†These authors contributed equally.
Academic Editor: Fazle Elahi
Background: Systemic lupus erythematosus (SLE) is a chronic multisystem
autoimmune disorder affecting almost any organ system without effective
treatment. Based on accumulating evidence, activated T cells are key cause
promoting the pathogenesis of SLE. A traditional clinic Langchuangding formula
(LCD) is an effective clinical traditional Chinese medicine prescription for SLE
with few side effects and good patient compliance. However, the mechanism of how
LCD affects SLE remains unclear. Methods: Targets related to LCD and SLE
were predicted and overlapped to construct protein-protein interaction (PPI) for
screening core target. Subsequently, flow cytometry analysis and Western-blot
method were used to verify the expression levels of target gene in LCD serum
treated-Jurkat T cells. The main compounds of LCD were identified by HPLC-MS and
further docked with the core targe. Results: 283 protein targets in LCD,
1498 SLE targets and 150 common targets were obtained to construct
protein-protein interaction (PPI). Network pharmacology results suggested that
LCD was closely related to CASP3 target. To verify the prediction of
pharmacological mechanism of LCD treatment for SLE, we investigated the
anti-proliferative effects of LCD-treated rat serum on
Systemic lupus erythaematosus (SLE) is a multisystem autoimmune disease that involves the skin, joints, tendons, kidneys and various other organs. It is characterized by overproduction of autoantibodies, immune complex deposition and activated T cells, causing excessive inflammation and damage [1, 2]. Uncontrolled T-cell activation plays key roles in the pathogenesis of SLE by promoting pro-inflammatory cytokine release and impairing the clearance of apoptotic cells [3, 4, 5]. The course of SLE is variable and unpredictable. Most patients with SLE require long-term treatment with glucocorticoids and immune-modulators. However, serious side effects occur, including central obesity, moon face, and buffalo hump [6, 7, 8]. Despite great progress in medical technology, the treatment of SLE is still a problem to be solved [9]. Traditional Chinese medicine (TCM) is a key component of complementary and alternative medicine that has been in China over a few millennia and has been increasingly integrated into conventional treatments, particularly for chronic autoimmune diseases such as SLE [10, 11].
Based on the basic theory of TCM, our team proposed the Langchuangding prescription (LCD) for SLE treatment [12, 13]. LCD consists of Cimicifugae Rhizoma, Paeoniae Radix rubra, Rehmanniae Radix, Trionycis Carapax, etc. As shown in our previous study, LCD, an effective prescription, exerts definite effects on treating SLE with fewer side effects and good patient compliance than conventional drugs such as corticosteroids and immune inhibitors [13]. However, the underlying therapeutic mechanism of LCD in treating SLE remains unclear.
Network pharmacology is a promising approach to explore the mechanism TCM formulae, which could provide a potential “network target” on a global level [14, 15]. It also could reveal the complicated relationship between herbs and diseases by constructing protein-protein interaction (PPI) networks [16, 17]. However, the accuracy of the network pharmacology analysis results remains limited due to lack of unified standards. Due to the multi-components and multi-targets of TCM, we should integrate multiple systems such as high-performance liquid chromatography tandem mass spectrometry (HPLC-MS) and molecular docking to investigate the interactions of targets and its active ingredients. Moreover, in vitro or vivo experiments were performed to validate the results [14, 18].
Here, in the present study, we predicted the potential mechanism of LCD treating
SLE based on network pharmacology. The target of LCD for treating SLE was further
predicted integrating HPLC-MS with molecular docking. To verify the target of LCD
in the treatment of SLE, we prepared different doses of LCD-treated rat serum and
then investigated their effects on activated Jurkat T cells by
HPLC-grade acetonitrile (CH3CN) and methanol were purchased from Sigma-Aldrich (St. Louis, MO, USA), while analytical grade formic acid was obtained from Huadong Chemical Reagent Co. Ltd. (Hangzhou, China). Deionized water was purified using a Milli-Q system (Millipore, Bedford, MA, USA). Other reagents were of analytical grade.
Ferulic acid (LOT#110773-200611, purity greater than 99.0%) and isoferulic
acid (LOT#11698-200602, purity greater than 99.0%) were purchased from the
National Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China). Antibodies against Caspase-3 (CASP3) and
LCD is composed of several medicinal herbs (Table 1) that were identified by Kong-long Chen and obtained from Medical Pieces Co., Ltd., Zhejiang Chinese Medical University (Hangzhou, Zhejiang, China). All herbs were soaked in distilled water for 1 h and extracted twice with distilled water under reflux for 2 h. The filtered extracts were concentrated to a condensed LCD decoction using a rotary evaporator at 50 °C and stored at 4 °C until use.
| Herb | Part used | Dosage used |
| Radix Rehmanniae (Shu Di Huang) | Root | 15 g |
| Trionycis carapax (Zhi Bie Jia) | Turtle | 12 g |
| Herba Artemisia annua (Qing Hao) | Herb | 12 g |
| Herba Hedyotis Diffusae (She Cao) | Herb | 15 g |
| Radix Paeoniae rubra (Chi Shao) | Root | 15 g |
| Herba Centellae asiaticae (Ji Xue Cao) | Herb | 12 g |
| Semen Coicis (Mi Ren) | Seed | 15 g |
| Citri sarcodactylis (Fo Shou) fructus | Fruit | 9 g |
| Rhizoma Cimicifugae (Sheng Ma) | Rhizome | 9 g |
| Radix Glycyrrhizae (Gan Cao) | Root | 6 g |
Potential targets from LCD and related SLE disease were obtained from 5 kinds of database such as TCMSP (http://www.tcmsp-e.com), Genecards (https://www.genecards.org/) OMIM, TTD and Drugbank. We further used human protein-protein STRING database (https://www.string-db.org/) to construct the protein-protein interaction (PPI) networks of LCD and SLE and visualized using Cytoscape 3.7.1 (Oracle, Austin, TX, USA). The key targets were input into Webgestalt website (http://www.webgestalt.org) for the enrichment analysis of Kyoto Encyclopedia of Genes and Genomics (KEGG) pathways.
Animal treatment: Male Sprague-Dawley rats (300
LCD serum sample preparation: Blood was collected from the orbital vein of each
rat on the 3rd day 1 h after the last drug administration, allowed to clot for
approximately 45 min at room temperature and then centrifuged at 3000 rpm for 10
min. The obtained LCD serum samples were prepared for analyses as follows: 400
We integrated HPLC-MS analysis with molecular docking to further validate the
interactions between CASP3 target and LCD. Different doses of LCD treated serum
were prepared and identified with HPLC/MS analysis. LCD treated-rat serum were
identified using an ion trap mass spectrometer equipped with an electrospray
ionization (ESI) source (Thermo Fisher, USA) under the control of Xcalibur
software (version 1.4, Thermo Fisher, USA) [16]. Chromatographic separation was performed using
reverse-phase HPLC on a Dionex C18 column (4.6
The HPLC eluents were analyzed with a mass spectrometer (Bruker, MA, USA) connected to an Ultimate 3000 HPLC system (Thermo Fisher Scientific, Wilmington, DE, USA) via an ESI interface. High purity nitrogen was used as the nebulizer and auxiliary gas, and argon was the collision gas. The Q-TOF-MS was operated in positive ion mode with a spray voltage of 4.5 KV and a collision-induced dissociation voltage of 25 V.
To further predict the possible component-target interaction, we used molecular docking simulation in this study. Structure information of the components was obtained from the NBCI PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The three dimensional (3D) structures were optimized by Chem3D (ChemDraw Professional software 17.0, Cambridge, USA) and saved in MOL2 format. We obtained the 3D structure of protein target from the RCSB Protein Data Bank (PDB, https://www.rcsb.org/) [19] in PDB format and then used PyMOL software (Schrodinger, Inc., New York, USA) to remove water and separate ligand from the 3D protein structure. Finally, PDB format was saved as PDBQT files, further select Grid Box’s range in AutoDock software and run Autodock vina (http://vina.scripps.edu/) for virtual molecular docking [20].
Jurkat T cell viability was evaluated using a CCK-8 assay in triplicate [21, 22]. Briefly, cells (2
Jurkat cells (2
Expression levels of the CASP3 mRNA in T cells were analysed using quantitative
real-time PCR (RT–PCR). Briefly, Jurkat cells (1
Expression levels of the CASP3 protein were measured using western blot
analysis. Briefly, Jurkat cells (1
The results are presented as the means
To screen the potential targets of LCD treating SLE, we used network
pharmacology technology. All components of LCD and the related potential targets
were collected from TCMSP database as shown in Supplementary Tables 1,2.
283 potential targets of LCD, 1498 SLE related targets and 150 common targets
were obtained from 5 kinds of database using network-based method shown in Fig. 1a. We further constructed PPI networks with 150 overlapping targets between
targets obtained from LCD effective components and SLE disease targets. The
targets were selected (combine score
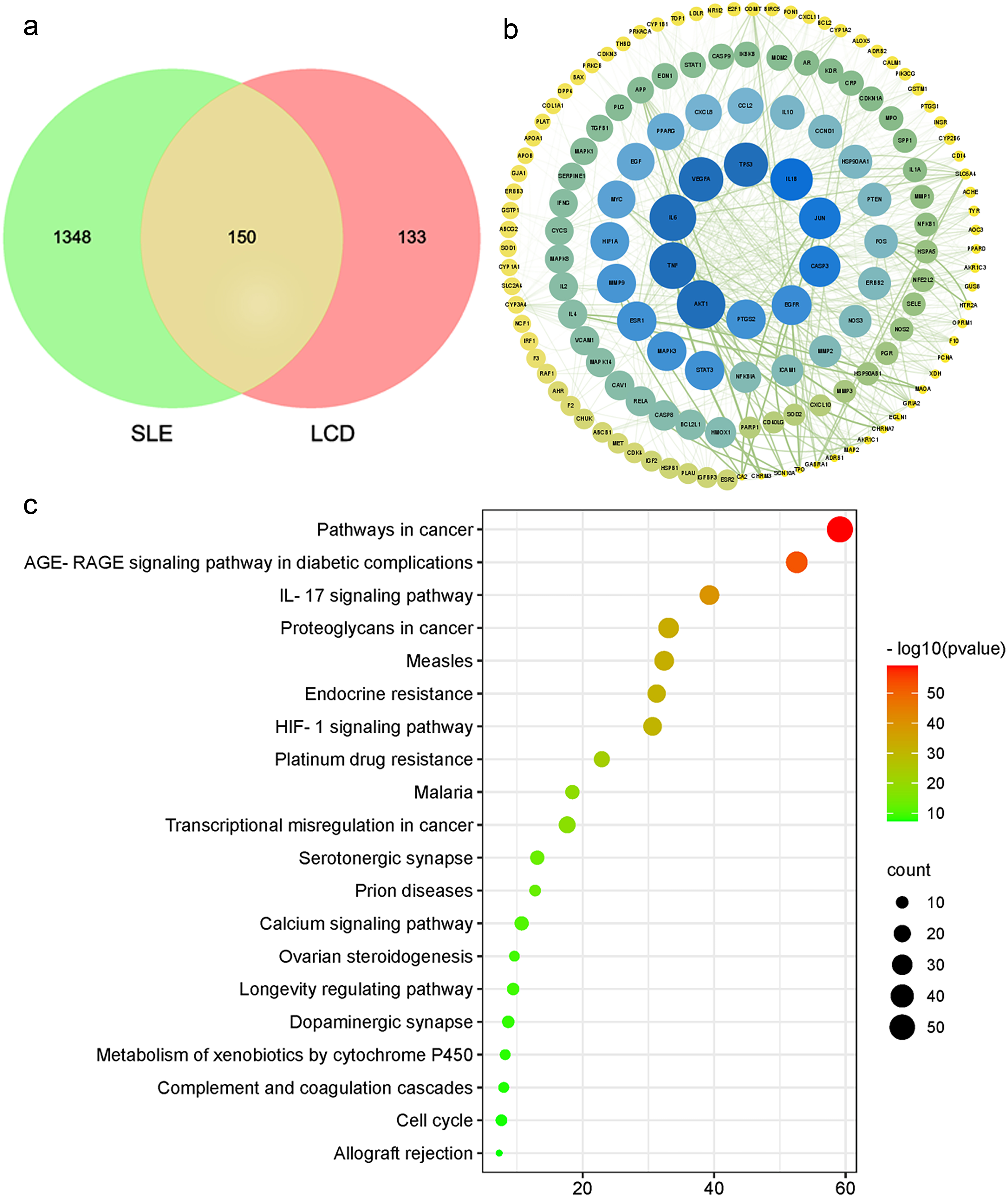 Fig. 1.
Fig. 1.Protein interactions network and KEGG pathway enrichment analysis between LCD component targets and SLE related targets. (a) Veen diagram. (b) PPI network. (c) The KEGG pathway enrichment analysis of the key targets.
To further predict CASP3 target of LCD treating SLE, we integrated HPLC-MS
analysis with molecular docking. Different doses of LCD treated serum were
prepared and identified with HPLC/MS analysis. According to the conditions
described above, two components of ferulic acid (Fig. 2a) and isoferulic acid
(Fig. 2b) in LCD serum were tentatively identified and showed better sensitivity
in the positive ESI-MS analysis (Supplementary Table 4), which yielded
protonated molecules at m/z 195. Based on comparisons of blank serum spiked
reference standards (Fig. 2c) and LCD treated serum samples (Fig. 2d), we further
performed a quantitative analysis including accuracy and precision. The
linear-regression line of standards was shown in Table 2. Under the analytical
conditions, the results of accuracy, intra-day precision and inter-day precision
were shown in Supplementary Table 5. The specificity of the method was
further supported based on the comparisons of chromatograms, retention times and
reference standards. Based on the linear regression curves of standards, the
contents of ferulic acid in high, middle and low doses of LCD serum (H, M and L)
were 10.18
 Fig. 2.
Fig. 2.Positive MS
| Standard compounds | Linear range ( |
Regression equation | Correlation coefficient |
| Ferulic acid | 0.15 |
y = 373895.4453x – 21277.8268 | 0.9999 |
| Isoferulic acid | 0.25 |
y = 31949.5905x – 87446.3771 | 0.9994 |
Molecular docking combined with molecular dynamics simulation were employed to reveal the detailed interactions between main compounds and potential protein targets. The potential target CASP3 with high degree and its main compounds of ferulic acid and isoferulic acid were selected for molecular docking based on the above results. Binding energies between CASP3 and compounds of ferulic acid and isoferulic acid were calculated in AUTODOCK vina. The binding energy less than zero mean spontaneous binding of targets and compounds. Lower docking energy indicating a stronger binding affinity and higher docking score. The lower the energy is, the binding is stable. It is generally accepted that the binding energy less than –5.0 kcal/mol was selected as the screening criteria. As shown in Fig. 3, ferulic acid (Fig. 3a) and isoferulic acid (Fig. 3b) buried in the binding pocket of the CASP3 target protein formed by amino-acid residues based on the hydrogen and hydrophobic interactions. Both compounds of ferulic acid and isoferulic acid bind to CASP3, but their potency were different, despite their similar structures. Three key amino acids, ARG286, GLU240 and GLU324 were found in the CASP3 (PDBID:1RHJ) - ferulic acid complex. Four key amino acids, ARG179, ARG341, GLN283 and SER236 were found in the CASP3 (PDBID:1RHU) - isoferulic acid complex. The binding energies of ferulic acid and isoferulic acid compounds with CASP3 were –5.8 kcal/mol and –5.5 kcal/mol, respectively, which showed good docking affinity. The results suggested that ferulic acid and isoferulic acid exhibited certain activity and CASP3 could be considered as an effective target of LCD in the treatment of SLE. LCD might exert protective effects against SLE through inducing CASP3 activities.
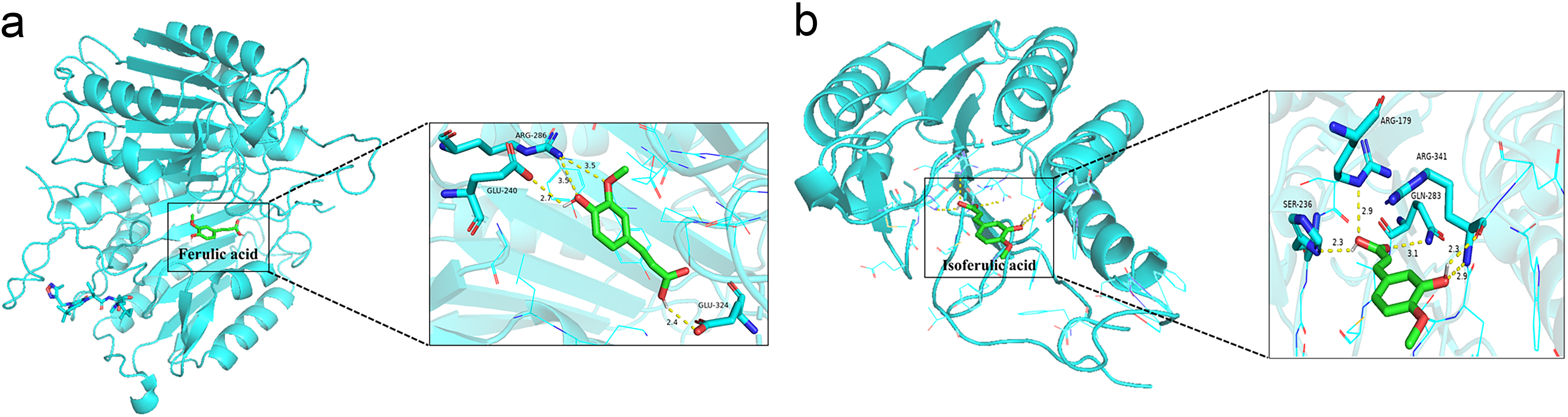 Fig. 3.
Fig. 3.The molecular docking of CASP3 target with components of ferulic acid and isoferulic acid. (a) Ferulic acid–CASP3. (b) Isoferulic acid-CASP3.
We activated Jurkat T cells with
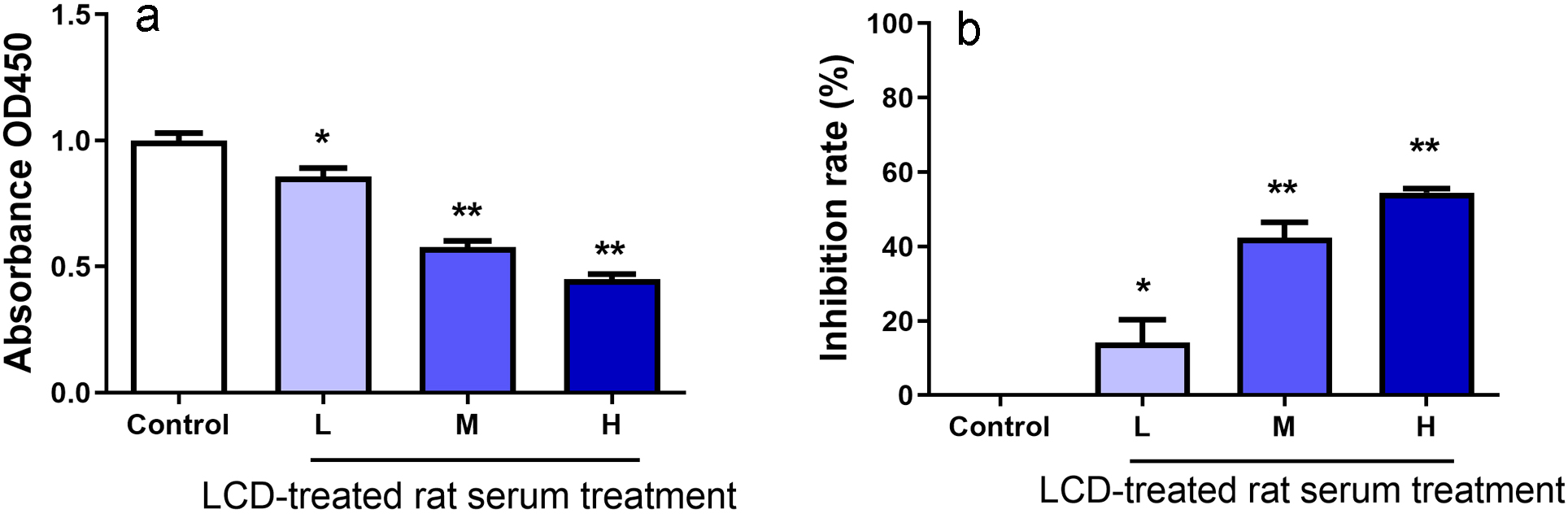 Fig. 4.
Fig. 4.Effects of LCD-treated rat serum on the inhibition rate of Jurkat T cells determined using the CCK-8 assay. Absorbance at OD450 (a) and T cell inhibition rate after treatment with different doses of LCD serum (H, M and L) (b). Values are presented as the mean
To explore whether LCD serum induced apoptosis in Jurkat T cells, we stained and
detected apoptotic cells with annexin V-FITC and PI. The percentage of apoptotic
cells was quantified using flow cytometry and an annexin V/PI Apoptosis Assay
Kit. Very few apoptotic cells were observed in the untreated control group (Fig. 5a). In contrast, the percentage of apoptotic cells increased gradually by 6.8%,
16.5% and 26.8% (Fig. 5b–d) after treatment with different doses of LCD serum
(L, M and H) along with
 Fig. 5.
Fig. 5.Representative flow cytometry profile of Jurkat T cells treated
without (a) or with low (b), middle (c) and high (d) doses of LCD-treated rat
serum along with
The early and late apoptosis rates increased gradually after treatment with low, middle and high doses of LCD serum (Supplementary Fig. 1). The increased apoptotic rates may be associated with LCD-induced apoptosis. The annexin V/PI double staining assay confirmed that LCD induces apoptosis in Jurkat T cells by initiating the apoptotic cascade.
We detected the expression of one important apoptosis-related protein, CASP3,
using western blot analysis to study the potential mechanism by which LCD induced
apoptosis. CASP3 is one of the executioner caspases activated by proteolytic
cleavage during apoptosis. The levels of
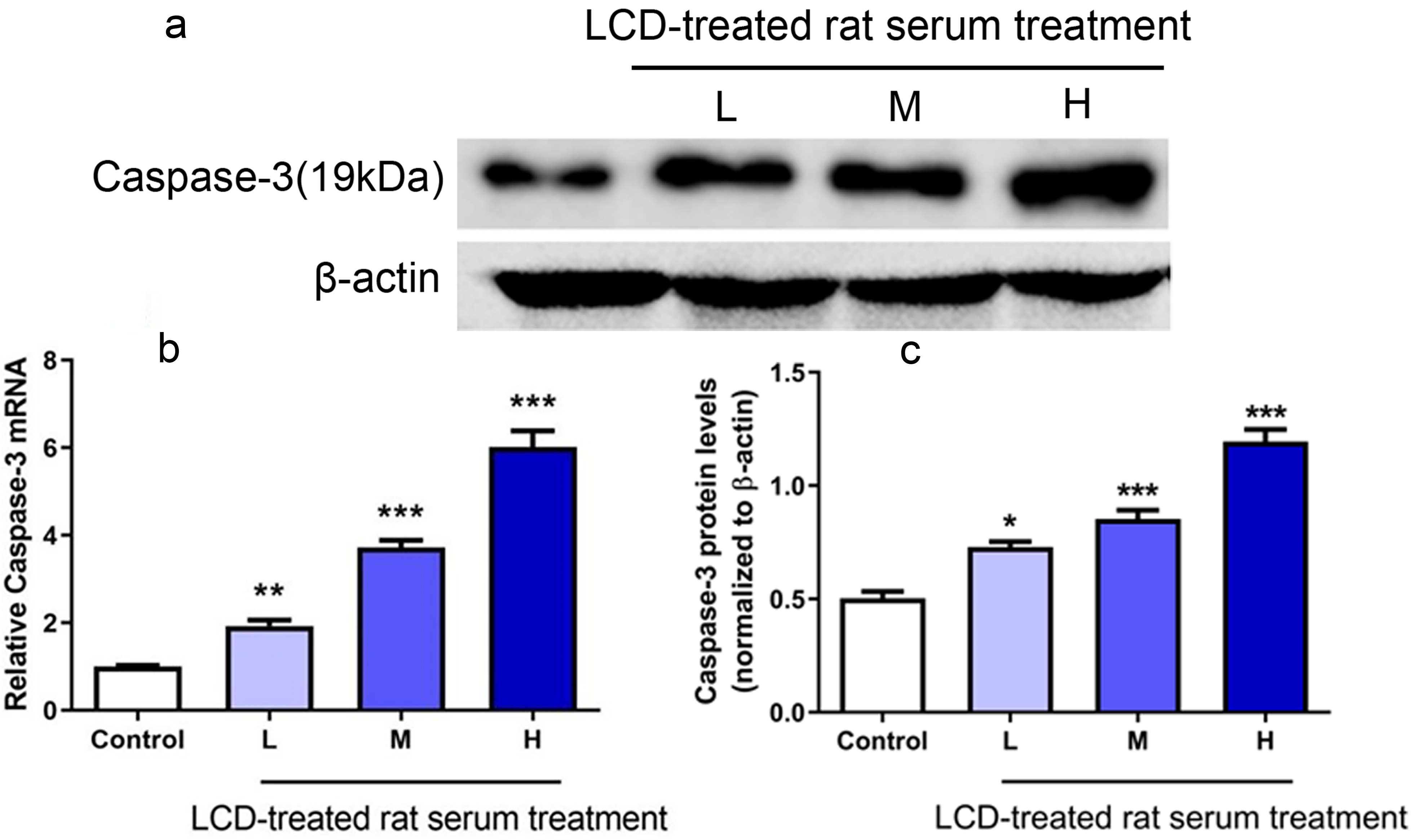 Fig. 6.
Fig. 6.Effects of LCD-treated rat serum on the caspase-3 (CASP3) mRNA
and protein expression levels in Jurkat T cells. Cells were seeded in 6-well
plates and treated with different doses of LCD serum (H, M and L) along with
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease characterized by uncontrolled T cell activation, resulting in damage to multiple organs or systems [5, 24, 25]. Glucocorticoids are the main drug treatment for SLE and effectively promote the apoptosis of lymphocytes [26, 27]. However, many serious effects and high recurrence rates are the main difficulties encountered in the clinic [6, 8, 28]. LCD, an effective TCM prescription in clinics, exerts obvious effects on treating SLE, such as alleviating SLE related clinical manifestations, reducing the SLE activity score, and improving the quality of life of patients. However, compared with modern medicine, the complex of multi-targets and mechanism in TCM hinder its acceptance and recognition in the western mainstream. Therefore, we need a well-understood method to demonstrate the effects of LCD on SLE for application to clinical popularization. In the present study, we integrated HPLC-MS analysis, network pharmacology and molecular docking approach to predict and validate the potential mechanism and target of LCD in treating SLE via suppressing activated T cells. We screened 283 protein targets from LCD and 1498 SLE - related targets from 6 kinds of database using network pharmacology. The PPI results with 150 common targets showed that CASP3 with high degree could be a key effective target.
Increasing evidence showed that defective control of T cells plays important
roles in the pathogenesis of SLE by stimulating auto-antibody production,
promoting inflammation and causing damage [3, 5, 29]. Apoptosis is the key
process that regulates the death of activated T cells. Consequently, aberrations
in T cell apoptosis are responsible for the progression of SLE. Oestrogen
triggers SLE activity, which correlates with a defect in T cell apoptosis [30, 31]. Therefore, to simulate the pathogenesis of SLE in the current study, we used
The LCD formula is composed of Radix Rehmanniae, Rhizoma Cimicifugae, Semen Coicis, etc. Due to the diversity of chemical constituents and the complexity of Traditional Chinese Medicine (TCM) formula, it is difficult to assess which components contribute to therapeutic effects. Chemical composition of the prescription are the main problems of an empirical LCD formula for the treatment of SLE. In our previous report, we identified three main components from LCD, which are ferulic acid, isoferulic acid and paeoniflorin, respectively with RP-HPLC [32]. Serum medicinal chemistry is an effective method to screen active components of TCM formula, which could analyze the components absorbed into serum after oral administration with less interference of other components [33]. In the present study, we found that LCD serum contained ferulic acid and isoferulic acid based on the retention time, accurate molecular weight and fragment ion peaks of the reference substances. Furthermore, the contents of ferulic acid and isoferulic acid in LCD serum were relatively high and varied greatly in different doses of LCD treatment. Thus, we selected ferulic acid and isoferulic acid for molecular docking to predict the target CASP3 of LCD for treating SLE. They showed good docking affinity. Although it is still unclear that which chemical components of LCD contribute to its inhibitory effect on the over-activated Jurkat T cells in the present study, we predicted and validated the potential mechanism of LCD formula in the treatment of SLE through initiating CASP3 activation for the first time. Some studies have shown that ferulic acid and isoferulic acid exert anti-inflammatory, antioxidant and apoptosis effects, consistent with our results. Our previous studies showed that LCD formula could exert obvious therapeutic effect in treating SLE patients and suppresses inflammatory activity of MRL/lpr Mice [12, 13, 34, 35, 36]. The present study aimed to explore the target of LCD for treating SLE via suppressing activated T cells. In the further research, we would identified more active compounds and proved the mechanism by animal model.
In conclusion, we revealed the potential mechanism of LCD formula in treating SLE via suppressing activated T cells integrating network pharmacology with HPLC-MS analysis and molecular docking. Based on a systematic prediction and experimental validation, LCD exerts an inhibitory effect on the proliferation of activated T cells in the treatment of SLE, which is potentially attributed to inducing activated T cells apoptosis by initiating CASP3 activation. LCD may be a promising choice for SLE supplementation treatment and its target CASP3 is worthy of in-depth study in our future research.
HLv drafted the manuscript. HLv, QL, JS, JT, HLi, JZ, HG and ZX, were responsible for the acquisition and analysis of data. ZX and HLv were responsible for the design and supervision of the work. All authors have read and approved the final version of the manuscript.
All animals were humanely cared in Zhejiang Chinese Medical University [SCXK (Yu)-2014-0005, Zhejiang Province, P.R. China]. The study was guided and approved by Animal Ethical and Welfare Committee of ZCMU.
We appreciate the experimental support from the Public Platform of Medical Research Center and the Public Platform of Pharmaceutical Research Center, Academy of Chinese Medical, Academy of Chinese Medical Science, Zhejiang Chinese Medical University.
This research was supported by grants from the National Natural Science Foundation of China (81973829, 81873266), the Key Supported Projects of the Joint Fund of the National Natural Science Foundation of China (U21A20402) and the Natural Science Foundation of Zhejiang Province (LY18H270014).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
