Academic Editor: Kevin Cianfaglione
Background: Phosphorus is an essential component of fertilizers and feed and in recent decades has become one of the main sustainability issues as a non-renewable resource. In plant seeds, the main reserve of phosphorus is phytic acid, a strong anti-nutritional factor for monogastrics and a pollutant of cultivated lands. The reduction of phytic acid in cereal seeds has become a major challenge in breeding programs to increase the nutritional quality of foods and feeds and to improve the environmental phosphorus sustainability in agriculture. In maize (Zea mays L.), four low phytic acid (lpa) mutations have been isolated and lpa1-1 is the most promising. However, the reduction of phytic acid in lpa1-1 leads to many adverse pleiotropic effects on the seed and in general on plant performance. A seed weight reduction and a consequent yield loss were previously described in this mutant. Method: In this work, a field experiment to study seed weight and yield was conducted for two years in two different genetic backgrounds (B73 and B73/Mo17). Furthermore, the greater susceptibility of lpa1-1 to drought stress was also investigated: a dedicated field experiment was set up and measurements were carried out under optimal water conditions and moderate drought stress. Results: From the first experiment it emerges that under high-input conditions, lpa1-1 seems to have comparable or even better yield than the relative control. The main problem of this mutant remains the reduced field emergence (~40%). In the study of drought stress it was found that the increased sensitivity in the mutant is mainly caused by an altered stomatal regulation, but not by a less developed root system, as previously reported. When the stress occurred, the parameters measured did not significantly change in the wild-type, while they dropped in the mutant: the net photosynthesis decreased by 58%, the transpiration rate by 63% and the stomatal conductance by 67%. Conclusions: Some possible solutions have been proposed, with the aim of developing a commercial variety, which remains the main goal to exploit the nutritional benefits of low phytic acid mutants.
In recent decades, phosphorus (P) has become one of the main sustainability issues as a non-renewable resource. P is a crucial element for animal and plant production and is involved in many physiological and biochemical processes. It was the first element to be recognized as an essential mineral nutrient for plants, and its functions cannot be replaced by any other mineral nutrient [1]. Being an essential component of fertilizers and feed, the increasing production of food has increased the rate of mobilization of reserves, and the price of this mineral is continuously rising. It is estimated that in the world, there will be reserves accessible with current technology for another 90 years at current consumption rates. However, its consumption will increase as the population grows [1].
In plant seeds, the main reserve form of P is phytic acid (PA, myo-inositol-1,2,3,4,5,6-hexakisphosphate) [2], an insoluble phosphate compound considered a strong anti-nutritional factor, the degradation of which occurs during germination by a group of enzymes called phytases [3].
Only ruminants can degrade phytic acid thanks to the presence of phytases in their digestive tract. Phytic acid is poorly digested by monogastrics: as it is not assimilated, PA is expelled with animal excrement, becoming a pollutant of cultivated land and contributing to water surface eutrophication. Since it is a compound poorly assimilated by monogastrics, farmers must add mineral phosphate to the animal feed, thus leading to an increase in costs [4]. Hence, the reduction of PA in cereal seeds has become a significant challenge in breeding programs to increase the bioavailability of micronutrients and improve seed nutritional quality, mainlyfor the benefit of populations whose diet is based on these staple crops. In the last decades, many low phytic acid (lpa) mutants have been isolated in several important crops, such as maize [5, 6, 7, 8], wheat [9], barley [10, 11, 12], rice [13, 14], soybean [15, 16, 17] and common bean [18, 19]. In maize (Zea mays L.), there are three different lpa mutations (lpa1, lpa2, and lpa3) depending on the step of the biosynthetic pathway they affect, with lpa1 showing the lowest PA content in the seed, followed by a proportional increase in inorganic P [5, 6].
In maize (Zea mays L.), lpa1 mutations are caused by lesions in the ZmMRP4 gene. Until now, four low phytic acid mutations have been isolated in the ZmMRP4 PA transporter: lpa1-241 [20, 21], lpa1-7 [7], lpa1-1 [22] and lpa1-5525 (not fully characterized) [8]. lpa1-241 and lpa1-7 are lethal in the homozygous state, displaying an 80%–90% decrease in PA, while lpa1-1 is the only one viable in homozygosity, showing a lower reduction in phytic acid (66%). Although it is not lethal, reducing PA in lpa1-1 leads to many adverse pleiotropic effects on the seed and, in general, on plant performance [23]. Among these agronomic defects, a seed weight reduction ranging from 8 to 23% was found in this mutant [5]. This decrease appeared to be mainly caused by endosperm loss, resulting in an agronomic yield reduction. Moreover, it was also observed that lpa1-1 was more susceptible to drought stress, probably due to an alteration in mature root system development [7]. Colombo and coworkers recently excluded this hypothesis: in recent work conducted in controlled or semi-controlled conditions, it emerged that the drought stress in the mutant lpa1-1 seemed to be caused by a reduced photosynthetic efficiency and not by a shallower root system [24].
Maize’s root system architecture is composed by embryonic and post-embryonic roots [25, 26]. The former, which includes the primary root and a variable number of seminal roots, is important for seedling vigor in the early stages of development [27, 28]; the post-embryonic roots dominate the mature root system and are formed by crown roots (at underground nodes of the shoot) and one or more whorls of brace roots [26, 29]. All these roots generate post-embryonic lateral roots, mediating the absorption of water and nutrients from the soil [30].
In maize, a hypothetical root system ideotype for optimizing water and nitrogen uptake was presented by Lynch and is called the SCD (“steep, cheap and deep”) ideotype [31]. It was reported that one whorl of brace roots is preferable to multiple whorls: in fact, the brace roots from younger whorls appear later in the development and are less useful for soil resource acquisition. Furthermore, it was reported that the first above-ground node should have high occupancy and should be entirely occupied by brace roots that reach the soil, thus giving stability to the plant [31].
Based on these data, the present work aimed to study two of the main pleiotropic effects that characterize the mutant lpa1-1 in maize (Zea mays L.), i.e., the seed weight reduction and the greater susceptibility to drought stress. Here we report the results of a field evaluation performed for two years in two different genetic backgrounds, highlighting a good yield of lpa1-1 under high-input conditions and limiting the problem of this mutant to the field emergence. Furthermore, in another trial, we collected different epigeal and hypogeal measurements to study drought stress and to confirm in the field what was previously reported by Colombo and coworkers under controlled conditions.
In this work, we used four different genetic materials which were compared pairwise. The first pair was lpa1-1/lpa1-1 vs. +/+ control in “B73” genetic background kindly provided by Dr. Victor Raboy, USDA ARS, Aberdeen, ID, USA. B73 is an inbred line used in the 80’s for the hybrid production, now widely used as a benchmark. The second pair was lpa1-1/lpa1-1 vs. +/+ control in “B73/Mo17” genetic background. These two genotypes were obtained by crossing lpa1-1/lpa1-1 B73 with “Mo17” inbred line provided by the germplasm bank at DISAA, Department of Agricultural and Environmental Sciences—Production Landscape, Agroenergy, University of Milan. The F1 obtained was selfed, generating an F2 population scored by Chen’s assay and genotyped to isolate lpa1-1/lpa1-1 and +/+ genotypes (as described in the following sections). Following three cycles of sib crossing, we obtained two synthetic populations differing only for the presence of the lpa1-1 in homozygous status (Fig. 1).
 Fig. 1.
Fig. 1.Pedigree scheme used to obtain the two synthetic populations in B73/Mo17 geneticbackground. The two synthetic populations differ only for the presence of the lpa1-1 in homozygous status.
The Chen assay is a colorimetric method used to distinguish the HIP (high
inorganic phosphate) phenotype. The HIP phenotype is diagnostic for the presence
of the lpa1 mutation. We used the qualitative Chen assay with some
modifications [32]. The seeds were ground in a mortar using a pestle, and 100 mg
of the flour obtained was placed in a microtiter plate with 1 mL of 0.4 M HCl
solution. After 1 h at room temperature, 100
DNA was extracted from wild-type and mutant leaves as described by Dellaporta
et al. [33]. PCR was performed with the primers 13L
(5′-CTTCATGATCTGCGGTCACG-3′) (forward primer, position +5293) and 51R
(5′-AAGCATCAGCTTCGGGTAATGT-3′) (reverse primer, position +6460) and 2
Experiments were carried out in 2020 and 2021 in the experimental field of the
University of Milan located in Landriano (PV), Italy (N45°18
The following agronomic data were collected in the two backgrounds in both years:
Plant height: the distance between the base of the male inflorescence (panicle) and the ground level was measured considering more than 30 plants chosen randomly in the three plots. Plant height was measured in mid-July during full flowering using a measuring rod.
Ear height: insertion height of the first ear measured considering the distance between the ground level and the insertion point of the first ear in the stem. These data were collected from the same plants used for plant height.
Moreover, five representative ears for each plot (15 ears for each genotype) were randomly selected for grain yield estimation in the two backgrounds in both years. The ears were harvested on September 12th, and the seeds were immediately dried to 12–13% of relative humidity. Later, the following parameters were collected:
Ear length: measured with a ruler on 15 ears for each genotype.
Average single seed weight: single representative seeds were selected from each ear and were measured using an analytical weight scale (Precisa XB 220A, 0.1 mg). The test was performed in triplicate.
The number of seeds per ear: evaluated by counting all the seeds of each sampled ear.
Seeds weight/ear: measured by weighing all the seeds that make up an ear.
In parallel, 200 seeds of the inbred line B73 and 200 seeds of the relative lpa1-1 mutant were sown in a non-irrigated part of the field to study drought stress in field conditions.
In particular, root sampling was carried out in the season 2021, and the root system architecture of the wild-type was compared with the relative lpa1-1. Only two days prior to sampling, the fields were irrigated with 12 mm of water to facilitate the excavation of roots. At flowering, ten roots per genotype were excavated in the first 30 cm using standard shovels. The excavated roots were shaken to remove the main fraction of soil adhering to the roots, and the remaining soil particles were removed by vigorous rinsing at low pressure, according to Trachsel et al. [34]. On the cleaned roots, different parameters were collected: number of above-ground whorls occupied with brace roots (BW); brace root number (BO); angle of the first arm of brace roots originating from the first whorl in relation to horizontal (BA1); culm diameter at the base of the sampled plant (CD).
Later, a crown root and a brace root were detached from each root system to estimate the root density. The roots of each sample were scanned (using a high-resolution digital scanner). The images were processed with Adobe Photoshop software: the shadows of the roots and the background of the images were removed, and the color of the roots was changed to green and made uniform. The processed images were analyzed using ImageJ 1.52 [35] and Easy Leaf Area software (University of California, Davis, CA, USA) [36] to calculate the root area/root length ratio.
Different parameters in the epigeal part of the plant were collected: net
photosynthesis (P
Stable carbon isotope analysis was carried out on flag leaves of both B73 and
lpa1-1/lpa1-1 plants grown in the field and sampling was done
on the 14th of July. Harvested leaves were preventively dried at 80 °C
and then ground to a fine powder by using a grinder. Samples were prepared by
adding 1 mg of dry powdered plant tissues into 5
The
Calibration was performed using three secondary reference materials provided by
IAEA: NBS18 (
The isotope ratio
which expresses the part per thousand deviation of the isotope ratio
For the quantification of abscisic acid (ABA), 100 seeds of the two genotypes (B73 and the relative lpa1-1 mutant) were germinated under controlled conditions. After 2 weeks, the plant leaves were sampled, and the following method was applied for the quantification of ABA.
Sample preparation and extraction method: samples were ground by an
electric blender and an amount of 5.0
Reagents and standard: the reagents used for the determination and
quantification of ABA were analytical-grade water, methanol and acetic acid
(Carlo Erba, Italy). Standard abscisic acid (purity
Apparatus: high performance liquid chromatography analysis of ABA was
performed on a Shimadzu instrument (Shimadzu, Milan, Italy) equipped with two
pumps (10AD vp), a diode array detector (SPD-M10A vp), a system controller
(SCL-10A vp) and a Rheodyne 20 mL injection loop (Thomas Scientific, Cotati,
Swedesboro, NJ, USA). The HPLC pumps and diode array system were controlled by
computer using a LC Solution version 1.25 SP5 workstation program (Shimadzu,
Milan, Italy). The analytical column employed was a Kinetex XB-C18 (150 mm
Chromatographic conditions: the mobile phase consisted of (A)
water/acetic acid (99:1 v/v) and (B) methanol. The operating conditions were as
follows: 0–30 min linear gradient from 10% B to 100% B, 30–50 min isocratic
elution 100% B, 50–70 min linear gradient from 100% B to 10% B, and 70–80
min isocratic elution 10% B. The flow rate was 0.4 mL
Analytical quality assurance: the abscisic acid content was quantified
using the calibration curve. A stock standard solution of ABA was prepared
dissolving the compound in methanol (concentration 1.0 mg
In this work, we used four different genetic materials compared pairwise. The first pair was lpa1-1/lpa1-1 vs. +/+ control in B73 genetic background. The second pair was obtained by crossing lpa1-1 B73 with Mo17 inbred line provided by our germplasm bank. The F1 obtained was selfed, generating an F2 population scored by Chen’s assay and genotyped to isolate lpa1-1/lpa1-1 and +/+ genotypes (as described in the following sections). Following three cycles of sib crossing, we obtained two synthetic populations differing only for the presence of the lpa1-1 in homozygous status (Fig. 1).
The presence of the lpa trait in the material used in the following experiments was confirmed through chemical and molecular methods. The qualitative Chen assay for free phosphate allowed us to rapidly distinguish the lpa1-1 mutants with respect to the wild phenotype: the former were colored blue in contact with the Chen reagent, while the wild-types remained white due to the low content of free phosphate (Fig. 2A). Later, Sanger sequencing was used to molecularly confirm the presence of the mutation (Fig. 2B): in comparison with the wild-type allele, the coding sequence of the lpa1-1 allele is characterized by the presence of a Single Nucleotide Polymorphism (SNP), C to T, in position 5759 with respect to the starting codon on the genomic sequence (Fig. 2C).
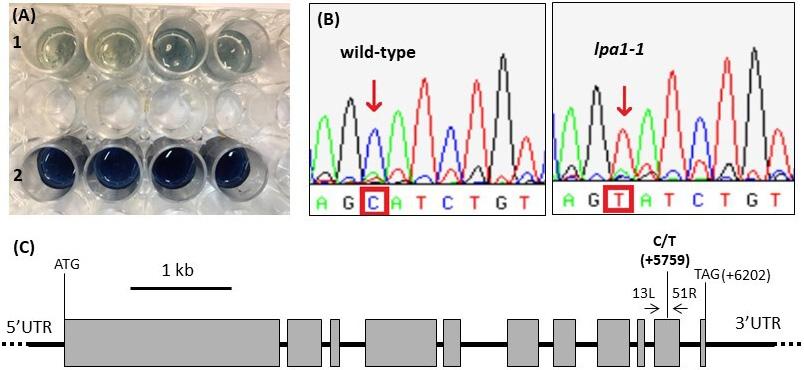 Fig. 2.
Fig. 2.Chen assay for free phosphate. (A) microtiter row 1 showed the results of the assay performed on four wild-type seeds; in row 2, the blue-colored phosphomolybdate complex was visible and represented four mutant seeds. (B) The presence of the mutation (C to T) was molecularly confirmed by Sanger sequencing. (C) Structure of the ZmMRP4 gene and position of the Single Nucleotide Polymorphism (SNP), C to T.
The field experiment was organized in randomized blocks; plants were grown under high-input conditions, and water was not a limiting factor during the season. Field emergence percentage was calculated by counting the number of plants present between the V2 and V4 stages of development and dividing by the total number of seeds. This parameter was measured in both the backgrounds and years and appeared to be reduced in lpa1-1 compared to the wild phenotype, as reported in Table 1. This value was much lower than the germination of the same seeds under controlled conditions at the optimal temperature and humidity (data not shown).
| Genotype | Field emergence (%) | 95% Fiducial Limit | Field emergence (%) | 95% Fiducial Limit |
| 2020 | 2021 | |||
| B73 | 100 | 0.00 | 100 | 0.00 |
| lpa1-1 (B73) | 37 | 6.67 | 45 | 6.91 |
| B73/Mo17 | 100 | 0.00 | 100 | 0.00 |
| lpa1-1 (B73/Mo17) | 36 | 6.71 | 43 | 6.88 |
Furthermore, the plants of lpa1-1 grown under field conditions were characterized by a lower plant and ear height compared to the relative wild-types in both the years analyzed and in both the backgrounds (Fig. 3A,B). In the control line B73, the plant height reached an average of 189.6 cm in 2020 and 198.3 cm in 2021, while the mutant lpa1-1 introgressed in B73 was 184.3 and 181.1 cm in height, respectively (Fig. 3A). On the same plants used for plant height, ear height was also measured. Ear height was statistically higher in B73 than lpa1-1 in both the years (127.1 vs. 93.4 cm in 2020 and 134.8 vs. 94.3 cm in 2021) (Fig. 3B).
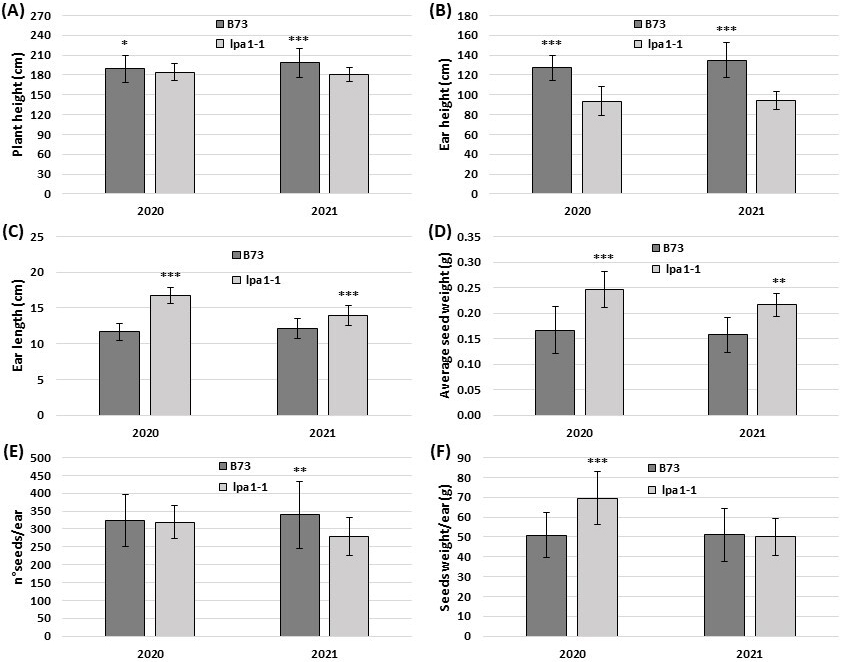 Fig. 3.
Fig. 3.Agronomic parameters collected in the field experiment on the
first genetic background (B73). Plant height (A) and ear height (B) measured at
flowering in B73 and in its relative lpa1-1. The data represent the
means of n
The same trend was recorded in the synthetic population B73/Mo17, although, in general, these plants were taller than in the first background due to the hybrid vigor. The height of B73/Mo17 plants reached 234.6 cm in 2020 and 243.2 cm in 2021, while the mutant lpa1-1 introgressed in B73/Mo17 was 189.6 and 190.1 cm high, respectively (Fig. 4A). Also, ear height was statistically higher in the wild-type than lpa1-1 in both the years (142.9 vs. 103.7 cm in 2020 and 147.8 vs. 105.3 cm in 2021) (Fig. 4B).
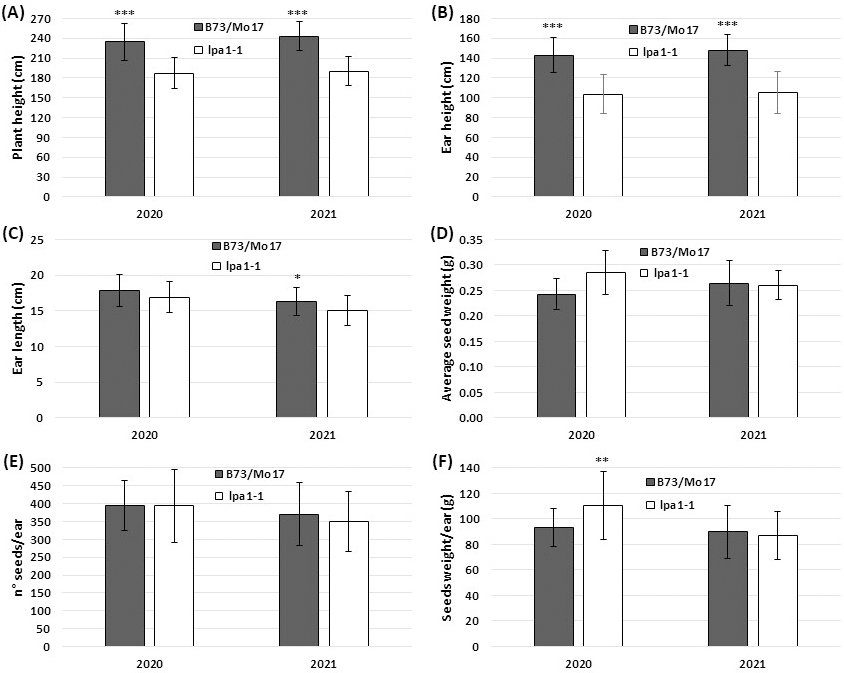 Fig. 4.
Fig. 4.Agronomic parameters collected in the field experiment
on the second genetic background (B73/Mo17). Plant height (A) and ear height (B)
measured at flowering in B73/Mo17 and in its relative lpa1-1. The data
represent the means of n
After collecting these agronomic data at flowering, other parameters (ear
length, average single seed weight, number of seeds/ear, and seeds weight/ear)
were measured at harvesting for grain yield estimation, collecting n
Under optimal growth and water conditions, lpa1-1 showed equal or superior performance compared to the inbred line B73. In particular, the ear length and the average seed weight were statistically higher in the mutant in both the years (Fig. 3C,D). In contrast, the number of seeds per ear was statistically the same in 2020 and slightly higher in B73 in the following year (Fig. 3E). An important parameter in grain yield estimation is the seed weight per ear, which was statistically superior in lpa1-1 in 2020 and the same between the two genotypes in 2021 (Fig. 3F).
These results were confirmed in the other genetic background, the synthetic population B73/Mo17. Most of the parameters measured were statistically the same in the wild-type and in the relative mutant, and even the seed weight/ear of lpa1-1 in 2020 was statistically higher than in the control (110.24 vs. 93.38 g).
Another experiment on lpa1-1 was conducted in the field in order to study its response to drought stress conditions. In this case, the plants of B73 and lpa1-1 were grown in a non-irrigated part of the field. Ten roots per genotype were sampled at flowering and then cleaned by vigorous rinsing (Fig. 5A). Photos of the two genotypes were taken (Fig. 5B), and among the parameters collected, no significant differences were found regarding the brace roots, as indicated in Table 2. Also, the diameter of the culm, an epigeal parameter measured at the base of the sampled plant, was statistically the same between lpa1-1 and the wild-type (Table 2).
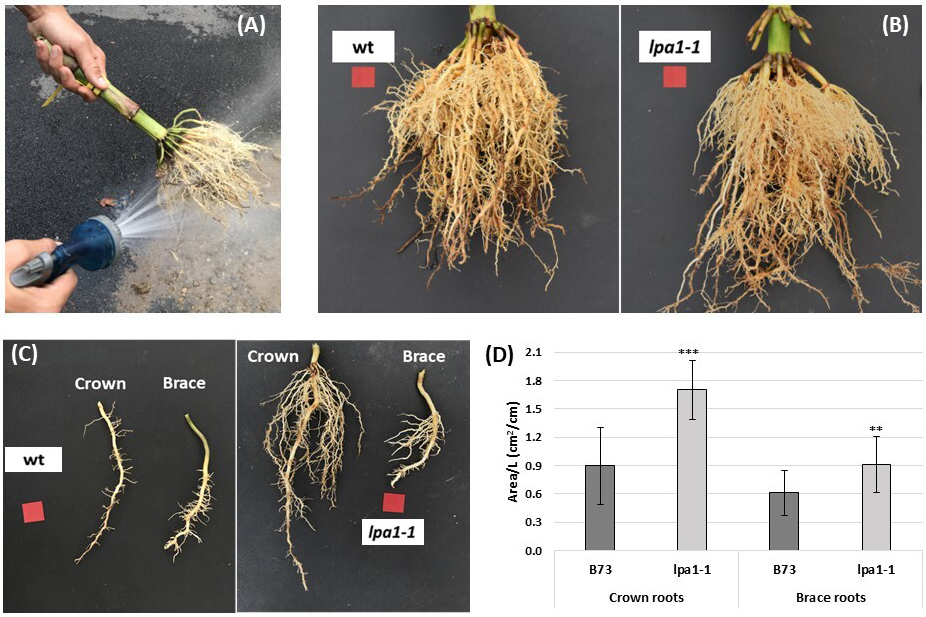 Fig. 5.
Fig. 5.Schematic representation of the root sampling and
collected data. Ten roots per genotype were sampled in the field through a
shovel and the soil was removed with a vigorous rinsing (A). An example of B73
and lpa1-1 root system architecture (B). A representative crown root and
a brace root were detached from each root system (C) and the area occupied per
unit of length was calculated with Easy Leaf Area software (D). Significant
differences between the wild-type and lpa1-1 were assessed by Student’s
t-test (*p
| Genotype | Brace whorls (BW) | Brace number (BN) | Brace angle (BA1) | Culm diameter (CD) |
| B73 | 1.40 |
15.44 |
49.00 |
27.95 |
| lpa1-1 | 1.70 |
16.60 |
50.39 |
26.74 |
| Values represent the mean and the standard deviation of ten biological
replicates. For each parameter, comparisons were made in column. Significant
differences between the wild-type and lpa1-1 were assessed by Student’s
t-test (*p | ||||
Furthermore, a crown root and a brace root were detached from each root system
to estimate root density (Fig. 5C). Images were processed, and the area occupied
per unit of length was calculated, as indicated in Fig. 5D. This ratio was
statistically higher in the mutant than in the wild-type in both types of
post-embryonic roots: in the case of crown roots, the area reached 1.70
cm
The results obtained so far seem to exclude the root system as the leading cause
of the drought stress in lpa1-1. Therefore, our research focused on the
epigeal part of the plant, and several measurements were made at two different
moments: under optimal water conditions (16% of volumetric soil moisture) and
under moderate drought stress (6% of volumetric soil moisture). In Fig. 6A it is
possible to observe how the leaves of the two genotypes appeared when the
measurements under moderate drought stress were carried out: the leaves of the
mutant appeared altered, stressed, and curled upwards. The three parameters
collected with the CIRAS-2 Portable Photosynthesis System highlighted that under
optimal water conditions the transpiration rate and stomatal conductance were
significantly higher (by 31% in both cases) in lpa1-1 compared to the
control line, revealing a greater stomatal opening in the mutant (Fig. 6C,D). Pn
did not significantly differ in the two genotypes under non-stressful conditions,
although the mean was higher in lpa1-1 than in the wild-type (29.70
vs. 24.70
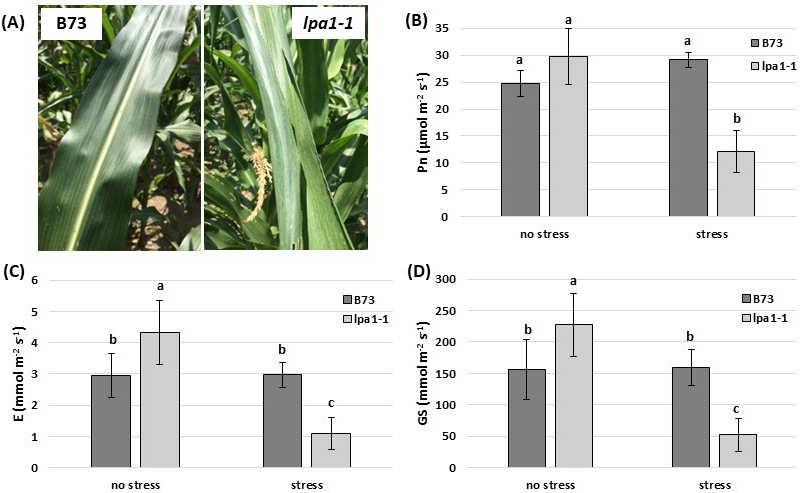 Fig. 6.
Fig. 6.Measurements carried out in the field at two different moments,
under optimal water conditions (no stress) and under moderate drought stress.
(A) Under moderate drought stress, the leaves of lpa1-1 were stressed
and curled upwards compared to the wild phenotype. The photos were taken the same
day. The CIRAS-2 Portable Photosynthesis System was used in the field to measure
the net photosynthetic CO
To further support this hypothesis, we measured the carbon stable isotope
composition of the maize flag leaves of the two genotypes grown under optimal
water conditions — i.e., when both transpiration rate and stomatal conductance
were higher in lpa1-1 than in the wild-type — since stomatal conductance
may significantly alter
Phytic acid represents the main P reserve in mature seeds and is degraded during germination by the activity of a group of enzymes called phytases [2, 3]. It is well known that phytic acid is characterized by remarkable chelating properties, and besides making P unavailable, it binds different minerals (such as Fe and Zn), making them unavailable for monogastric animals due to the lack of phytases in their digestive systems. Despite being an anti-nutritional factor for all these reasons, PA is considered an important antioxidant compound: by chelating iron cations, phytic acid can counteract the formation of reactive oxygen species (ROS), thus preserving seed vitality [40, 41, 42]. In recent decades, phosphorus has also become a topic of primary importance because it is a non-renewable resource. In fact, phosphorus is widely used in agriculture and the increased food production has increased the rate of reserve mobilization. The price of the mineral is continuously rising and at current consumption rates it has been estimated that phosphorus reserves will run out in 90 years [8].
In this context, the reduction of PA in cereal seeds has become a major challenge in breeding programs to increase the bioavailability of micronutrients and improve seed nutritional quality. In the last decades, many low phytic acid (lpa) mutants have been isolated and characterized in all major crops and lpa1-1 is the most promising in maize. However, the reduction of PA in seeds can affect the vitality of the seeds and in general the agronomic performance of the plants [23]. Among these adverse pleiotropic effects, a seed weight reduction was previously described [5] and a major susceptibility to drought stress was observed in lpa1-1 [7].
In the first part of this work, we report the results obtained in a field study conducted for two years in two different genetic backgrounds. In this way it was possible to investigate the first of the two agronomic defects previously reported, i.e., the reduction in seed weight and the consequent yield loss that characterize lpa1-1. To our knowledge, after the isolation of this mutant by Raboy and collaborators in 2000 [5], no trials in field conditions have been conducted in recent years to evaluate its yield in comparison to the wild phenotype. In this experiment, plants were grown under high-input conditions and water was not a limiting factor during the season.
In both the backgrounds, lpa1-1 showed a reduced stature compared to the wild-type (Figs. 3,4A,B), confirming that the shortening of the internodes could be caused by an alteration in the auxin polar transport, as occurs in other known maize mutants, such as brachytic 2 (br2) and brevis plant1 (bv1) [43, 44, 45]. For grain yield estimation, different parameters were measured at harvesting and lpa1-1 surprisingly showed equal or superior performance to the inbred line B73 (Fig. 3C,D,E,F). To confirm what was measured in the first background, the same parameters were collected in the synthetic population B73/Mo17 and also here lpa1-1 showed a good yield (Fig. 4C,D,E,F). This was not the first case in which a low phytic acid mutant had a similar performance to its respective wild-types: in barley, the majority of lpa mutations appeared to have little or no effect on yield [12, 46], although the cultivation in more stressful environments remains problematic [47]. In the same way, the lpa1 mutants in common bean do not exibit negative agronomic effects, described in mutants affected by the orthologous gene [42], thanks to the presence of the PvMRP2 paralog gene, which is able to complement the function of PvMRP1 in organs different from the seed [19]. From the data collected here, it emerged that the biggest problem connected with this mutant remains the reduced field emergence, which limits the interest of breeders.
As discussed above, the agronomic defects may be caused by an increased amount of free iron cations present in the mutant seeds and the consequent higher level of toxic ROS [41]. A possible approach to defend any plant cell from reactive oxygen species consists in scavenging these toxic radicals by means of antioxidant molecules. Thus, with the aim of improving the agronomic performance of these mutants, classical breeding could be used to introgress the ability to synthesize and accumulate natural antioxidants (such as polyphenols) in the living tissues of the seed.
In the second part of this work, we focused on the increased susceptibility to drought stress observed in lpa1-1. This pleiotropic effect was previously studied under controlled and semi-controlled conditions by Colombo et al. [24] and it was hypothesized that the drought stress appeared to be caused by a reduced photosynthetic efficiency and not by a shallower root system, as initially hypothesized by Cerino Badone et al. [7]. To confirm this hypothesis in non-controlled conditions, in this work different epigeal and hypogeal measurements were collected in the field. In this experiment, the plants of B73 and its relative lpa1-1 were grown in a non-irrigated part of the field. At flowering, ten roots per genotype were sampled and then cleaned by vigorous rinsing (Fig. 5A). Among the parameters collected, the root area occupied per unit of length was statistically higher in lpa1-1 than in the wild phenotype (Fig. 5D). Therefore, the root density was higher in the mutant due to a greater development of the lateral roots, which are essential for the uptake of water and nutrients, particularly in stressful conditions. Thus, the mutant root system did not appear to be the main cause of drought stress in lpa1-1 and for this reason our research shifted to the epigeal part of the plant, where measurements were carried out in two different conditions: under optimal water conditions and under moderate drought stress. In the latter case, the leaves of the mutant appeared stressed and curled upwards compared to the wild phenotype, as shown in Fig. 6A.
The three parameters collected with the CIRAS-2 Portable Photosynthesis System
revealed a greater stomatal opening in the mutant than in the wild-type under
optimal water conditions: in fact, the transpiration rate and the stomatal
conductance were significantly higher in lpa1-1 compared to the
wild-type (Fig. 6C,D). These results were in line with what was reported by
Colombo et al. [24] in controlled conditions – where lpa1-1
exhibited a lower leaf temperature and a greater stomatal opening compared to the
wild-type—but also in accordance with the results presented here, obtained by
analyzing the carbon stable isotope composition (
Conversely, when water stress occurred, the net photosynthetic transpiration rates, as well as the stomatal conductance did not significantly change in the wild-type, while they dropped dramatically in the mutant: the net photosynthesis decreased by 58%, the transpiration rate by 63% and the stomatal conductance by 67%, revealing an alteration of stomatal regulation in lpa1-1 under drought stress. This experiment conducted in open field conditions strongly supports the hypothesis that the increased sensitivity to drought stress in the mutant is mainly caused by an altered stomatal regulation efficiency, not by a less developed root system.
Phytic acid not only plays a central role in phosphate storage but is also
involved in several plant processes such as biotic and abiotic stress response,
hormonal responses, P homeostasis and signal transduction [48, 49]. The
interaction of inositol phosphates with auxins seems to explain the shortening of
the internodes that occurs in our mutant. Another plant hormone, abscisic acid
(ABA), appears to interact with the inositol phosphate pathway and seems to have
an important role in the plant’s response to drought stress. In fact, a typical
effect of ABA is to reduce the leaf water loss by closing the stomata and at the
same time defending itself from microbes by limiting their entry through the
stomatal pores [50]. In this work, a quantification of ABA was conducted on
seedlings grown for 14 days in controlled conditions. This preliminary experiment
gave us an unexpected result, since the mutant showed a significantly higher
level of ABA compared to the wild-type (9.8
In conclusion, this work highlights the possible advantages of low phytic acid1-1 mutant in increasing the nutritional quality of foods and feeds and in improving the environmental sustainability of phosphorus in agriculture.
From the data collected in the field, it emerged that the biggest problem of this mutant remains the reduced field emergence, which consequently limits the yield. Furthermore, it was found that the increased sensitivity to drought in the mutant is caused by an altered stomatal regulation, but not by a less developed root system, as previously reported. Several studies are now under way with the aim of reducing the negative pleiotropic effects in lpa seeds. Among the possible solutions, traditional breeding could be used to introgress the lpa1-1 allele into a new genetic background (e.g., a commercial hybrid) with the aim to select plants with a higher field performance and a better stress response. Furthermore, another possible strategy is represented by seed priming, a pre-sowing treatment that allows the seeds to germinate with a higher efficiency, also increasing the tolerance to abiotic and biotic stresses and individual plant performance.
Overall, the study of these possible solutions is now under way, but more work is still necessary before the development of a commercial variety, which always remains the main goal to exploit the nutritional benefits of low phytic acid mutants.
FC and RP designed the research study. FC performed the research. SS, AA, SKS, MB, FT, FFN provided help and advice in the experiments conducted. FC, MB, FT, FFN analyzed the data. FC, FFN, RP wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We wish to thank Davide Reginelli for his hard work in the field and Lesley Currah for her editing and suggestions.
This research received no external funding.
Given his role as Guest Editor, Roberto Pilu had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Kevin Cianfaglione. The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
