Academic Editors: Giuseppe Ingravallo and Graham Pawelec
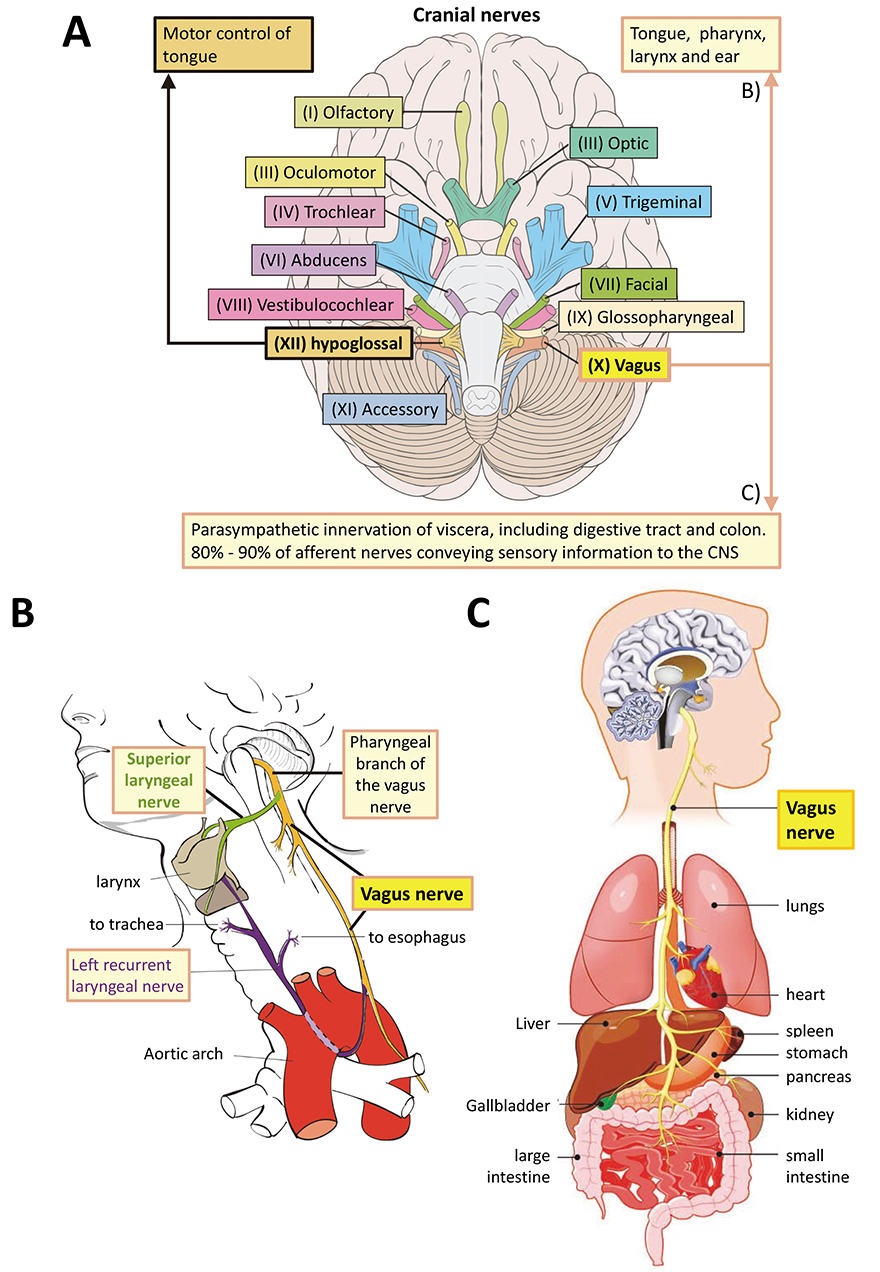
The title usage of Latin Quo vadis ‘where are you going’ extends the question Unde venisti from where ‘did you come?’ posed in the accompanying paper and extends consideration of how ancient eukaryotic and eumetazoan functions of progesterone receptor membrane component (PGRMC) proteins (PGRMC1 and PGRMC2 in mammals) could influence modern human health and disease. This paper attempts to extrapolate to modern biology in terms of extensions of hypothetical ancestral functional states from early eukaryotes and the last eumetazoan common ancestor (LEUMCA), to relativize human metabolic physiology and disease. As novel cell types and functional specializations appeared in bilaterian animals, PGRMC functions are hypothesized to have continued to be part of the toolkit used to develop new cell types and manage increasingly complex tasks such as nerve-gut-microbiome neuronal and hormonal communication. A critical role of PGRMC (as one component of a new eumetazoan genetic machinery) is proposed in LEUMCA endocrinology, neurogenesis, and nerve-gut communication with possible involvement in circadian nicotinamide adenine dinucleotide synthesis. This model would explain the contribution of PGRMC to metabolic and differentiation/behavioral changes observed in age-related diseases like diabetes, cancer and perhaps aging itself. Consistent with proposed key regulation of neurogenesis in the LEUMCA, it is argued that Alzheimer’s disease is the modern pathology that most closely reflects the suite of functions related to PGRMC biology, with the ‘usual suspect’ pathologies possibly being downstream of PGRMC1. Hopefully, these thoughts help to signpost directions for future research.