Academic Editor: Marcello Iriti
Introduction: Boesenbergia rotunda is a famous
culinary/medicinal herb native to Southeast Asia region and it is traditionally
used in the treatment of several diseases. This study investigated the
anti-diabetic properties of Boesenbergia rotunda polyphenol extract
(BRE) in high fructose/streptozotocin-induced diabetic rats. Method: The
in vitro antioxidant activity was evaluated using DPPH and ABST
colorimetric assays, while the Folin-Ciocalteu method was used for the total
phenolic content of BRE. For diabetes induction, a combination of high fructose
solution and streptozotocin was administered to the rats and diabetic rats were
orally administrated with BRE (100 and 400 mg/kg) for 5 weeks. The fasting blood
glucose, body weight gain, food and water consumption were determined during the
treatment period. Results: BRE showed excellent in vitro DPPH
and ABTS scavenging activity with high phenolic content. BRE significantly
lowered fasting blood glucose level, HbA1c, lipid profile, hepatorenal
biochemical parameters and ameliorated the IPGTT in diabetic rats. Additionally,
BRE reversed body weight loss, attenuated food and water intake, serum insulin
level, pancreatic
Diabetes mellitus is a chronic metabolic disease which has evolved over the years as a major global public health issue [1]. The increase in the prevalence of the disease has been largely attributed to globalization, environmental factors as well as changes in lifestyle and behaviours [2]. Typically, diabetes mellitus is principally depicted by excessive blood glucose concentration (hyperglycemia), together with alterations in lipid, carbohydrate and protein metabolism [3, 4]. Out of the three different types of diabetes, type II diabetes mellitus is the most rampant accounting for approximately 90–95% of all global cases [4]. Insulin resistance, pancreatic beta cell dysfunction and insufficient insulin production are the characteristics hallmarks of type II diabetes mellitus [5, 6]. Hyperglycaemia is the major clinical feature in diabetes and it is responsible for the multiple tissue and organ damages associated with the disease. Hyperglycemia through oxidative-inflammatory outburst forms the pathological and physiological basis for all diabetic complications including nephropathy, neuropathy, cardiovascular diseases, retinopathy and Alzheimer’s disease [6, 7].
While significant progress has been made in the development of synthetic antidiabetic drugs, unfortunately adverse side effects as well as the inability of these drugs to impede or slow down the development of diabetic associated comorbidity have necessitated the search for effective antidiabetic alternatives [8, 9]. Medicinal plants have been enormously explored for their health promoting roles and low toxicity especially in the treatment of diabetes and its associated complications. Numerous bioactive components from medical plants have been found as excellent antidiabetic agents through their inhibitory effects on several diabetic related pathways [3, 10]. Besides, medicinal plants possesses excellent antioxidant and anti-inflammatory properties which makes them significantly relevant in tackling key components of diabetes and its complications [3, 11]. For instance, Tiliacora triandra was reported to showed significant attenuative effect against diabetic nephropathy and testicular dysfunction via improving antioxidant defense and alleviating oxidative stress [12]. In addition, several other medicinal plants including Solanum macrocapon Linn, Bridelia ferruginea Benth and Alchemilla mollis have been reported to show protective effects against diabetic induced complication via modulation of oxidative stress and enhancement of antioxidant activities [13, 14, 15].
Boesenbergia rotunda is a medicinal and culinary herb that originated
from Southeast Asia and China. It is called “Krachai” or finger roots
and famously used as a culinary herb in Thai dishes [16]. Traditionally,
B. rotunda is used as an aphrodisiac (Thai ginseng), as well as in the
treatment of ulcer, rheumatic diseases, wound, bacteria inflections, muscle pain
and gastrointestinal disorders [17, 18, 19]. Aside the ethno-pharmacological
properties of B. rotunda, scientific investigations have shown that the
extracts/bioactive compounds from the plant have displayed significant
antimicrobial, wound healing, antioxidant, anti-inflammatory, neuroprotective,
in vitro antidiabetic, antiobesity and anticancer properties [16, 20, 21].
B. rotunda is rich in flavonoids and polyphenolic compounds, notably
flavones, and these compounds have been extensively implicated in the bioactivity
of the plant including its effect on diabetes, diabetic neuropathic pain,
inflammation and obesity [22, 23]. Our earlier studies showed that B.
rotunda ameliorated diabetic peripheral neuropathy [22], additionally, it has
been shown that B. rotunda showed anti-glycation,
B. rotunda roots were purchased from Saburi Medicinal Herbs Store, Hat Yai, Songkla, Thailand in March 2021. After taxonomic authentication (voucher specimen:BR-TT-050), the specimen was kept at the herbarium of Faculty of Thai Medicine, Prince of Songkla University. The dried roots of B. rotunda were pulverized to fine powder and exhaustively macerated with 95% ethanol. The extract was dried using a rotary evaporator and the dried dark brown extract was suspended in distilled water successively extracted with n-hexane and ethyl acetate. The ethyl acetate fraction was subsequently evaporated to dryness and stored in tightly closed glass bottles at 4 °C until further use.
To assess the total phenolic content (TPC) of BRE, 20 mg of BRE was solubilized
in 10 mL of DMSO and 25
The DPPH working solution was prepared by diluting the DPPH stock solution (50
% DPPH inhibition = [(Ac-As)/Ac]
Where: Ac = absorbance of control
As = absorbance of sample solution
The DPPH radical scavenging capacity of BrE was expressed as IC
ABTS (2,2-azino-di-3-ethylbenzothialozine-sulphonic acid) was employed for the
determination of ABTS radical scavenging assay. ABTS stock solution (7 mM) was
prepared through the reaction of 7mM ABTS and 2.45 mM of potassium persulphate as
the oxidant agent (1:1 v/v). The working solution of ABTS+
The male Sprague-Dawley rats used in this study were six weeks old with body
weight between 140–180 g. The animals were accommodated in stainless steel cages
(6 rats/cage) in a temperature, relative humidity and light/dark cycle controlled
room (22
After acclimatization, the rats were equally allotted into four groups: normal control (NOR-C) and diabetic model group (DIAB-C), both groups were treated with 5% DMSO, and two BRE intervention groups at 100 and 400 mg/kg (DIAB-BRE-100 and DIAB-BRE-400, respectively). Prior to the induction of diabetes, the rats in the diabetic model and BRE intervention groups were given 30% fructose solution for 4 weeks, while the counterparts in the normal control group received normal water. Thereafter, the all the rats were fasted overnight and the rats fed with 30% fructose received an intraperitoneal injection of streptozotocin (STZ, 35 mg/kg, solubilized in 0.1 M citrate buffer; pH 4.5). The normal rats were also injected with citrate buffer solution. The fasting blood glucose concentration of all the rats was determined three days after administration of STZ from the blood taken from the tail end of the rats. All rats with fasting glucose values greater than 250 mg/dL were adjudged as diabetic. After conformation of diabetes, the rats were treated as stated above for 5 weeks, during this period, daily water and food intake was evaluated, while the blood glucose concentration was measured weekly.
At the end of the treatment, intraperitoneal glucose tolerance test was performed by injecting 2 g/kg of glucose solution to all the rats and the blood glucose concentration of each of the rats was periodically evaluated over a time course of 2 hours (0, 30, 60, 90 and 120 mins).
The rats were fasted overnight, euthanized with 150 mg/kg of sodium thiopental and peripheral blood samples were taken through cardiac puncture. Thereafter, all the rats were sacrificed by cervical dislocation. The pancreas and liver were immediately excised, cleaned with physiological saline.
After high speed centrifugation of the blood samples collected, the serum
obtained was used for analysing blood chemistry parameters including liver
function parameters (AST, ALT and ALP), kidney function (BUN, creatinine, uric
acid), lipid profile (HDL, LDL-c, total cholesterol and triglyceride) and
glycated haemoglobin (HbA1c) using automated chemistry analyzer. Fasting serum
insulin levels was also evealuted using ELISA kits. Homeostasis model assessment
insulin resistance (HOMA-IR) was calculated as HOMA-IR = FBG (mmol/L)
The pancreas specimens were homogenized (10% w/v) in 0.1 M PBS (pH 7.4),
centrifuged (3000 rpm for 10 min at 4 °C) and the supernatant collected
was evaluated for the levels of malondialdehyde (MDA), tumor necrosis factor
(TNF-
The liver tissues were also homogenized in 0.1 M Tris-HCl buffer (pH 7.5), centrifuged (3000 rpm for 10 min at 4 °C) and the supernatant obtained was used for the determination of hepatic hexokinase (HK), glucose 6-phosphatase (G6Pase) and fructose 1,6-bisphosphatase (FBP1) content using previous reported methods [24, 25, 26, 27, 28].
A portion of the pancreas harvested from the rats was fixed in 10% neutral buffered formalin solution, embedded using paraffin and sliced into sections. The sections were deparaffinized, mounted on microscope slides, routinely stained with haematoxylin and eosin and visualized under light microscope.
The immunostaining of anti-insulin in the pancreatic tissues was performed on deparaffinized tissue sections. In brief, the pancreatic tissues were incubated in antigen retrieval buffer for 15 min at 95 °C. Thereafter, the sections were incubated with primary anti-insulin overnight at 4 °C. The sections were further incubated with biotinylated secondary antibody for 1 h at room temperature. All tissue sections were visualized under light microscope
Data were compared using one-way ANOVA followed Tukey multiple comparison post
hoc test. All data analyses were performed with GraphPad Prism version 5.0
software (San Diego, CA, USA). p values
The total phenolic content and in vitro antioxidant activity of BRE are
shown in Table 1. BRE showed high content of total phenols of 23.50
| Sample | Total phenolic content (mg GAE/g) | DPPH (IC |
ABTS (IC |
| BRE | 23.50 |
205.97 |
34.97 |
| Ascorbic acid | - | 6.61 |
2.83 |
According to the body weight measurement after the 8th week of treatment, the
average body weight of the animals in the NOR-C group was 435
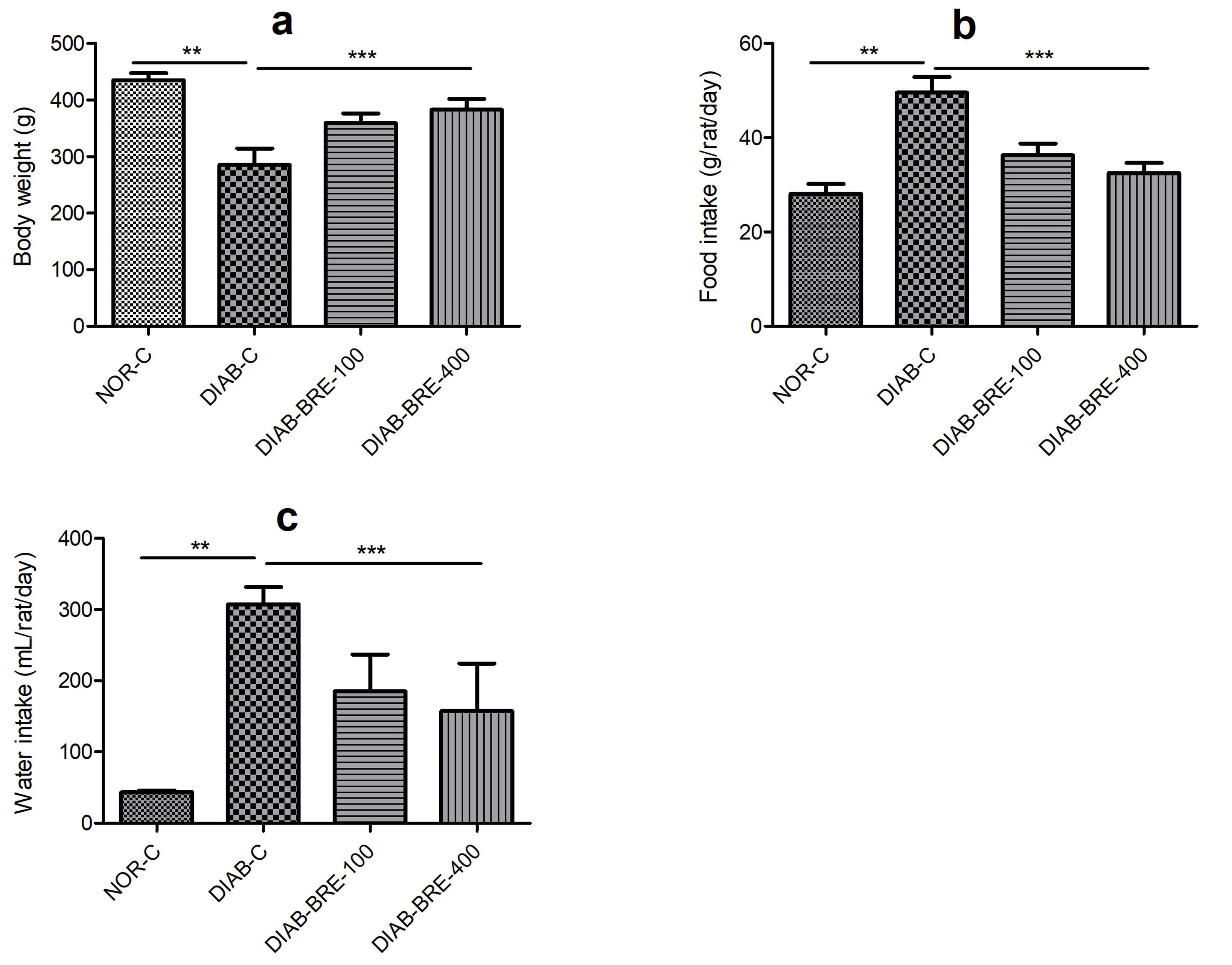 Fig. 1.
Fig. 1.Effect of BRE on (a) body weight (b) food intake (c) water
intake in diabetic rats. Data are displayed as the mean
As indicated in Fig. 1b,c, the results showed that there were significant differences in the food and water consumption of the DIAB-C rats compared to the NOR-C rats. Nevertheless, the increase in water and food intake observed in the DIAB-C group were significantly improved in the rats administered with BRE (Fig. 1b,c).
Compared with NOR-C group, the rats in the DIAB-C group developed severe
hyperglycemia throughout the duration of the study. There was marked increase in
fasting blood glucose concentration of the DIAB-C group 72 h after STZ injection
to the end of the 8th week in comparison to NOR-C rats (p
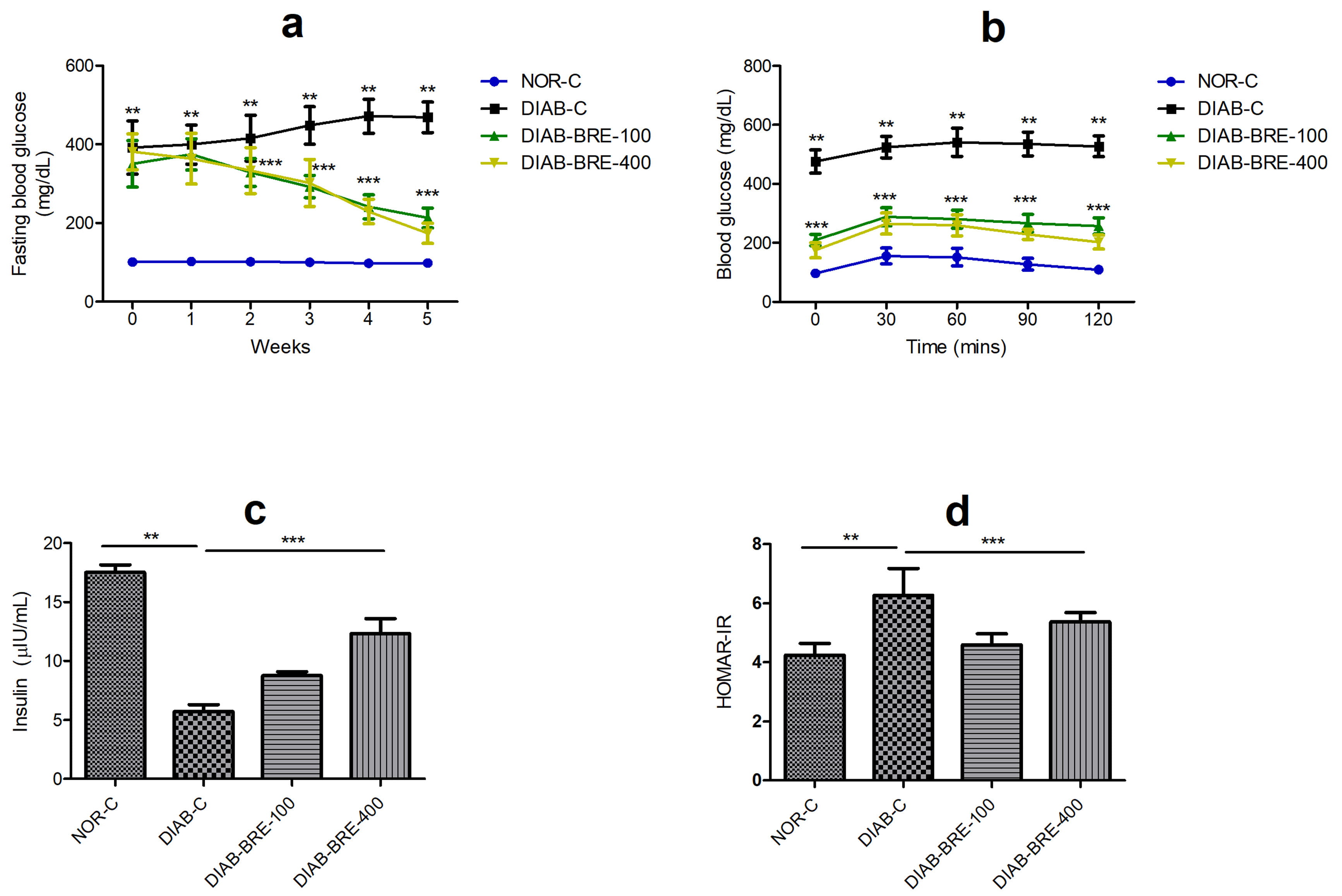 Fig. 2.
Fig. 2.Effect of BRE on (a) fasting blood glucose (b) intraperitoneal
glucose tolerance (c) insulin (d) HOMA-IR in diabetic rats. Data are displayed
as the mean
Additionally, in the IPGTT, it was observed that the DIAB-C group had significantly higher blood glucose level at all the measured time point compared to the corresponding values from the NOR-C rats, while BRE treated rats showed significantly reduced blood glucose concentration from 0–120 mins post glucose administration when compared to the DIAB-C rats (Fig. 2b). The fasting insulin levels (Fig. 2c) in the DIAB-C rats was significantly decreased, while HOMA-IR (Fig. 2d) was notably increased compared to the NOR-C rats. However, the fasting insulin levels of were markedly increased, while HOMA-IR was down-regulated in BRE intervention groups compared to the DIAB-C group (Fig. 2c,d).
The serum levels of biomarkers related to kidney and liver function including
BUN, creatinine, uric acid, AST, ALP and ALT were observably increased in the
DIAB-C rats compared with NOR-C rats (p
| Groups/parameters | NOR-C | DIAB-C | DIAB-BRE-100 | DIAB-BRE-400 |
| Creatinine (mg/dL) | 0.32 |
0.93 |
0.59 |
0.41 |
| Uric acid (mg/dL) | 1.91 |
4.05 |
2.93 |
2.33 |
| BUN (mg/dL) | 21.0 |
59.80 |
42.0 |
31.50 |
| ALP (U/L) | 89.0 |
177.80 |
121.67 |
110.16 |
| ALT (U/L) | 53.83 |
88.50 |
63.0 |
59.5 |
| AST (U/L) | 117.90 |
211.66 |
161.0 |
147.5 |
| Cholesterol (mg/dL) | 78.83 |
162.50 |
110.33 |
89.16 |
| Triglyceride (mg/dL) | 110.0 |
202.60 |
140.50 |
128.30 |
| LDL-c (mg/dL) | 32.67 |
60.0 |
43.17 |
32.67 |
| HDL (mg/dL) | 24.00 |
9.50 |
15.60 |
18.50 |
| Data are shown as mean | ||||
In the DIAB-C group, serum total cholesterol, triglycerides and LDL-c were significantly increased, while HDL was reduced compared with the NOR-C rats (Table 2). Whereas, after treatment with BRE, the total cholesterol, triglycerides and LDL-c were notably reduced while HDL-c was significantly increased in all the treated groups compared to the DIAB-C group (Table 2).
The results shown in Fig. 3 showed histological appearances of the pancreas of
the normal, diabetic and diabetic treated rats. The histopathological appearances
of pancreas of the normal rats appeared normal without any tissue degeneration.
Whereas, the severity of pancreatic degeneration in the DIAB-C group was
highlighted as characterized by loss of acini, inflamed
 Fig. 3.
Fig. 3.Effect of BRE on pancreatic histology in hematoxylin and eosin
staining of diabetic rats. Brown arrow: beta cells. Magnification 200
Regarding the immunohistochemical staining of insulin-secreting islet beta
cells, the results indicated that diabetic rats showed significantly reduced
insulin positive stain when compared to the normal rats that displayed large
insulin positive
 Fig. 4.
Fig. 4.
Effect of BRE on insulin immunostaining (brown colour) in
diabetic rats
As shown in Fig. 5, the activities of HK was significantly decreased, while the activities of FBP1 and G6Pase were markedly increased in the DIAB-C (Fig. 5a–c). Whereas, the treatment of diabetic rats with BRE significantly attenuated the activities of these enzymes compared to the DIAB-C rats.
 Fig. 5.
Fig. 5.Effect of BRE on (a) hexokinase (b) glucose-6 phosphatase (c)
fructose 1,6-bisphosphatae in diabetic rats. Data are displayed as the mean
According to the data shown Fig. 6, the DIAB-C rats displayed marked decrease in pancreatic levels of antioxidants enzymes (SOD, GSH and CAT) compared to the NOR-C rats (Fig. 6a–c). After 5 weeks of BRE supplementation, the levels of these antioxidant enzymes were significantly enhanced compared to the DIAB-C group (Fig. 6a–c). A similar but opposite trend was observed for the lipid peroxidation (MDA), the DIAB-C group showed significantly elevated MDA level when juxtaposed to the NOR-C rats, while treatment with BRE markedly reduced pancreatic MDA level (Fig. 6d).
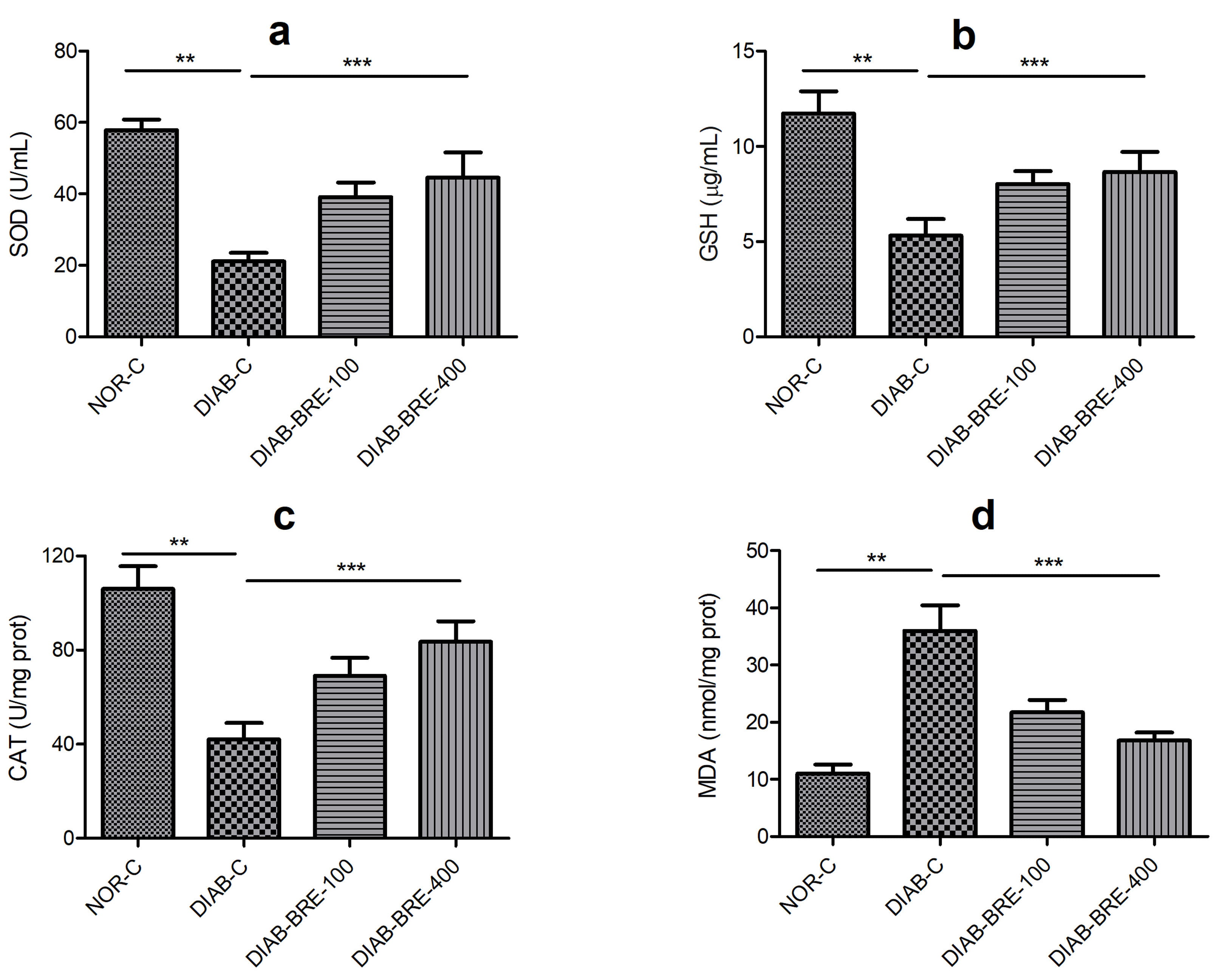 Fig. 6.
Fig. 6.Effect of BRE on pancreatic (a) superoxide dismutase (b)
glutathione (c) catalase (d) malonaldehyde in diabetic rats. Data are displayed
as the mean
There were significant increases in pancreatic TNF-
 Fig. 7.
Fig. 7.Effect of BRE on pancreatic (a) tumor necrosis factor alpha (b)
interleukin 1 beta in diabetic rats. Data are displayed as the mean
In recent years, the use of natural products for the treatment of diabetes has attracted a lot of attention, due to the huge financial burden associated with the use of synthetic antidiabetic drugs as well as the increasing number of complications associated with diabetes among several other reasons [12, 29]. Thus, this study investigated the antidiabetic effects of the polyphenol rich fraction of B. rotunda in high fructose/streptozotocin induced diabetic rats.
High sugar/fat and streptozotocin have been extensively used as one of the most
preferred choice for inducing type 2 diabetes mellitus due to similarities to the
pathogenesis of type 2 diabetes in humans. High fat/sugar diet can promote the
development of insulin resistance and hyperinsulinemia, while a low-dose of STZ
results in modest impairment in insulin secretion, which bears close resemblance
to human type 2 diabetes. Thus the combination of these two approaches mimics the
phenotype and pathogenesis of type 2 diabetes in humans [30, 31]. STZ, an
antibiotic from Streptomyces achromogenes causes
Diabetes has been shown to be a major contributor to the high prevalence of cardiovascular disease. In fact, cardiovascular disease is one of the main cause of mortality and morbidity in diabetic patients, as diabetic patients have higher risk of cardiovascular disease than non-diabetic people [41]. Diabetic dyslipidemia characterised by increased lipids profiles including triglyceride, total cholesterol, low density lipoprotein cholesterol and low HDL are markers of endothelial dysfunction and cardiovascular risk in diabetes [4, 5, 42, 43]. Dyslipidemia in diabetes occurs due to hyperglycemia and insulin impairment resulting in reduced efficiency of lipase enzyme culminating into lipolysis, lipotoxicity and hyperlipidemia [3, 4]. The elevated lipid profiles (TC, TG and LDL-C) in this study was remarkably reduced by BRE in the treated diabetic rats, which is consistent with the previous reports that Boesenbergia rotunda regulated the lipid metabolism [23].
Diabetes has been extensively reported to cause hepatorenal toxicity characterized by increased serum levels of liver and kidney function enzymes including ALT, AST, ALP, BUN, creatinine and uric acid [4, 44]. Hepatorenal cell necrosis induced by systemic inflammation leads to the release of these enzymes into the blood stream [40]. Treatment of diabetic rats with BRE significant reduced hepatorenal toxicity, as evidenced by attenuated serum levels of ALP, ALT, AST, BUN, creatinine and uric acid in the treated rats. These results suggested that BRE possesses hepatorenal protective effect in diabetes.
The results from this study indicated that BRE improved chronic inflammation and
oxidative stress. Insulin resistance and persistent hyperglycemia results in
excessive production of reactive radicals (ROS and RNS) which promotes oxidative
stress as well as alterations in the lipids and protein. The effects of these
oxidative cytotoxic events can initiate glycoxidation, lipid peroxidation,
inflammation and oxidative damages [33, 45]. The vicious cycle between oxidative
stress triggered increase in inflammatory cytokines and vice versa in diabetes is
majorly responsible for most secondary complications associated with diabetes
[3, 33]. Accumulating studies have shown that diabetes is associated with reduced
antioxidant enzyme capacities, increased lipid peroxidation and proinflammatory
cytokines [5, 12, 44]. In line with these previous studies, treatment with BRE
significantly restored pancreatic SOD, GSH, CAT and reduced MDA, TNF-
Previous studies have suggested that insulin deficiency is associated with increased HOMA-IR, which correlated with altered pancreatic histopathological observation including reduced islet mass and number, hypocellular cells and congested blood capillaries which could be an explanation for the significant reduction in serum insulin level of the untreated diabetic rats [4, 46, 47]. Furthermore, immunohistochemical images showed that the untreated diabetic rats had significantly reduced area of insulin immunoreactivity, which further suggests impaired insulin secretion and beta-cell function owing to loss of beta-cell mass and function in the untreated diabetic animals. BRE treated animals showed significant pancreatic tissue regeneration and protective effects on pancreatic islet function.
Diabetes have been shown to significantly increase glycogenolysis, gluconeogenesis and glycolysis due to excessive hepatic glucose production. The liver plays a vital role in maintaining glucose homeostasis and the activation of key carbohydrate metabolising enzymes involved in these pathways, resulting in continuous glucose production, thus aggravating hyperglycemia [48, 49]. Furthermore, insulin insufficiency/resistance stimulates these enzymes, resulting in carbohydrate metabolism dysfunction and hyperglycemia, since insulin physiologically impedes gluconeogenesis and glycogenolysis via the alteration of post translational changes in the gene expression of these enzymes [50, 51]. In addition, the bye-products of glycolysis including protein kinase C and AGEs have been associated to several diabetic complications [52]. Hyperglycemia is mainly driven by excessive glucose generation through gluconeogenesis and glycogenolysis. Glucose-6-phosphatase is the main enzyme responsible for controlling gluconeogenesis, thus limiting glucose outflow while frucose-1,6-bisphosphatase regulates gluconeogenesis by catalysing the irreversible stage of gluconeogenesis [53, 54]. The untreated diabetic rats displayed increased levels of glucose-6-phonphatase and fructose-1,6-bisphosphatase in the hepatic tissues, suggesting glycogenolysis and gluconeogenesis, which corresponds with high blood glucose level and low insulin secretion in the untreated diabetic rats. Treatment with BRE significantly reduced the activities of these enzymes which also corresponds with decrease in blood glucose and increase in insulin levels in the treated rats.
Phenolic compounds have been extensively reported as excellent antioxidant,
antidiabetic, antiglycation and anti-inflammatory secondary metabolites that are
abundantly present in medicinal plants [10, 55, 56]. Previous reports have
indicated that BRE is a remarkable reservoir of polyphenolic compounds, primarily
flavanones, flavones, prenylated flavonoids and chalcones [16, 57]. In agreement
with these previous studies, we identified and isolated six flavonoids including
pinocembrin, boesenbergin A, panduratin A, pinostrobin, cardamonin and alpinetin
from BRE. The details regarding the isolation of these compounds was extensively
reported in our previous study [22]. Regarding the potentials of BRE polyphenols
in the treatment of diabetes, Chatsumpun et al. reported the ability of
panduratin A, isopanduratin A and hydroxypanduratin A to inhibit
In conclusion, the study revealed that Boesenbergia rotunda extract
(BRE) displayed antidiabetic, antihyperlipidemic, antioxidant and
anti-inflammatory effects in fructose-streptozotocin induced diabetic rats.
Treatment with BrE led to significant reduction in blood glucose level, serum
lipid profiles (LDL-c, TC and TG), serum hepatorenal parameters (BUN, creatinine,
uric acid, AST, ALP and ALT), enzymes involved in carbohydrate metabolism
(glucose 6-phosphatase and fructose 1,6 biphosphatase), and increased serum
insulin levels. Furthermore, BrE administration increased pancreatic antioxidant
enzyme activity (SOD, GSH and CAT), decreased pancreatic inflammatory markers
(TNF-
TW and OJO conceived and designed this study; TW, CL and OJO performed the experiments; SS and QZ analyzed the data; TW, CL, SS and QZ interpreted the results of the experiments; OJO wrote and revised the manuscript. All authors read and approved the final manuscript.
The animal experiment received approval from the Ethics Committee of Anhui Medical University (AHYKDX-LLWYH-2021-1008).
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
