Purpose: In the present study, to achieve high paclitaxel (PTX) loading
in a conjugated drug delivery system with minimal long-term side effects, we
formulated a novel degradable stereocomplexed micelle-like particle with a
core-shell structure. Materials and methods: In this system, methoxy
polyethylene glycol (MPEG) acted as the hydrophilic shell, and the stereocomplex
of polylactic acid with PTX (SCPLA-PTX) acted as the hydrophobic core. The
MPEG-SCPLA-PTX micelle-like particles were synthesized via the self-assembly of a
MPEG-poly L-lactic acid (PLLA) copolymer with a PTX-poly
D-lactic acid-PTX copolymer. The resultant copolymers and their
intermediates were characterized using
Paclitaxel (PTX) is a tetracyclic diterpene compound that exhibits considerable
antineoplastic activity against various solid tumors, including ovarian cancer
[1], breast cancer [2, 3], head and neck carcinomas [4, 5], and non-small cell
lung cancers [6]. However, it can easily undergo oxidation, and storage is
difficult. Its poor water solubility (
Several types of PTX formulations are available, including liposomes [14, 15], cyclodextrin inclusion compounds [16, 17], PTX prodrugs [18, 19, 20], PTX nanoparticles, and microspheres [21]. Notably, amphiphilic polymer particles have attracted considerable research interest in pharmaceutical formulation development owing to their excellent performance as drug carriers [22]. Amphiphilic macromolecule polymers can readily self-assemble in aqueous solution to form nanoparticles with a hydrophobic inner core and hydrophilic shell, encapsulating lipophilic drugs in the hydrophobic core to increase drug solubility owing to the lower critical micelle concentration (CMC) of amphiphilic macromolecule polymers [23]. The hydrophilic shell also protects particles from being recognized by the reticuloendothelial system and removal from the body [24]. Polyethylene glycol–polylactic acid (PEG-PLA) is an amphiphilic biodegradable material approved by the U.S. Food and Drug Administration (FDA) for biomedical applications and is widely used as a pharmaceutical drug carrier [25, 26]. We selected MPEG-PLLA as the target product to achieve green and controllable synthesis.
Amphiphilic PEG-PLA chains are used as carriers of PTX; however, the drug loading content is limited by the compatibility between drug molecules and the hydrophobic PLA segment, resulting in undesirable rapid drug release [27, 28, 29, 30]. For chemical cross-linking methods, such as linking by ester bonds, the complex architecture of polymers tends to increase synthetic difficulties. The introduction of harmful and non-degradable ingredients is inevitable, which results in long-term systemic side effects [31, 32]. A biodegradable stereocomplex composed of polylactic acid (SCPLA) is formed by the selective combination of dextral polylactic acid (PDLA) and levo-polylactic acid (PLLA) molecular chains. A few researchers have previously explored its potential application in drug delivery [33, 34] and obtained satisfactory results. In our designed micelle-like particles, we chemically linked PTX to PDLA and utilized the stereocomplex structure to combine it with methoxy poly (ethylene glycol)-PLLA (MPEG-PLLA). Accordingly, we achieved a relatively high PTX-loading efficiency using biodegradable PLA-based particles.
In the present study, MPEG-SCPLA-PTX particles were synthesized via a two-step self-assembly process. In the first step, PTX-PDLA-PTX and MPEG-PLLA formed MPEG-SCPLA-PTX through the stereocomplex formed by PDLA and PLLA. In the second step, MPEG-SCPLA-PTX self-assembled into particles under a hydrophobic effect. Characterization and tumor cell inhibition tests confirmed the successful chemical embedding of PTX into particles and the effectiveness of MPEG-SCPLA-PTX particles.
MPEG (molecular weight: 5000 Da and 2000 Da) was purchased from Sigma-Aldrich
(Burlington, VT, USA). l-lactide (LLA) was purchased from Shenzhen Esun
Industrial Co. Ltd. (Shenzhen, China), and PTX was obtained from J&K Scientific
Ltd. (Guangzhou, China). Tetrahydrofuran (THF) was purchased from Tianjin Kemi
Europe Chemical Reagent Co., Ltd. (Tianjin, China). Diethyl ether, succinic
anhydride (99%), N,N-dimethylpyridin-4-amine (DMAP; 99%), and DCC
(99%) were obtained from Alfa Aesar (Shanghai, China).
Zn(Oct)
As shown in Fig. 1a, MPEG-PLLA copolymers were synthesized via ring-opening
polymerization (ROP) [35]. l-lactide and MPEG were used as raw materials
for the synthesis of amphiphilic diblock copolymers. The molecular weight of the
MPEG-PLLA copolymer was regulated by the molar ratio of the monomers (molecular
weight and ratio are listed in Supplementary Table 1 in the
Supplementary material). A specific amount of MPEG, LLA, and
Zn(Oct)
 Fig. 1.
Fig. 1.Synthesis route for the formation of (a) MPEG-PLLA, (b) PTX-PDLA-PTX, and (c) MPEG-SCPLA-PTX particles.
Synthesis route for PTX-PDLA-PTX is shown in Fig. 1b. The typical procedure was as follows: 230 g of d-lactic acid was added to a
four-necked flask. The flask equipped with a mechanical stirrer and reflux
condenser was connected to a vacuum-argon system, and the flask
was evacuated and charged with argon three times. The mixture was heated to 100
Purified PDLA (3 g, 1.5 mmol), succinic anhydride (0.3002 g, 3 mmol), and DMAP
(0.3665 g, 3 mmol) were dissolved in 1, 4-dioxane (20 mL) and reacted in a
nitrogen atmosphere for 48 h at room temperature. The product was precipitated in
n-hexane, and the polymer precipitate was
dried under a vacuum for 48 h at room temperature. The polymer was reconstituted
using THF and poured into a large amount of
distilled water. The resultant precipitate was filtered and dried under vacuum at
room temperature for 48 h. A dicarboxylic PDLA (DI-PDLA) with terminal carboxyl
groups was obtained. (M
DI-PDLA (0.222 g) and PTX (126.5 mg) were dissolved in anhydrous methylene
chloride (30 mL) in a dried flask. DCC (30.6 mg) and DMAP (18.1 mg) were added at
0
Synthesis route for MPEG-SCPLA-PTX particles is shown in Fig. 1c. PTX-loaded particles were prepared using a solvent displacement method with a
THF/H
Blank particles were prepared in a manner similar to PTX-loaded particles. MPEG-PLLA (22 mg, 0.002 mmol) and DI-PDLA (12.6 mg, 0.006 mmol) were first dissolved in THF (10 mL), and the following steps were identical to those previously described.
GPC measurements were conducted using a PL-GPC120 instrument (Polymer
Laboratories, Shropshire, UK) equipped with an Agilent MIXED-B column and a
differential refractometer detector. THF (with 0.02–0.03 wt% BHT stabilizer)
was used as the eluent and maintained at a flow rate of 1 mL/min at 40
The drug loading efficiency (LE) was calculated as follows:
The calculation formula could be further specified to:
where M
Based on the dosages of MPEG-PLLA and PTX-PDLA-PTX (Supplementary Table 5), the total theoretical PTX loading of the particles can be calculated as:
Physical embedding ratio can be calculated as:
where m
Considering that PTX is loaded only by physical or chemical methods, the chemical embedding ratio is calculated as:
CMC represents the lowest concentration of the block copolymer required in
solution to form polymer micelles [36], which is used to characterize the
stability of micelle-like particles. The CMC of the PTX micelle-like particles
and blank micelle-like particles was determined using a fluorescence technique
with pyrene as a hydrophobic probe. Steady-state fluorescence spectra were
obtained using a PerkinElmer LS50B luminescence spectrometer (ALT, Norwalk, CT,
USA). A predetermined amount of pyrene solution in acetone was added to a
volumetric flask, and the acetone was completely evaporated. The flask containing
the solid pyrene was filled with DI water up to
the calibration line. The pyrene concentration in the final solution was
12
In vitro PTX release from block copolymer particles was investigated in
PBS (pH = 7.4) [38]. Typically, 10 mL of 5-6 particles at a concentration of 1.4
mg/mL, 2-6 particles at a concentration of 1.7 mg/mL, and 5-2 particles at a
concentration of 1.9 mg/mL were placed in a dialysis bag (MWCO = 2500 Da). The
release experiment was initiated by placing the end-sealed dialysis bag in 40 mL
PBS solution at 37
HeLa cells were maintained in modified Eagle’s medium (MEM) with 10% fetal
bovine serum (FBS) under standard cell culture conditions (37
The viability of HeLa cells was determined using CCK-8 assay. MK571 and PSC833
(10 mM), known inhibitors of multidrug resistance-associated proteins
(MRPs/ABCCs), and a subfamily of ATP-binding cassette transporters (ABC
transporters), were used as positive controls. After 24 h exposure to different
concentrations of PTX-loaded particles, 10
Intracellular ROS levels were assessed based on the increased fluorescence
values of the DCF cell dye. To reduce errors due to loss of cell numbers during
exposure to PTX-loaded particles, Hoechst 33342 (YeaSen Biotechnology, Shanghai,
China), a fluorescent dye used to stain DNA, was employed to measure the number
of HeLa cells remaining in each well and normalize the DCF fluorescence value.
After PTX exposure, DCF (final concentration: 10
Mitochondrial membrane potential, an early indicator of cell apoptosis, was measured using a JC-1 detection kit [41]. After exposure to PTX for 24 h, JC-1 solution (final concentration: 20 nM) was added to each well of a 96-well plate, and the plate was incubated for 25 min. The fluorescence intensity of JC-1 was measured using a microplate reader. Two fluorescence values were observed for JC-1. The excitation and emission wavelengths for green fluorescence were 485 and 530 nm, respectively, and those for red fluorescence were 530 and 590 nm, respectively. The ratio of red to green fluorescence was used to determine the mitochondrial membrane potential.
Lysosomes in HeLa cells were determined using LysoTracker Deep Red (Molecular Probes)( Thermo Fisher Scientific, Shanghai, China), which indirectly indicates the cellular uptake of PTX [42]. Similar to the DCF assay, Hoechst 33342 was used to normalize the fluorescence value of LysoTracker. After exposure to PTX for 24 h, LysoTracker solution (final concentration: 50 nM) was added to each well, and the cells were incubated for 30 min. Hoechst 33342 was added and the cells were incubated as described above. Finally, the cells were analyzed using a microplate reader. The excitation and emission wavelengths for LysoTracker were 647 nm and 668 nm, respectively.
CAM was used to assess ABC transporter activity using a dye accumulation assay
[43, 44]. After exposure to PTX for 24 h, CAM at a final concentration of 0.25
In the present study, MPEG reacted completely without residual monomers, as the characteristic MPEG peak was not observed in the GPC traces (Fig. 2a,b). A single peak with a relatively narrow distribution indicates the successful synthesis of MPEG-PLLA, DI-PDLA, and PTX-PDLA-PTX (Fig. 2).
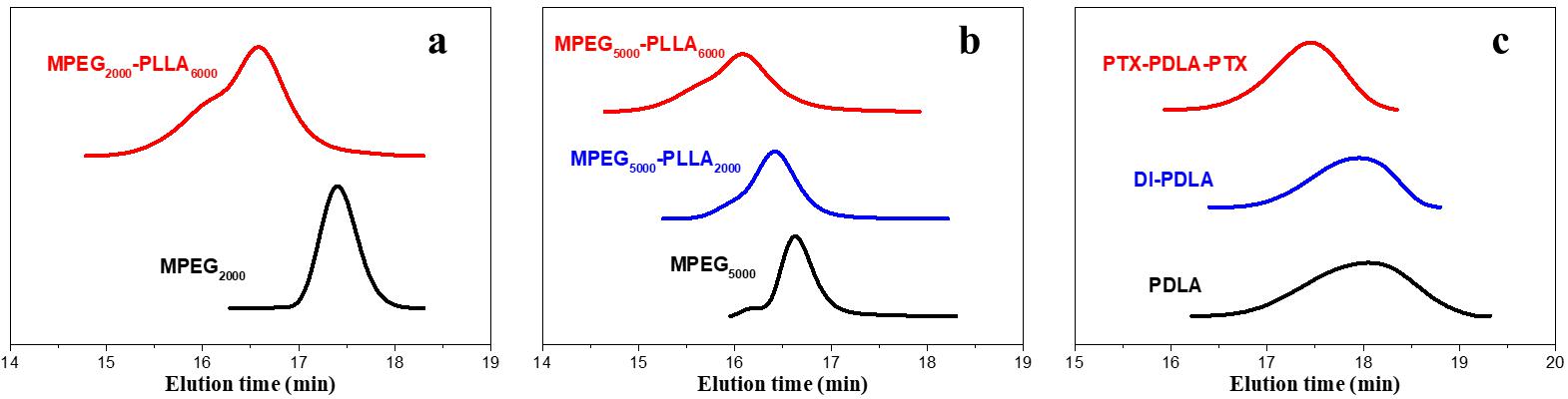 Fig. 2.
Fig. 2.GPC traces of (a) MPEG2000 and MPEG2000-PLLA6000; (b) MPEG5000, MPEG5000-PLLA2000, and MPEG5000-PLLA6000; (c) PDLA and its derivative. Abbreviation: GPC, gel permeation chromatography.
The
 Fig. 3.
Fig. 3.
The
 Fig. 4.
Fig. 4.
The specific molecular weights and distribution coefficients of PDLA and its
derivatives are listed in Supplementary Table 2. For the
molecular weight, a specific difference can be observed between the results
calculated from
The thermodynamic properties of the micelle-like particles and MPEG-PLLA were
measured by DSC (Fig. 5), and the results confirmed the self-assembly of
enantiomeric PLA-based copolymers. The peak at a relatively high temperature
corresponds to the melting point of the PLA chain. Owing to the formation of
SCPLA, the melting point of the PLA chain in 5-6 particles and 2-6 particles
increased from 151.6
 Fig. 5.
Fig. 5.DSC curves of (a) MPEG
The particle size and distribution of the drug-loaded and blank copolymer particles were investigated by DLS, as shown in Table 1 and Fig. 6. The DLS results of drug-loaded and blank copolymer particles all displayed narrow, single peaks and a normal distribution at a concentration of 0.5 mg/mL, indicating that the particle size distribution was relatively concentrated. The average particle size of 5-6 blank particles, 2-6 particles, 5-6 particles, and 5-2 particles was 78.8 nm, 141.3 nm, 111.4 nm, and 132.4 nm, respectively. The mean particle size of 5-6 blank particles was substantially smaller than that of the other particles owing to a lack of PTX.
 Fig. 6.
Fig. 6.Particle size and distribution of drug-loaded and blank copolymer particles.
| 5-2 particles | 5-6 particles | 2-6 particles | 5-6 blank particles | |
| Average size | 132.4 nm | 111.4 nm | 141.3 nm | 78.8 nm |
The relative molecular ratio of the components in the copolymer demonstrated a
marked impact on the micellar size. Comparing 5-6 particles with 2-6 particles,
the length of PLA at the hydrophobic end was consistent, and accordingly, the
hydrophobic core of the micellar particles was equally large. The 5-6 particles
presented a longer MPEG chain that greatly improved the particle hydrophilicity;
hence, the particle size was less than that of 2-6 particles (111.4 nm
TEM images of 5-6 particles, 2-6 particles, 5-2 particles, and 5-6 blank particles are shown in Fig. 7a–d, and the SEM images are shown in Fig. 7e–h. The as-formed particles have a spherical morphology with an approximate diameter of 100 nm. The size measured by DLS analysis was slightly larger than that determined by TEM and SEM images, and some particles with diameters larger than 200 nm were observed in the SEM images (Fig. 7g). This could be attributed to the dehydration of the samples during the preparation. Dehydration of the hydrophilic MPEG shell particles resulted in smaller particles. However, micellar aggregation resulted in several larger particles.
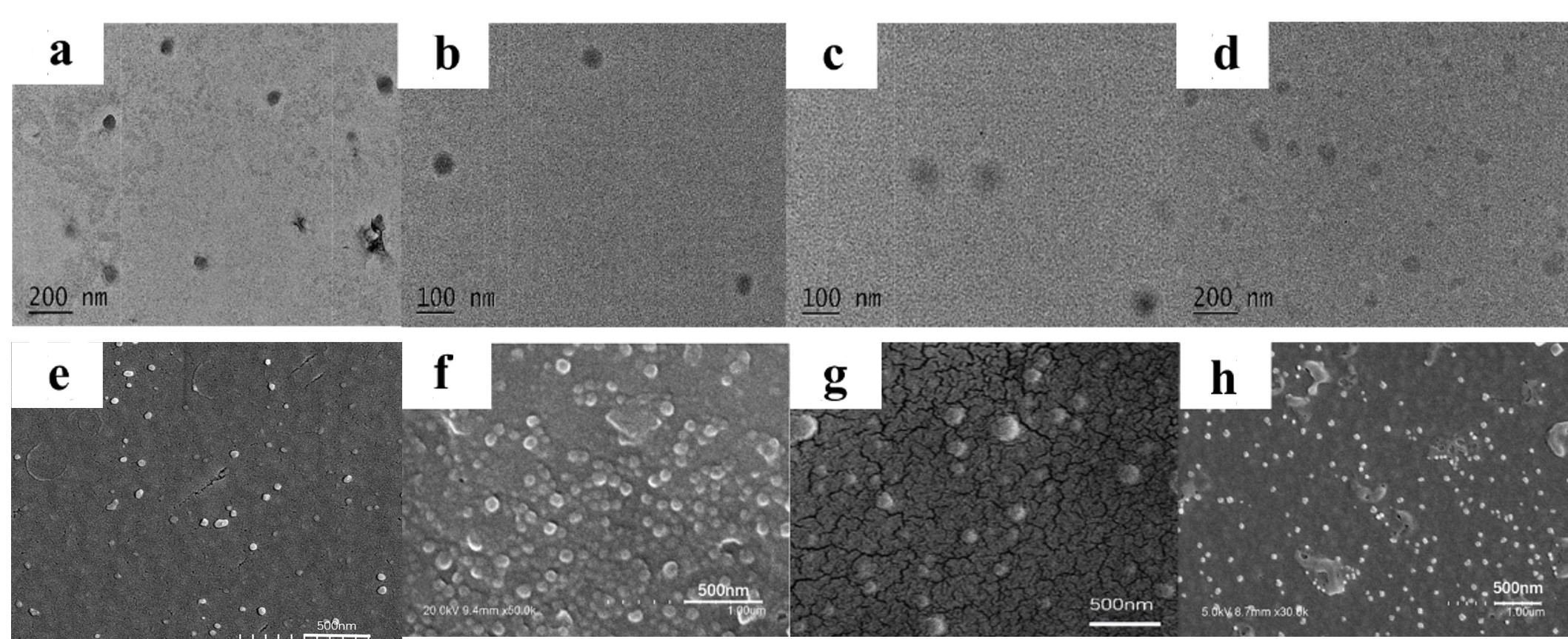 Fig. 7.
Fig. 7.TEM a-d and SEM e-h images of particles. (a,e) 5-6 particles; (b,f) 2-6 particles; (c,g) 5-2 particles; (d,h) 5-6 blank particles. Abbreviation: TEM, transition electron microscopy; SEM, scanning electron microscopy.
Based on these calculations, the PTX loading efficiencies for different particles are listed in Table 2. The physical loading of PTX in 5-6 particles, 2-6 particles, and 5-2 particles is shown in Table 3. The loading content of PTX in 2-6 particles reached 20.11%. Drug loading ratio of the chemical method On comparing 5-6 particles with 5-2 particles, it was observed that the larger the molecular weight of PLA, the higher the drug loading content of PTX.
| 2-6 particles | 5-2 particles | 5-6 particles | 5-6 blank particles | |
| TTLE (%) | 20.11 | 11.65 | 17.22 | 0.0 |
| Drug loading ratio | 5-6 particles | 5-2 particles | 2-6 particles |
| Physical embedding method (%) | 5.94 | 4.89 | 5.74 |
| Chemical embedding method (%) | 94.06 | 95.11 | 94.26 |
The fluorescence emission spectra of the pyrene probe in 5-6 particles, 2-6 particles, 5-2 particles, and 5-6 blank particles at different polymer solution concentrations are shown in Supplementary Fig. 3. The maximum peak intensity ratio I335/I333 in the excitation spectrum was plotted against the logarithm of the polymer concentration, and each sample exhibited an S-shaped curve, as shown in Fig. 8.
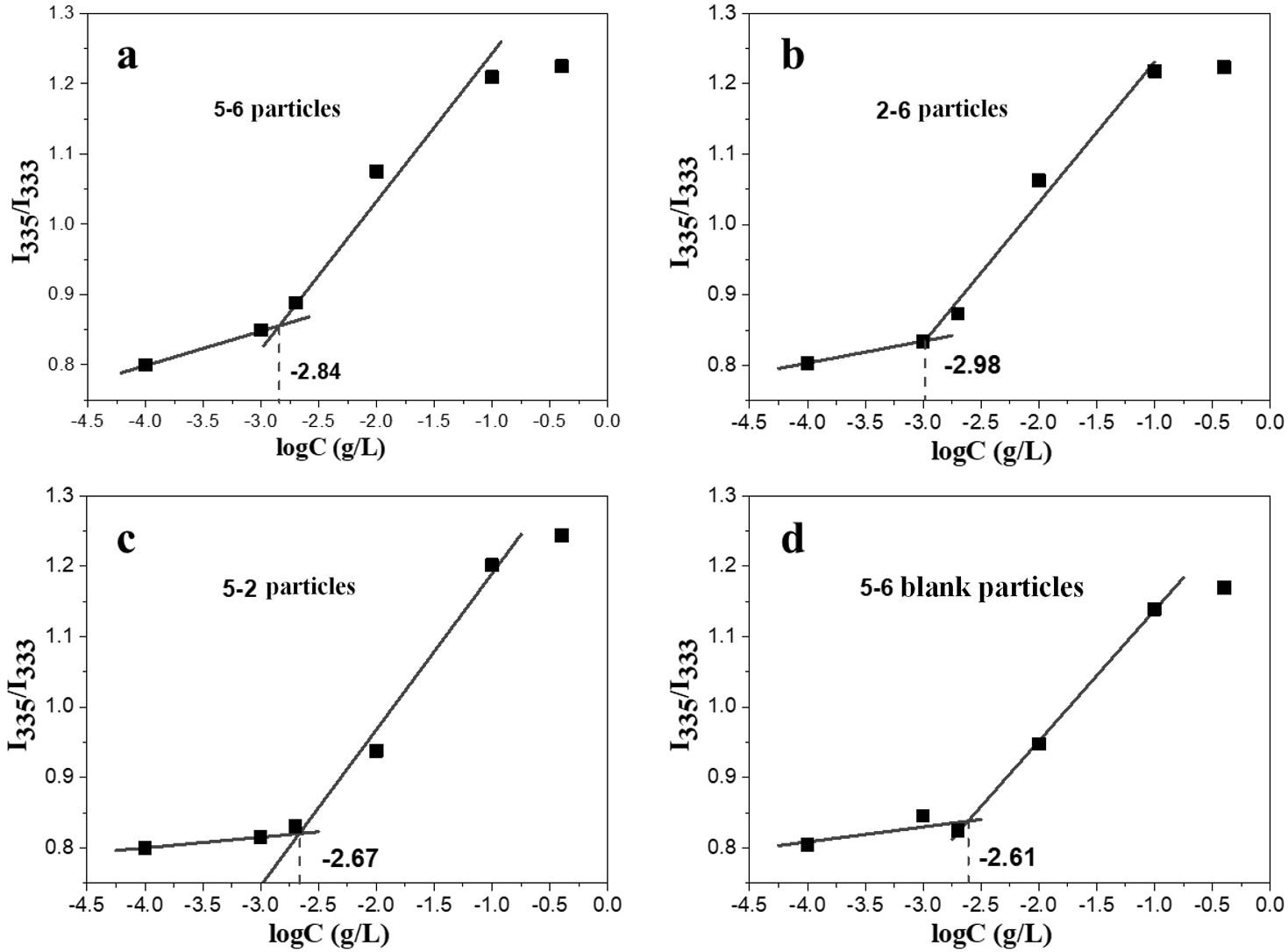 Fig. 8.
Fig. 8.Curves of maximum excitation peak intensity ratio I335 nm/I333 nm of micellar aqueous solution on the logarithm of concentration. (a) 5-6 particles. (b) 2-6 particles. (c) 5-2 particles. (d) 5-6 blank particles.
The CMC of drug-loaded 5-6 particles, 2-6 particles, 5-2 particles, and 5-6
blank particles were 1.4
As shown in Fig. 9 and Table 4, 5-2 particles had the largest cumulative PTX
release percentages of 52.0% at 216 h. The cumulative release rate reached
20%–30% of the drug-loaded particles in the first 50 h, meeting the clinical
need for achieving an effective blood concentration of 0.01
 Fig. 9.
Fig. 9.Cumulative release percentage-release time curve of the in vitro release of 5-2, 5-6, and 2-6 particles at 216 h.
| 5-6 particles | 5-2 particles | 2-6 particles | |
| Release | 51.5% | 52.0% | 37.7% |
By comparing 5-6 particles with 2-6 particles, we observed that 5-6 particles presented a faster release of PTX than 2-6 particles. This may be attributed to the improved hydrophilicity of micellar nanospheres with longer MPEG segments. Improved hydrophilicity promotes hydrolysis of ester bonds between PTX and PDLA, facilitating the release of PTX. On comparing 5-6 particles with 5-2 particles, we found that the molecular weight of PLA minimally influenced the release rate. Thus, increasing the molecular weight of MPEG promoted the release of PTX.
The performances of the different types of PTX-loaded particles are listed in Table 5 (Ref. [30, 32, 46, 47, 48, 49]) [46, 47, 48, 49, 50, 51]. In the present study, relatively high loading efficiency and controlled release of PTX were achieved by preparing biodegradable PLA-based particles. Moreover, the amount of PTX loaded can be adjusted by modulating the PLLA chain length, and the release rate can be adjusted by adjusting the chain length of the MPEG in the MPEG-PLLA segment.
| PTX loading method | Particle size (nm) | CMC (g/L) | Release rate | Maximum drug load (%) | |
| Chemical methods | Click chemistry of azide-alkynyl groups | 10–100 | / | 65% at 72 h | 23.2 [32] |
| Ester bond | 180–210 | 2.5 × 10 |
/ | 16.0 [46] | |
| Ester bond | 130 | 6.3 × 10 |
50–55% at 216 h | 10.0 [47] | |
| Physical methods | Lipophilicity of PTX | 2–3 × 10 |
/ | / | 10.8 [48] |
| Lipophilicity of PTX | 96 | 0.8 × 10 |
95–100% at 45 h | 10.6 [30] | |
| Lipophilic of PTX | / | / | 74–88% at 24 h | 4.6 [49] | |
| Particles in this paper | Ester bond | 110–130 | 1.0–2.5 × 10 |
37–52% at 216 h | 20.11 |
Cell viability, excessive ROS generation, increase in mitochondrial depolarization, and cellular uptake of drugs were used to characterize the tumor cell inhibitory effects of PTX and PTX-loaded particles (Fig. 10).
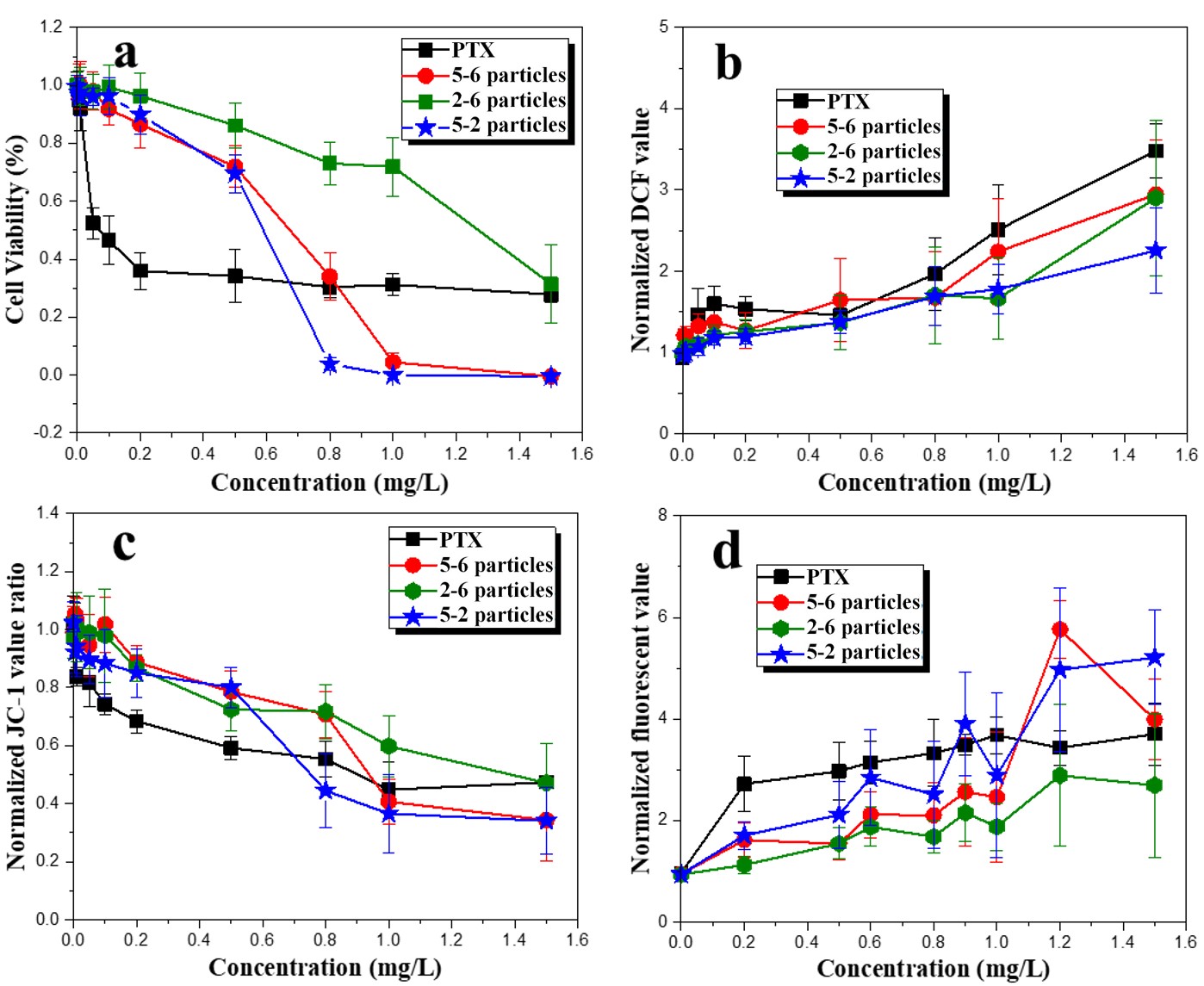 Fig. 10.
Fig. 10.(a) Cell viability, (b) normalized DCF fluorescence values, (c) mitochondrial membrane potential, and (d) normalized LysoTracker fluorescence values after 24 h exposure to PTX, 5-6 particles, 2-6 particles, and 5-2 particles.
As shown in Fig. 10a, PTX significantly decreased cell viability at 0.05 mg/L and higher concentrations. And 5-6 particles, 2-6 particles, and 5-2 particles decreased cell viability at 0.1 mg/L, 0.5 mg/L and 0.2 mg/L, respectively. At a concentration of 0.6 mg/L and lower, PTX induced greater cytotoxicity than the drug-loaded particles due to its burst release effect, which is undesirable in clinical applications. 5-6 particles and 5-2 particles induced higher cytotoxicity than PTX and 2-6 particles at concentrations above 0.8 mg/L with increasing exposure concentrations, demonstrating the controlled release process of drug-loaded particles.
Excessive ROS generation, an important indicator for monitoring cancer cells, can induce cell damage by destroying cellular proteins, lipids, and nucleic acids, affecting normal cellular signaling pathways and gene regulation [50]. Intracellular ROS generation was measured using DCF fluorescence (Fig. 10b). Following exposure to PTX and PTX-loaded particles, intracellular ROS generation significantly increased. The lowest effective concentration of ROS generation for PTX and three drug-loaded particles were 0.05 mg/L and 0.5 mg/L.
The increase in mitochondrial depolarization was determined by the decrease in the red/green fluorescence intensity ratio (Fig. 10c). PTX and other particles all individually decreased the red/green ratio of JC-1 value ratio at 0.01 mg/L, 0.5 mg/L, and higher concentrations, indicating the occurrence of mitochondrial depolarization and potential cell apoptosis.
The cellular uptake of drugs was measured using the cell dye LysoTracker, which can indirectly determine the number of lysosomes (Fig. 10d). The results showed that exposure to PTX and drug-loaded particles significantly increased the number of lysosomes in HeLa cells. For PTX, the lowest effective concentration was 0.2 mg/L. For 5-6 particles, 2-6 particles, and 5-2 particles, the lowest effective concentrations were 0.6 mg/L, 0.5 mg/L, and 0.5 mg/L, respectively.
Generally, the lowest effective concentrations for the three particles are 0.5 mg/L, approximately. Furthermore, 2-6 particles caused lower cytotoxicity than 5-6 particles and 5-2 particles, which might be attributed to the lower release percentage (Table 4) and larger size (Fig. 6), which limits the number of drugs that enter the cell.
In the present study, MK571 and psc833, inhibitors of MRPs and P-gp transporter
activity, were selected as positive controls. According to the significance test
results (P
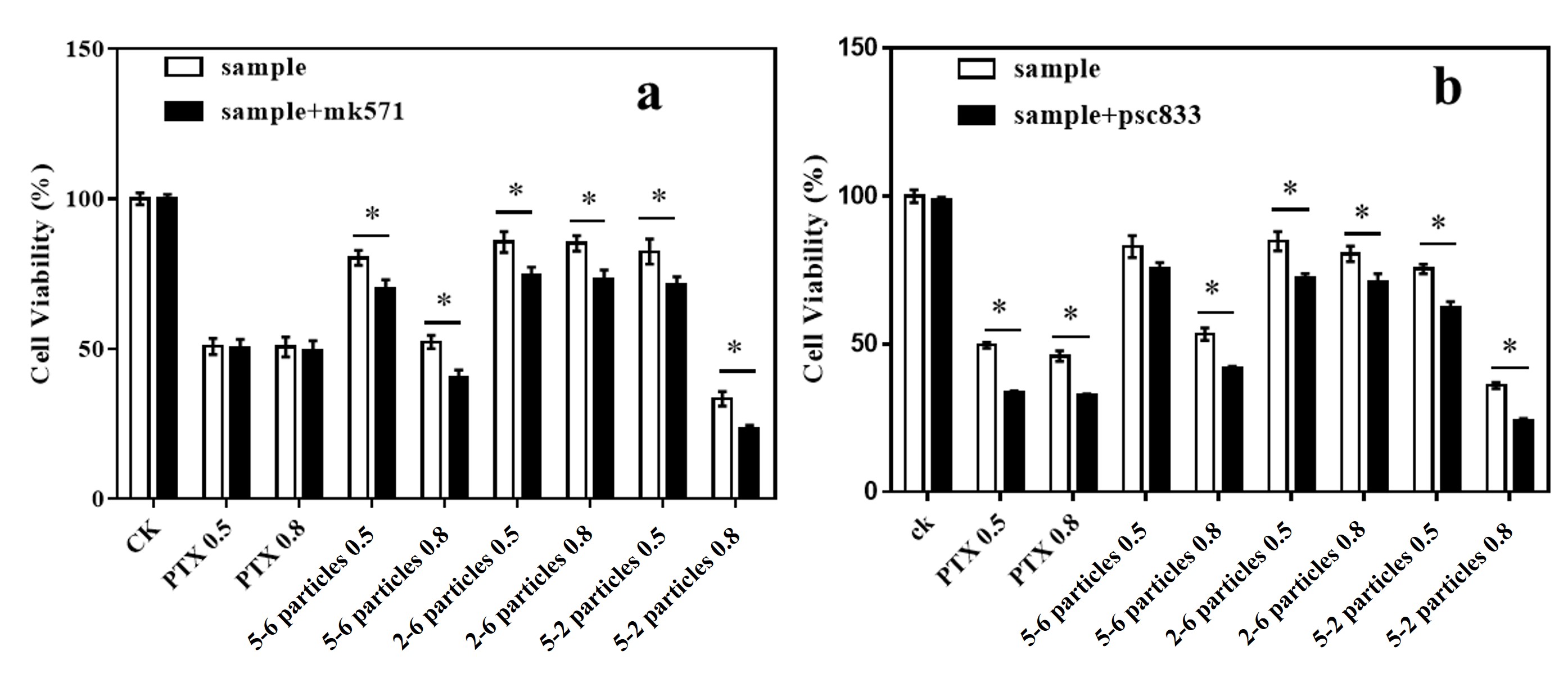 Fig. 11.
Fig. 11.Cell viability comparison with and without positive inhibitors. (a) MK571 as a positive inhibitor; (b) psc833 as a positive inhibitor.
CAM is a substrate of ABC transporters [51] and can be metabolized into calcein, which is not an ABC transporter substrate and is fluorescent; thus, it gets trapped in the cytoplasm. If the activity of ABC transporters is inhibited, calcein tends to accumulate, increasing the fluorescence values. The results of CAM accumulation induced by carrier material of PTX, PTX, 5-6 particles, 2-6 particles, and 5-2 particles exposure in HeLa cells are shown in Fig. 12.
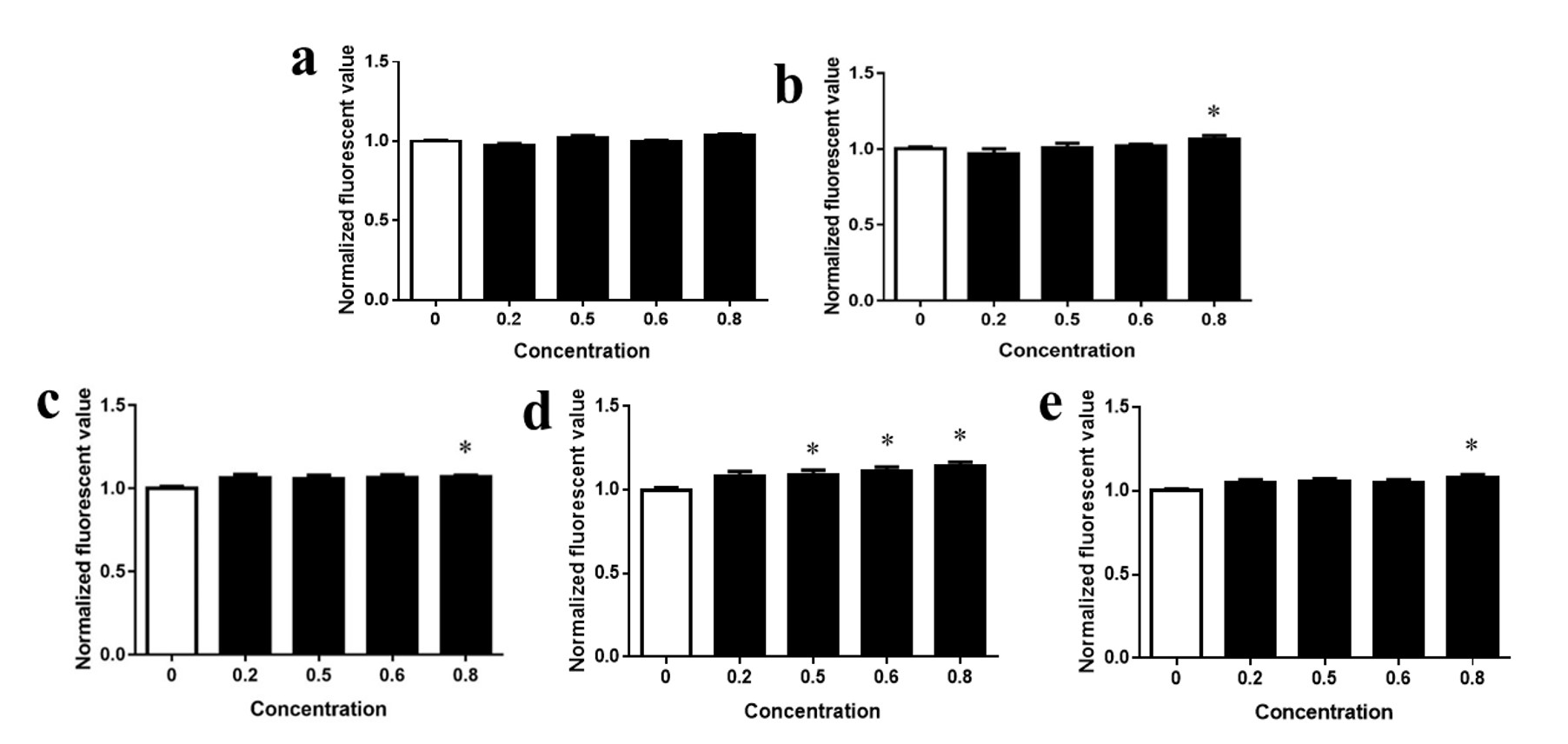 Fig. 12.
Fig. 12.Relative CAM fluorescence of (a) carrier material; (b) PTX; (c) 5-6 particles; (d) 2-6 particles; and (e) 5-2 particles. Abbreviation: CAM, calcein-AM; PTX, paclitaxel.
When the carrier material is exposed to cells alone, CAM accumulation does not
occur (Fig. 12a). Exposure of HeLa cells to PTX, 5-6 particles, 2-6 particles,
and 5-2 particles increased the fluorescence values of CAM (Fig. 12c–e),
indicating inhibition of ABC transporter activity. Furthermore, PTX, 5-6
particles, 2-6 particles, and 5-2 particles showed highly statistically
significant differences (P
It could be postulated that when the drug-loaded particles enter cells, they are engulfed by lysosomes (Fig. 13), releasing PTX from the loading materials. The drug and drug-loaded particles entering cells induce oxidative stress by disturbing the balance between oxidant and antioxidant processes, such as the glutathione system. Subsequently, the generated ROS destroy the mitochondria and stimulate the production of lysosomes, inhibit cancer cell proliferation, and promote apoptosis.
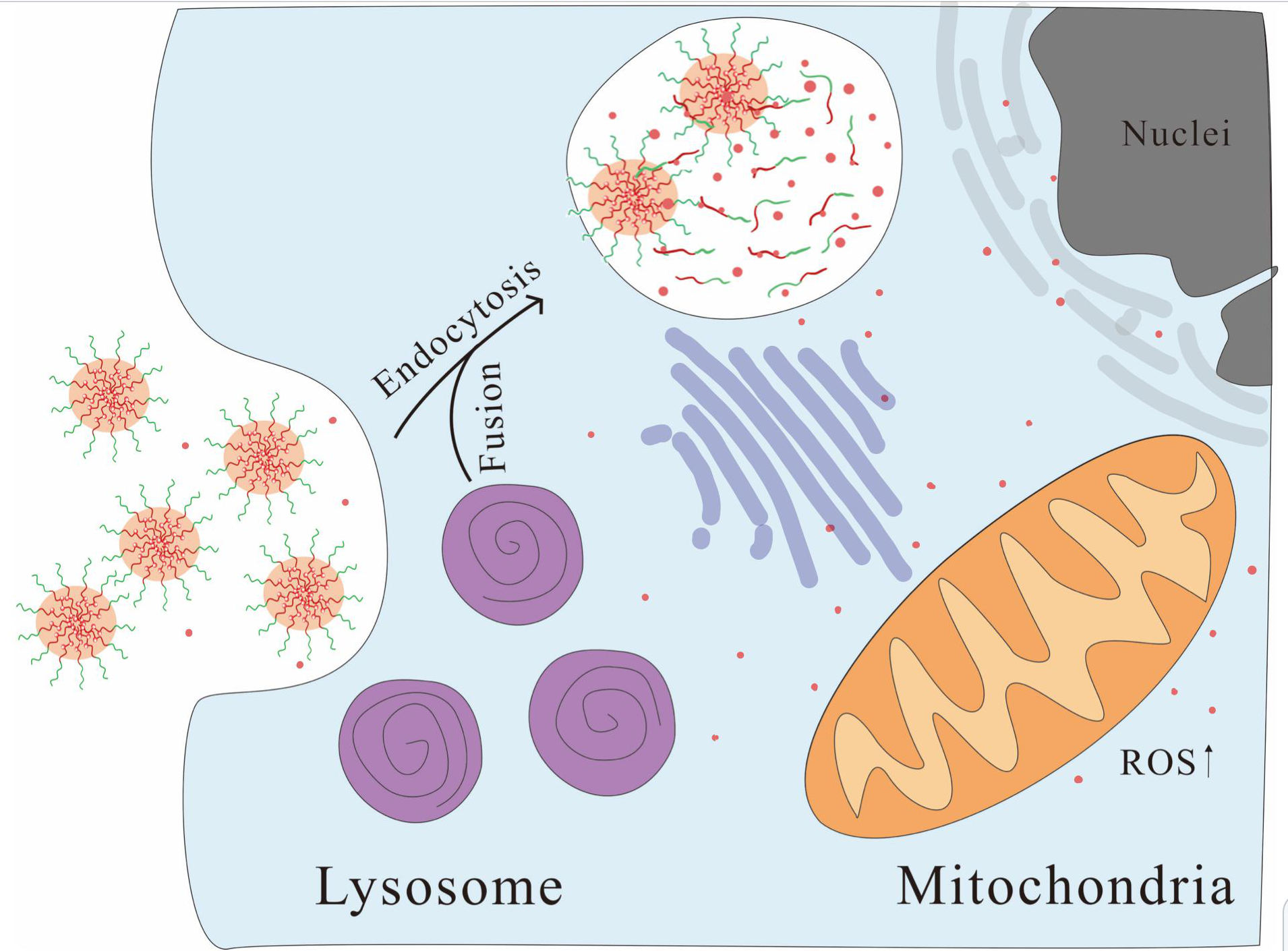 Fig. 13.
Fig. 13.Controlled release of PTX by MPEG-SCPLA-PTX particles in HeLa cells.
This is the first study to propose a method for combining PTX-PDLA-PTX and
MPEG-PLLA to prepare MPEG-SCPLA-PTX drug-loaded particles via a combination of
chemical embedding and stereocomplexation. The average particle size was
approximately 100 nm, which is suitable for human administration. Its low CMC
(1.0–2.5
SC conceived and designed the experiments; SC, YW performed the experiments; SC, YW, BW, WJ analyzed the data; WJ and QZ contributed reagents and materials.
Not applicable.
Authors’ are very grateful to Miss Jing Yu for her assistance in the Tumor cell experiment.
This work was supported by the National Natural Science Foundation of China (NSFC) (21304045).
The authors declare no conflict of interest.
