Introduction: A loss of endogenous stem cells capable of tissue repair and regeneration drives the biological process that we recognize as “aging”. Recovery of stem cell-mediated repair and regenerative functions in aged animals has been reported in murine heterochronic parabiosis experiments. Objectives: Herein we will review how pregnancy is an unusual form of heterochronic parabiosis, as the placenta prevents the exchange of most blood cells between parabionts. Instead, plasma and its content, including small extracellular vesicles, can readily cross the placental barrier. These nanosized extracellular vesicles are readily produced by the placenta, amnion, fetus and mother, and are essential for fetal organogenesis, growth and the progression of a healthy pregnancy. If defective, these extracellular vesicles can cause havoc such as in the case of peripartum cardiomyopathy. We will also review how these extracellular vesicles impact the mother substantially (including cardiac function) in the parabiosis of pregnancy. Conclusion: Extracellular vesicles generated during the course of a healthy pregnancy are essential for organogenesis and fetal growth, and also for maternal tissue repair and regeneration, and might be defective or deficient in pregnancies that result in peripartum cardiomyopathy.
Parabiosis is the research experiment where two immunologically matched organisms are connected through a patch of derm tissue and blood capillaries are shared by the pair, thus bridging circulation between the two organisms (parabionts). In heterochronic parabiosis (hetero, different; chronic, age; para, together; biosis, living) experiments one organism is old, while the other is young, leading to improvement of tissue hemostasis and repair of the old organism by systemic factors produced by the young organism [1]. According to Conboy et al. [1, 2], this phenomenon occurs “through boosting the regenerative capacity of (endogenous) tissue stem cells”. With pregnancy, a fetus and a mother are connected similarly, except that the two heterochronic organisms are not immunologically matched, and a placenta stands in-between mother and fetus, connecting them, instead of a shared derm patch (Fig. 1).
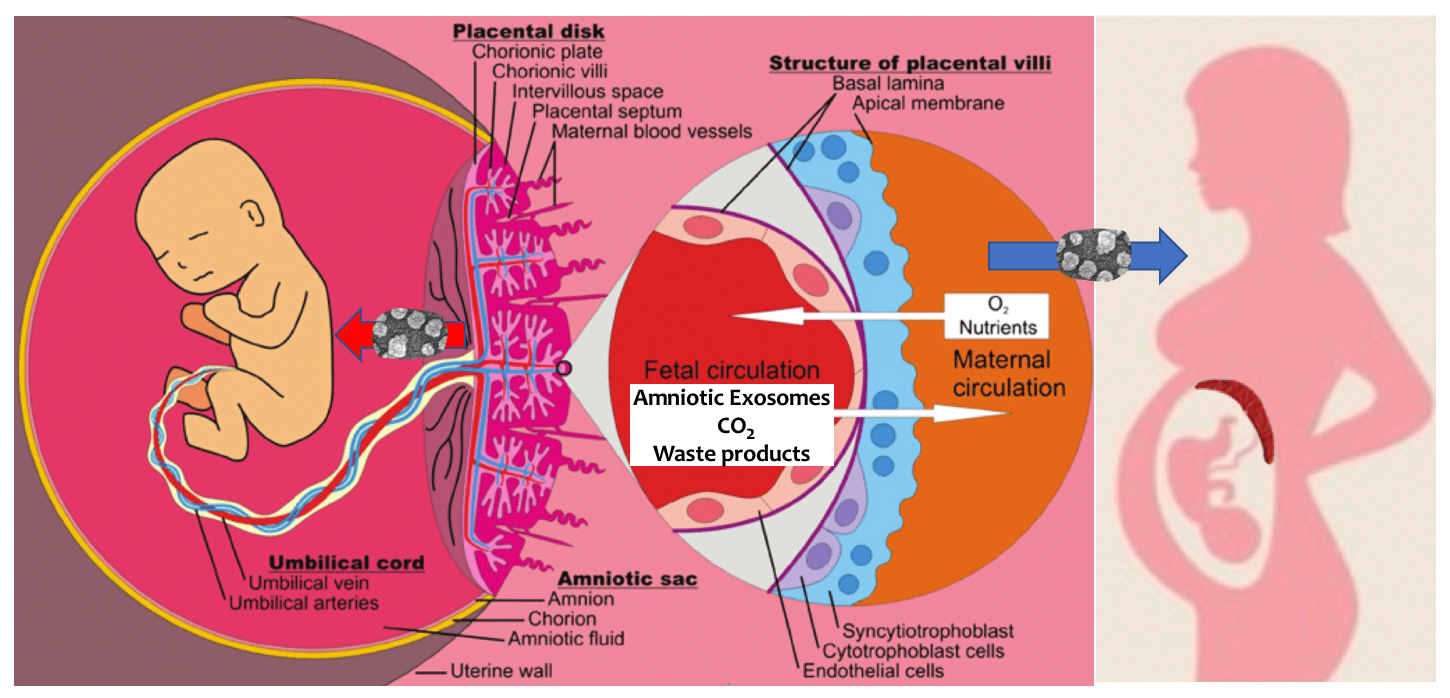 Fig. 1.
Fig. 1.Parabiosis of Pregnancy. It was known for some time that small extracellular vesicles (see electron microscopy on thick arrow) are released by the placenta and amnion to support the embryo then fetus. Less well known was the fact that amniotic extracellular vesicles also support the physiologic transformation of the mother (thick blue arrow). The placenta (and its components indicated on the left diagram) stands between the circulation of the fetus and that of the mother (right diagram, dark red crescent).
The placenta is a key organ allowing the transfer of nutrients and oxygen from the mother to the fetus. There are several mechanisms for these transfers such as passive diffusion, carrier-mediated uptake and transcytosis processes. While there is some cellular transfer through the placenta, cell transfer is limited and mainly from the side of the fetus to the mother [3]. In humans, such fetal cells could be involved in cell and tissue repair for the mother [3]. However, they are also believed to be responsible for pathological conditions such as autoimmune disorders [3, 4] and feto-maternal hemorrhage [5], and the presence of fetal cells in maternal blood has been used as non-invasive prenatal test for aneuploidy [6].
It has been shown that the placenta can mediate its many functions, including immune and metabolic regulation, via placental nanosized extracellular vesicles (or amniotic small extracellular vesicles) and also larger micro-vesicles [7]. Indeed, these extracellular vesicles appear early during the first trimester and their release into maternal circulation increases with gestation [8]. They may be involved in specific conditions of pregnancy such as gestational diabetes and pregnancy-induced hypertension [9]. Oxygen tension and glucose level seem to be major regulators of their production, and the provision of nutrients and vesicles like amniotic extracellular vesicles may play an important role in angiogenesis, cell proliferation, apoptosis and organ maturation for the fetus [7, 8, 9, 10, 11, 12].
Instructively, in standard heterochronic parabiosis, benefits for the older animal do not require for young cells to be transferred to the old animal, but rather they seem to be mediated by the plasma fraction of the blood and its nanometer-sized vesicle content [1, 2]. Indeed, with pregnancy, mostly smaller particles like amniotic extracellular vesicles, with a radius 100-times smaller than cells, can cross the placental barrier freely between the circulations of the two organisms [8]. Amniotic extracellular vesicles have several sources during pregnancy, and the multinucleated syncytiotrophoblast is the major source for these vesicles during the last four months of pregnancy [8]. Evidence that amniotic extracellular vesicles are shared between the mother and the fetus has provided a reasonable explanation for many physiological and pathophysiological events that can occur during pregnancy, which have, until recently, occurred without a comprehensive explanation [8, 9, 10, 11, 12].
Amniotic extracellular vesicles are controlling the immune system of the mother constantly, from conception to delivery, to prevent rejection of the fetus [8]. Indeed, pregnancy is supported by a unique process of immune-modulation aimed at protecting the fetus from allogenic rejection. Amniotic extracellular vesicles, and other vesicles, could suppress immune rejection against the fetus while maintaining concurrently an adequate inflammatory response by increasing pro-inflammatory cytokines, activating neutrophils and T-cell regulation (i.e., immune modulation) [8]. Amniotic extracellular vesicles also allow for adaptation of other maternal organs besides the immune system during pregnancy [13, 14, 15]. For example, the heart of the third trimester mother undergoes hypertrophy, even for mothers with sedentary lifestyle, its output and stroke volume are akin to that of elite swimmers or runners, except that it is racing for three months uninterrupted, instead of minutes to hours for Olympians [10]. Until recently, there was no explanation for such an extraordinary transformation.
Amniotic extracellular vesicles are carrying a cargo of lipids, proteins, complex sugars, and nucleic acids such as microRNAs (miRs), which support growing tissues in the fetus, and adapt the biology of several tissues in the mother [7, 8, 9, 10, 12]. Amniotic extracellular vesicles are produced by placental, fetal and amniotic cells, and are captured by cells of their target tissues via a process called “membrane ruffling” before fusing with target cells via receptor/ligand interaction [8]. While this process has evolved over more than 7,000,000 years for the human species, and in most pregnancies performs impeccably, few mothers experience pregnancy-related complications including a dreadful cardiac pathology described as peripartum cardiomyopathy (PPCM) [16, 17].
It is highly likely that PPCM, a pregnancy condition of unknown etiology, may develop as a result of an insufficient supply of amniotic extracellular vesicles and their essential cargo [8, 12]. A murine model of PPCM requires a cardiomyocyte-specific deletion of stat3, and presents in pregnant mice as a condition akin to human PPCM [18]. In this model, a 16-kDa fragment of prolactin (16K-PRL) cleaved by cathepsin D causes elevation of miR-146a within the vascular endothelium [19]. The endothelium-produced extracellular vesicles carrying miR-146a, which can then fuse with neighboring cardiomyocytes, results in the elevation of miR-146a levels within cardiac tissue [19]. Targets of miR-146a such as Erbb4, Notch1, and Irak1, are then downregulated in cardiomyocytes, resulting in impaired cardiac contractile function and symptoms [19]. These findings provide evidence for extracellular vesicle-based intercellular communication between endothelium and cardiomyocytes, communication that involves miRs carried by extracellular vesicles [19, 20]. From these studies, we conclude that extracellular vesicles have been actively selected throughout evolution for the purpose of critical intercellular communication and exchange between tissues and whole organisms, and next, we will address why they have been selected for such functions.
Fusion of cells in human physiology and pathology is surprisingly not a frequent event for most tissues. It happens mainly upon interaction between two bound cells of very different sizes, such as when a sperm fertilizes an oocyte, when satellite cells (myoblasts) fuse with multinucleated skeletal muscle fibers in a situation of muscle injury, repair, or growth, or in pathological circumstances where monocytes become activated into macrophages to destroy a much larger target cell (like smooth muscle cell of the arterial wall in atherosclerosis, for example) [21, 22]. In most of these situations, one of the two fusing cells is substantially smaller than its fusion partner. Likewise, amniotic extracellular vesicles, like any extracellular vesicles, are substantially smaller than their target cells (radius about 100-times smaller) [8, 12].
The reason for such discrepancy between fusion partners is best explained by the physics law that Pierre-Simon Laplace established more than two centuries ago (Fig. 2). Laplace was studying fluid mechanics and more specifically the relationship between differential pressure between the inside and the outside of a bubble, surface tension, thickness and radius of the bubble. Assuming rather constant thickness and tension for the detergent bilayer, or lipid bilayer in the case of live cells and extracellular vesicles, the major determinant of the pressure differential is 1/r where r is the radius of the bubble (or cell/extracellular vesicle). Hence, the smaller the vesicle, the higher the pressure, and therefore the inside pressure of an extracellular vesicles is much greater than for much larger target cells. When an extracellular vesicle binds to a cell via receptor/ligand interaction between membranes [23], the higher pressure inside the amniotic extracellular vesicles allows it to extrude its cargo of proteins, complex sugars, miRs into the cytoplasm of the target cell, while its membrane lipids (phospholipids, cholesteryl-ester, etc.), integrate within the plasma membrane of the recipient cell.
 Fig. 2.
Fig. 2.Laplace Law and SECV Pressure. Bubble bilayers are similar to plasma membranes, except that their bilayer is reversed (hydrophobic outside) compared to plasma membrane (hydrophilic outside). Laplace Law, contained in his book Celestial Mechanics, provides the pressure inside a bubble relative to the outside as an equation where the denominator is the radius of the bubble. Hence, assuming that thickness and tension of the plasma membrane are constant, then the smaller the bubble (or the extracellular vesicle) the greater the inside pressure.
In a situation of very fast growth, like the growth of the fetus during the last trimester of pregnancy, it is tempting to speculate that such cargo delivery is critical to providing the massive amounts of substrates that are required for successful completion of organogenesis and maturation of the fetus. It is also required for the adaptation of the mother to the substantial expansion of “her parabiont” (the fetus) in this unique case of heterochronic parabiosis [8].
The growth of the fetus is faster than that of an aggressive tumor, gaining
Indeed, the prescient parabiosis observations of Conboy, Rando et al. [2], were that “a broad improvement in tissue maintenance and repair can be promoted in an old mammal by young systemic factors through boosting the regenerative capacity of tissue stem cells” (Fig. 3A, Ref. [1, 2, 24, 25, 26, 27]) [1, 2]. It is indeed exposure to a young systemic environment that triggers the rejuvenation of aged progenitor cells and tissues [24].
 Fig. 3.
Fig. 3.Heterochronic Parabiosis and Pregnancy. (A) Conboy et al. [1, 2] have explained the effect of heterochronic parabiosis as a conditioning of the niche for endogenous stem cells (as in the bone marrow), such that an old niche, which does not support its stem cells effectively, can be rejuvenated to produce stem cells competent for the repair of damaged and aged tissues by the plasma fraction of the blood originating from the young mouse. (B) Consistently with Conboy’s hypothesis, Loffredo et al. [27] showed the heterochronic parabiosis can prevent age related cardiac hypertrophy. (C) We have reported that young, but not old Lin- cultured bone marrow cells from ApoE-KO mice could delay the development of atherosclerosis in response to high circulating cholesterol levels, but without reducing cholesterol [25, 26]. On this panel we show that injected Lin- cultured bone marrow cells (nuclear beta-galactosidase labeled) can implant on the arterial wall [25]. (D) Some of the implanted cells can display markers of endothelial cells like CD31 (PECAM-1) [25]. (E) Green dots and arrows are added artificially to the original image to illustrate production of extracellular vesicles (not visible at this magnification) by implanted stem cells, as a way to impact adjacent tissues (paracrine effect). (F) Distant cells/tissues (like cardiomyocytes/myocardium) can also be impacted by extracellular vesicles produced at a distance by the endothelium (see yellow dots added artificially to the image of a cardiomyocyte, to illustrate that these extracellular vesicles could also function via endocrine mechanisms.
Our team had previously reported that the repeated administration of cultured young bone marrow Lin-negative cells (Lin- cells), but not the same cells collected from old bone marrow, both obtained from apolipoprotein-E knock-out (ApoE-KO) mice on high fat diet, was able to prevent the development of inflammation and atherosclerosis [25, 26]. And this was despite very high blood cholesterol, which remained unchanged after bone marrow cell transplant (since the marrow donors were ApoE KO mice too).
Loffredo et al. [27] showed more recently, using heterochronic mouse parabiosis as a model, that hypertrophy of the heart of old mice could be mostly prevented by factors originating from the young mouse (Fig. 3B), amongst which they identified growth differentiation factor 11 (GDF11) as an important player. It is increasingly clear that extracellular vesicles contribute substantially to the observations of these studies. Indeed, we reported that young Lin- bone marrow cells cultured in the right conditioning medium (conditioned to develop gene expression markers of mesenchymal stem cells, i.e., consistent with pericytes differentiation) [26, 28], are able to bind to, and integrate within the arterial endothelium (Fig. 3C–D). Others have shown that these cells can produce extracellular vesicles that impact not only the adjacent endothelium, paracrine effect, but also more distant cells like cardiomyocytes, an endocrine effect (Fig. 3E–F). Hence, it is highly likely that amniotic extracellular vesicles are critical to the adaptation of the heart during pregnancy, i.e., a transformation of the heart with increase in stroke volume akin to that of elite runners and swimmers [7].
PPCM is a dreadful condition with an incidence of 4470 new cases per year [16, 17]. The definition of PPCM is “an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of heart failure can be found.” (Heart Failure Association of the European Society of Cardiology Working Group on PPCM) [16]. Risk factors associated with PPCM include advanced maternal age, African-American race, chronic hypertension, preeclampsia, multiple gestations, and prolonged use of tocolytic drugs [16, 17]. There is no known etiology, however, in mice the 16K-PRL causes endothelium elevation of miR-146a, blocks angiogenesis, and leads to cardiomyopathy akin to PPCM via extracellular vesicles enriched in miR-146a [18, 19, 20].
The 16K-PRL hypothesis has triggered much enthusiasm, and this prolactin fragment is one of several antiangiogenic prolactin fragments, together called vasoinhibitins [29]. In the original mouse study, bromocriptine was shown to reverse PPCM [18], however limited initial studies with bromocriptine showed mixed success, and bromocriptine can trigger significant side effects including cardiac problems [29]. However, a recent meta-analysis of randomized controlled trials indicated that addition of bromocriptine to standard heart failure therapy significantly improved the left ventricular ejection fraction of patients PPCM [30]. We propose that the administration of amniotic extracellular vesicles, especially those produced at the end of normal pregnancies (last trimester), could be a safe and effective adjunct therapeutic strategy in combination with bromocriptine [30]. And this approach would be in addition, of course, to existing measures to support the failing left ventricle (left ventricular assist devices and other technologies), the control of arrhythmia (including with automatic implantable cardioverter-defibrillator AICD placement), and a drug regimen adapted from the management of severe heart failure [16].
As demonstrated by Heterochronic Parabiosis Experiments, there is great potential for the use of extracellular vesicles derived from pregnancy in treating the various symptoms of “aging”. Due to their nano size and immune status these extracellular vesicles are freely able to pass from the fetus to the mother through the placental barrier and similarly they are able to cross the blood brain barrier [31]. There exists clear evidence that extracellular vesicles are essential for proper organogenesis in the fetus and in aiding the mother in cellular and tissue repair and organ plasticity. The transfer of extracellular vesicles from fetus to mother in pregnancy is akin to the validated heterochronic parabiosis experiments first described in mice, which contributes to immune modulation and also to maternal health and vibrance during gestation. Treatment of patients with extracellular vesicles derived from healthy pregnancies has great potential within healthcare for both pregnant women experiencing issues such as PPCM and elderly patients experiencing frailty, including cardiomyopathy, neurodegeneration and other common symptoms of aging.
PJGC wrote the initial draft of the manuscript, IAW and PJGC provided key concepts of the review. CH, AJPG and DLD have provided unique and essential contributions based on their particular expertise. All authors have contributed to the generation and final review of the manuscript.
Not applicable.
Not applicable.
NIH R01 AG023073 to PJG-C.
The authors declare no conflict of interest. Neobiosis is a company whose focus is on regenerative medicine.
