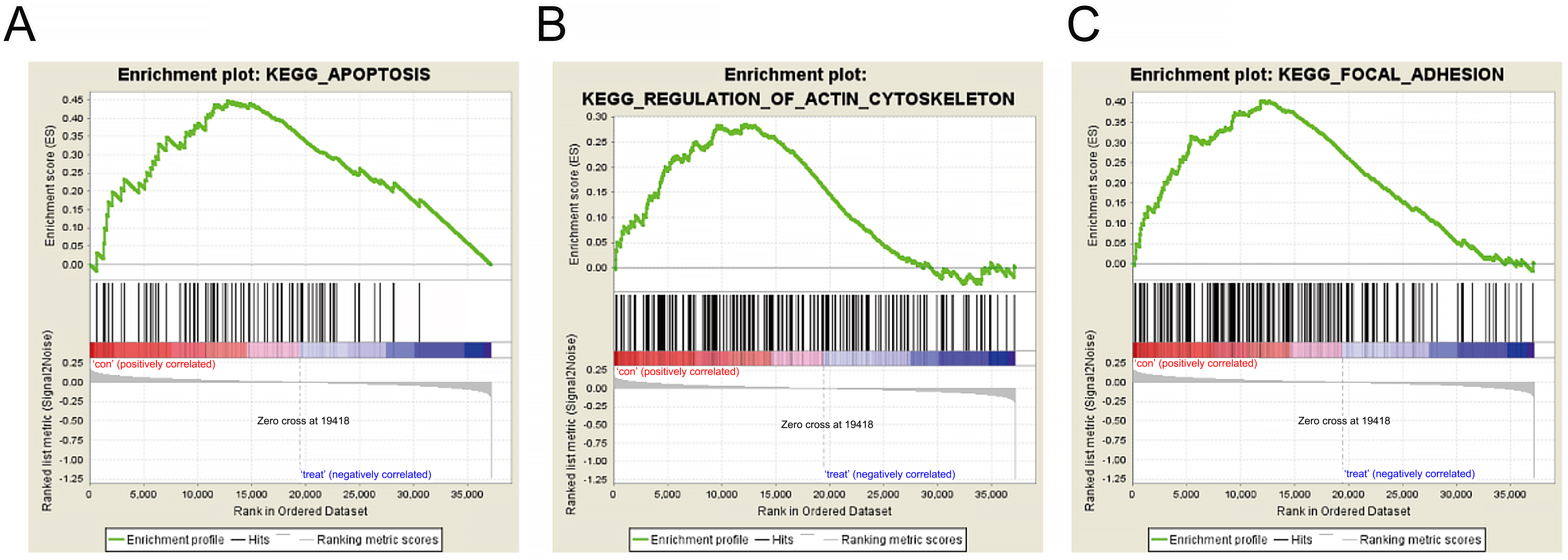 Fig. 3.
Fig. 3.KEGG analysis of TMEM98 gene-related biological function. (A) Enrichment plots of gene expression signatures for apoptosis according to TMEM98 expression levels. (B) Enrichment plots of gene expression signatures for cytoskeleton according to TMEM98 expression levels. (C) Enrichment plots of gene expression signatures for cell adhesion according to TMEM98 expression levels.
SKOV3 cells and IOSE80 cells were treated with siRNA-TMEM98 and TMEM98 overexpression plasmid respectively. After the cell silencing and overexpression treatment, cell viability was detected. The cell viability of the si-TMEM98 group was compared with the NC group, and that of the oe-TMEM98 group was compared with the NC group. The results showed that compared with the NC group, the cell viability in the si-TMEM98 group was increased (Fig. 4A,B), while the cell viability was inhibited in the oe-TMEM98 group (Fig. 4C,D).
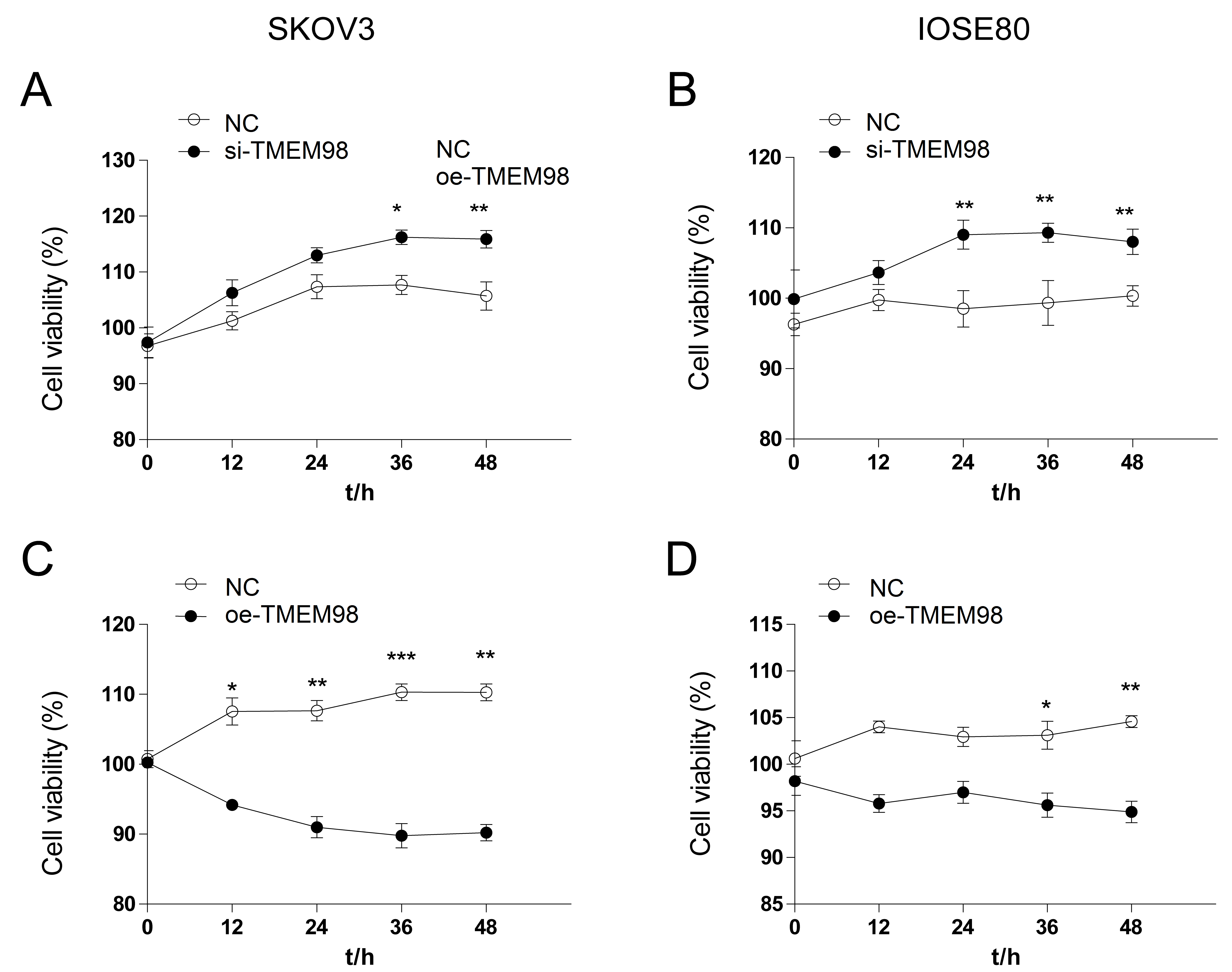 Fig. 4.
Fig. 4.Cell viability by down-regulation and up-regulation of TMEM98.
(A) Compared with the NC group, the cell viability of SKOV3 cells in the
si-TMEM98 group increased significantly. (B) Compared with the NC group, the cell
viability of IOSE80 cells in the si-TMEM98 group increased significantly. (C)
Compared with the NC group, the cell viability of SKOV3 cells in the oe-TMEM98
group decreased significantly. (D) Compared with the NC group, the cell viability
of SKOV3 cells in the oe-TMEM98 group decreased significantly (n = 3,
*p
Moreover, cell healing was observed and the mobility of each group was calculated to investigate the ability of cell migration between si-TMEM98 and oe-TMEM98 groups. The results showed that the migration rate of SKOV3 cells in the si-TMEM98 group was significantly higher than that in the NC group and the ability of cell migration was significantly inhibited in the oe-TMEM98 group compared to the NC group (Fig. 5A,B,E,F). Interestingly, although cell migration ability is a malignant behavior of tumor cells, the cell migration ability of human normal ovarian cell IOSE80 was similar to those of OC cells SKOV3. Compared with the NC group, the cell migration rate of IOSE80 in the si-TMEM98 group was significantly higher and was significantly lower in the oe-TMEM98 group (Fig. 5C,D,G,H). These results indicated that TMEM98 knockdown could promote the development of OC and enhance the migration ability of OC. In addition, in normal ovarian cells, si-TMEM98 may promote the normal ovarian cell to have a trend of OC development, and after overexpression of the TMEM98 could further weaken this ability.
 Fig. 5.
Fig. 5.Cell migration by down-regulation and up-regulation of TMEM98.
(A,E) Test the migration ability of SKOV3 cells by scratch test. (B,F)
Quantitative analysis of the migration rate of SKOV3 cells. (C,G) Test the
migration ability of IOSE80 cells by scratch test. (D,H) Quantitative analysis of
migration rate of IOSE80 cells (n = 3, *p
Furthermore, the Transwell chamber was used to determine the effect of TMEM98 on the invasion ability of SKOV3 cells. The results showed that compared with the NC group, the invasive ability of the si-TMEM98 group was significantly increased, while the invasive ability of the oe-TMEM98 group was significantly inhibited (Fig. 6A,B). These results indicate that knockdown of TMEM98 promotes the invasion ability of SKOV3 cells.
 Fig. 6.
Fig. 6.Invasion ability and vascular mimicry by down-regulation and
up-regulation of TMEM98. (A) The invasion ability of SKOV3 cells was detected by
the Transwell chamber. (B) Quantitative analysis of SKOV3 cell invasion ability
(n = 3, *p
In this study, the vascular mimicry model of OC was established by using a matrix glue simulation environment to investigate the effect of TMEM98 on the angiogenesis of OC cells. The results showed that compared to the NC group, the si-TMEM98 group had significantly increased vascular mimicry, and the cells were closely connected and had small microtubules, which could form a ring grid (Fig. 6C). Opposite results were found in the oe-TMEM98 group (Fig. 6D). The findings indicated that the low TMEM98 could significantly promote the angiogenesis of OC cells.
To further explore the effect of TMEM98 on the cell cycle. The effects of TMEM98 on the cell cycle of SKOV3 and IOSE80 were detected by flow cytometry. In the SKOV3 cells, the results showed that compared with the NC group, the proportion of G2 phase cells in the si-TMEM98 group was significantly increased (Fig. 7A), and the proportion of S phase and G2 phase cells in the oe-TMEM98 group were significantly decreased (Fig. 7C), which indicated that TMEM98 knockdown promoted the cycle progression. In the IOSE80 cells, compared with the NC group, the proportion of S phase and G2 phase cells in the si-TMEM98 group was significantly increased, which promoted the cycle progression (Fig. 7B). The proportion of S phase cells in the oe-TMEM98 group was significantly increased, and the proportion of G2 phase cells was significantly decreased (Fig. 7D), which indicated that overexpression of TMEM98 blocked the progress of IOSE80 from the S phase to the G2 phase. The proliferation index was calculated by using the formula proliferation index and combined with the results of the cell proliferation test, the results showed that TMEM98 knockdown improved the proliferation activity of IOSE80 cells and promoted the cycle progression. Moreover, overexpression of TMEM98 decreased the proliferative activity of SKOV3 cells and blocked the cycle progression (Fig. 7E).
 Fig. 7.
Fig. 7.Cell cycle progression by down-regulation and up-regulation of
TMEM98. (A) The cell cycle of SKOV3 cells in the NC group and si-TMEM98 group.
(B) The cell cycle of IOSE80 cells in the NC group and si-TMEM98 group. (C) The
cell cycle of SKOV3 cells in the NC group and oe-TMEM98 group. (D) The cell cycle
of IOSE80 cells in the NC group and oe-TMEM98 group. (E) Cell proliferation index
of NC group, si-TMEM98 group, and oe-TMEM98 group of SKOV3 cells and IOSE80
cells (n = 3, *p
The above results showed that TMEM98 could inhibit malignant proliferation and cell cycle progression, and enrichment analysis showed that TMEM98 was closely related to apoptosis. To explore whether TMEM98 can inhibit the proliferation of OC cells, we further explored the effect of TMEM98 on apoptosis. In the SKOV3 cells, the results showed that compared with the NC group, the number of early apoptotic cells in the si-TMEM98 group decreased significantly, the number of living cells in the oe-TMEM98 group decreased significantly and the number of early apoptotic and late apoptotic cells increased significantly in the oe-TMEM98 group. The results of IOSE80 cells showed that compared with the NC group, the number of late apoptotic cells in the si-TMEM98 group was significantly decreased, the number of living cells in the oe-TMEM98 group was significantly decreased, and the number of late apoptotic cells was significantly increased (Fig. 8A–H). The results showed that the knockdown of TMEM98 could inhibit the apoptosis of SKOV3 and IOSE80 cells. Similar results were found using the TUNEL staining in the SKOV3 cells (Fig. 8I,J).
 Fig. 8.
Fig. 8.Apoptosis status by down-regulation and up-regulation
of TMEM98. (A) Flow cytometry for apoptosis of SKOV3 cells. (B) The percentage
of viable SKOV3 cells in the NC group, si-TMEM98 group, and oe-TMEM98 group. (C)
The percentage of early apoptotic SKOV3 cells in the NC group, si -MEM98 group,
and oe-TMEM98. (D) The percentage of late apoptotic SKOV3 cells in the NC group,
si-TMEM98 group, and oe-TMEM98 group. (E) Flow cytometry for apoptosis of IOSE80
cells. (F) The percentage of viable IOSE80 cells in the NC group, si-TMEM98
group, and oe-TMEM98 group. (G) The percentage of early apoptotic IOSE80 cells in
the NC group, si-TMEM98 group, and oe-TMEM98 group. (H) The percentage of late
apoptotic IOSE80 cells in the NC group, si-TMEM98 group, and oe-TMEM98 group.
(I,J) TUNEL staining for apoptotic cells in the NC group and si-TMEM98 group (n
= 3, *p
To further explore the effect of TMEM98 on the malignant behavior of OC in vivo, 20 female nude mice were used in this experiment. SKOV3 cells were transfected and treated separately. The cells were collected and inoculated under the skin of nude mice to establish the model of OC heterotopic transplantation. The results showed that there was no tumor in the oe-TMEM98 group. Then the subcutaneous ovarian tumors of the NC group and si-TMEM98 group were taken out and compared with the volume, and then embedded sections were stained with it. The results showed that the volume of subcutaneous tumors in the si-TMEM98 group was significantly larger than that of the NC group at 4 and 8 weeks after inoculation. HE staining showed that there are many vacuoles and no obvious necrotic lesions in the tumor tissue (Fig. 9).
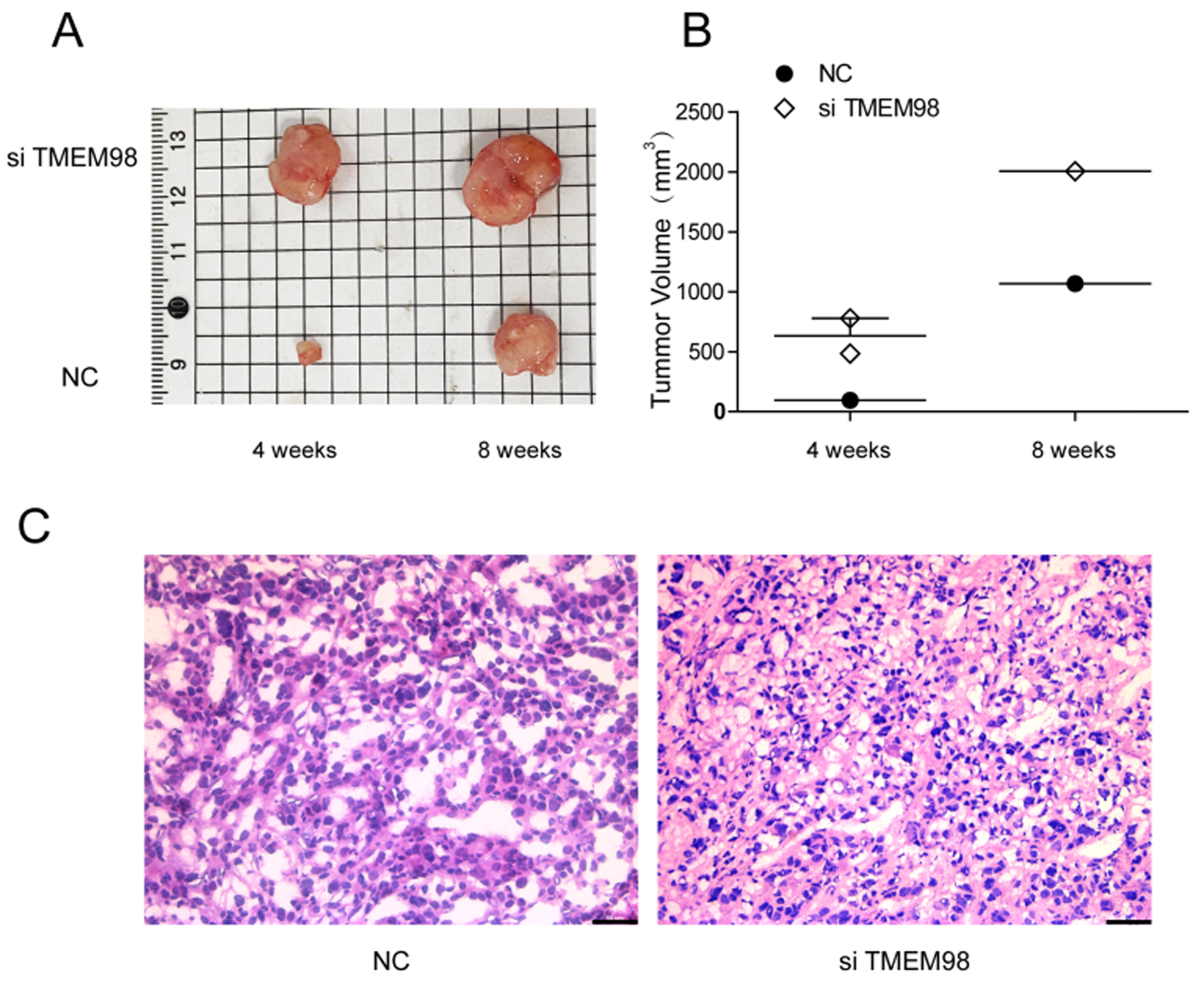 Fig. 9.
Fig. 9.Tumor growth of TMEM98 in OC. (A) The images of tumor injection with si-TMEM98 SKOV3 cells. (B) Knockdown of TMEM98 increased tumor volume. (C) Hematoxylin-eosin staining of tumor sections in NC group and si-TMEM98 group.
According to the above results and enrichment analysis, TMEM98 may inhibit the
development of OC by regulating apoptosis. It has been confirmed that PARP
activity is necessary for repairing single-strand DNA breakage by base excision
and repair. Therefore, when PARP function is inhibited, cell death increases.
PARP inhibitor can inhibit the repair of DNA damage, and then promote the
apoptosis of tumor cells. In addition, PARP is the substrate of most members of
the caspase family, and the shear fracture is considered as the symbol of
apoptosis and is also generally regarded as the indicator of Caspase-3
activation. To further explore the relationship between TMEM98 and apoptosis and
the preliminary study of its pathway, the correlation between the TMEM98 gene and
PARP family was analyzed online by the cbiportal website (Fig. 10). The results
showed that TMEM98 was significantly negatively correlated with PARP family
members, and the most significant correlation was PARP4, PARP9, PARP10, PARP12,
PARP14, and PARP15 (all p
 Fig. 10.
Fig. 10.TMEM98 expression is negatively correlated with PARP
expression. (A) TMEM98 expression was significantly negatively correlated with
PARP4 expression. (B) TMEM98 expression was significantly negatively correlated
with PARP9 expression. (C) TMEM98 expression was significantly negatively
correlated with PARP10 expression. (D) TMEM98 expression was significantly
negatively correlated with PARP12 expression. (E) TMEM98 expression was
significantly negatively correlated with PARP14 expression. (F) TMEM98 expression
was significantly negatively correlated with PARP15 expression (***p
Finally, we investigated the association between the expression level of TMEM98 and OC apoptosis in vivo. The expression of Caspase-3 and Bcl-2 in OC was detected by immunohistochemistry to assess the apoptosis status. The results showed that compared with the NC group, the expression of Caspase-3 was significantly downregulated and the expression of Bcl-2 was significantly increased in the si-TMEM98 group (Fig. 11). These findings suggested that down-regulation of TMEM98 could inhibit the apoptosis of OC cells in vivo and in vitro. Fig. 12 is the summary of this experiment.
 Fig. 11.
Fig. 11. Apoptosis status by down-regulation of TMEM98 in vivo. (A,B) Immunohistochemistry and quantitative analysis of Caspase-3 protein
expression. (C,D) Immunohistochemistry and quantitative analysis of Bcl-2
expression (n = 3, **p
 Fig. 12.
Fig. 12.Summary of this study.
In this study, we found that TMEM98 was a low expression in OC, and a low level of TMEM98 was associated with lower survival outcomes compared to those with a high level of TMEM98. The biological function study showed that low expression of the TMEM98 could promote proliferation, migration, invasion, vasculogenic mimicry, and cell cycle progression in OC cells. In addition, knocking down TMEM98 could inhibit apoptosis. Our study suggests that TMEM98 plays an important role in the development of OC.
In the TNMplot database [11] and the Human Protein Atlas database [12], the gene expression of TMEM98 in normal ovarian tissue is significantly higher than that in various normal human tissues, suggesting that TMEM98 has higher specificity in the human normal ovary. In our study, we found that TMEM98 expression was significantly lower in primary OC than in the normal ovarian tissue. The prognosis in patients with low TMEM98 expression was inferior to those with high TMEM98 expression, which showed that TMEM98 was involved in the development and metastasis of OC. However, TMEM98 was overexpressed in hepatocellular carcinoma and was significantly associated with advanced stage and poor prognosis [16]. In those with cervical adenocarcinoma [17], lung cancer [18], gastric cancer [19], and head and neck carcinoma [20], TMEM98 was also overexpressed in tumors compared to the normal tissues. In the TNMplot database, there were also significant differences in the expression of TMEM98 in different tumors. The above studies suggest that TMEM98 has high heterogeneity in various tumors.
Several studies have confirmed that TMEM98 was related to the differentiation of
T helper 1 cells [15], normal eye development [21], and atherosclerosis [22]. In
previous studies, TMEM98 was associated with the ERK, AKT/GSK3
In this study, we found that low expression of the TMEM98 promotes vasculogenic mimicry in OC cells. Although antiangiogenic therapy can inhibit tumor growth by inhibiting tumor angiogenesis. However, the antiangiogenic drug bevacizumab did not prolong overall survival in patients with OC as a first-line setting [23]. One theory is that antiangiogenic drugs are ineffective because tumors may have other blood supply patterns, known as “vasculogenic mimicry”. Antiangiogenic drug with bevacizumab promotes a hypoxic response and vasculogenic mimicry [24], which may be related to limited efficacy and poor response to antiangiogenic therapy in OC. Angiogenesis mimicry has recently been demonstrated in a variety of human malignancies such as hepatocellular carcinoma [25], gastric cancer [26], glioblastoma [27], breast cancer [28], and head and neck cancers [29]. Vasculogenic mimicry also exists in OC, which is closely related to tumor progression and poor survival outcomes [30, 31]. Therefore, targeting TMEM98 to inhibit vasculogenic mimicry may overcome the limited efficacy of antiangiogenic therapy for OC patients.
Using the KEGG function enrichment analysis, we found that TMEM98 had a closer relationship with apoptosis. Our study also showed that low expression of TMEM98 could inhibit apoptosis of OC in vivo and in vitro. The previous study has shown that PARP activity is necessary for repairing single-strand DNA breakage [32]. Therefore, inhibition of PARP function can lead to apoptosis of homologous recombinant tumor cells [33]. PARP inhibitors are the biggest advance in the treatment of OC in recent years [34]. In addition, PARP overexpression was associated with poor survival outcomes in patients with OC [35]. In our study, we found that TMEM98 was negatively related to the PARP family. PARP is a substrate of caspase, and PARP is the marker of apoptosis initiation by caspase [36]. Therefore, TMEM98 is closely related to the caspase apoptosis pathway. Our in vivo study showed that the expression of Caspase-3 was significantly downregulated and the expression of Bcl-2 was significantly increased in the si-TMEM98 group.
Our study has some limitations. First, we didn’t use the TMEM98 knockout cell line, which is more efficient and persuasive in exploring the role of TMEM98 in OC cell proliferation and apoptosis. Second, although we have proved that TMEM98 is related to the occurrence and development of ovarian cancer and has a tumor suppressor effect on ovarian cancer, the exact pathway is still unclear and requires further validation. Third, we did not conduct further studies on PARP and the role of PARP in TMEM98-dependent regulation of apoptosis is still not clear.
In conclusion, our study demonstrates for the first time that TMEM98 is downregulated in OC, and patients with lower TMEM98 mRNA levels show inferior survival. In addition, our study suggests that tumor-suppressor gene TMEM98 promotes proliferation and inhibits apoptosis of OC.
SGW, JYX, JL, and MH are lead authors who participated in manuscript drafting, tables/figures creation, and manuscript revision. SGW aided in data collection. JZ is the corresponding author who initially developed the concept and drafted and revised the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This work was partly supported by grants from the Commission Young and Middle-aged Talents Training Project of Fujian Health Commission (No. 2019-ZQNB-25 and 2021GGB027) and the Natural Science Foundation of Fujian Province (No. 2020J011240).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
