† These authors contributed equally.
Notwithstanding previous studies have proved the anti-apoptotic effect of Bcl-2 associated athanogene3 (BAG3) in myocardium, the structural domains PXXP and BAG responsible for its protection are not reformed. Since BAG3 in cardiomyocytes is a new target for inhibiting apoptosis induced by hypoxia/reoxygenation (H/R) stress, we demonstrated that over-expression of BAG3 reduced the injury induced by H/R in either neonatal or adult rat cardiomyocytes (NRCMs and ARCMs, respectively) and PXXP and BAG domains play an important role in cellular protection in H/R stress. Apoptosis in cardiomyocytes induced by hypoxia-reperfusion was evaluated with propidium iodide (PI) staining, cleaved caspase-3, and terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining in cultured NRCMS. Either increasing expression of BAG3 or its mutants was performed to manipulate the level of BAG3. Co-immunoprecipitation (Co-IP) was used to demonstrate the complex that BAG3 is binding to HSC70 and JNK. PXXP and BAG domains of BAG3 played an essential role in BAG3 attenuating cardiomyocytes apoptosis induced by H/R through the JNK signalling pathway. The cellular protection of BAG3 with its structural domain PXXP or BAG is associated with the binding with HSC70 and JNK. These results showed that the protective effect of BAG3 on apoptosis induced by H/R stress is closely related to its structural domains PXXP and BAG. The mechanism may provide a new therapeutic strategy for the patients suffering from ischemic cardiomyopathy and may be a critical role of its PXXP and BAG3 domains.
Cardiac ischemia can be intermittent and life-saving revascularisation is necessary, reperfusion that affected muscle could be from further muscle damage, which is an ischemia/reperfusion (I/R) injury [1] that is a major cause of mortality and disability worldwide [2]. Thus, it is necessary to protect cardiomyocytes against both ischemic and I/R injury to limit the size of the original myocardial infarction, cardiac apoptosis, and preserve cardiac function.
Bcl-2 associated athanogene3 (BAG3) is a member of the chaperone BAG family [3]
and is prominently expressed in cardiac and skeletal muscle tissues [4]. BAG3
variation causes family cardiac disease [5, 6] and over-expression of BAG3
preserves the cardiac contractile function in animal study [7, 8, 9] through
improving intracellular Ca
Since JNK signalling has been displayed in cellular injuries induced by hypoxia-reperfusion [22, 23, 24] and participated in BAG3 cellular function in cell death and cell proliferation [25, 26, 27], the present study explores the possible effects of PXXP and BAG domains of BAG3 on its cellular protection and to determine if JNK signalling is involved in its constructive protection of BAG3 using the cultured neonatal or adult rat cardiomyocytes (NRCMs and ARCMs, respectively) under H/R stress.
Neonatal (1-3 day) and adult SD rats were purchased from Animal Centre of Nantong University. The study was approved by The Board of Nantong University Animal Care and Use.
Rat cardiomyocytes were isolated as previously described [28, 29]. NRCMs were isolated by pancreatin digestion and cultured in the DMEM/F-12 complete medium containing 10% foetal bovine serum, 100 U/mL penicillin-streptomycin solution and 0.1 mmol/L 5-bromo-2’-deoxyuridine for three days before passaging. ARCMs were isolated by Langendorff perfusion, Type II collagenase digestion, and cultured in the serum-free Medium199 with 100 U/mL penicillin-streptomycin solution for 2-4 h before passaging.
Cells were cultured with a serum-free medium after pre-treatment such as drug
stimulation for 8–12 h, and then exposure to an oxygen-deficient environment with
humid 5% CO
RNA levels of BAG3 in NCRMs were quantified by qPCR. The total RNA of cultured
cardiomyocytes was extracted according to protocol of TRIZOL Reagent (WI).
Step-one Real-Time PCR System (CA) was used to amplified cDNA. Primer sequences
of BAG3 (Accession No. 293524) were 5’-GAGGTCCCAGTCTCCTTT-3’ (sense) and
5’-GACGGAGACTGAGATCGC-3’ (anti-sense), respectively. The temperature curve was
established as follows: 95
BAG3shRNA-Ad was constructed as previously described [30]. An RNAi-ready
pSIREN-DNR vector was set with a dsDNA oligonucleotide targeting specific BAG3
mRNA. After ligation, the shRNA expression box was transferred to the adenovirus
receptor vector pLP-Adeno-X-PRLS DNA (BD-Adeno-X Expression System 2), which
contains the
Full-length BAG3 (Ad-FLAG-BAG3-WT), mutations of BAG3
(Ad-FLAG-BAG3-
Cardiomyocytes were transfected with Adenovirus by 100 MOI (multiplicity of infection) in an Enhanced Infection Solution for 10–12 h. Cells were then fed with culture medium for 36–72 h.
Propidium iodide (PI) assay was used to quantify the death of cardiomyocytes
with propidium iodide (C0080, Solarbio, China). Cardiomyocytes were rinsed with
PBS after H/R treatment and incubated with Hoechst H33342 (B8040, Solarbio,
China) for 15–30 min at
TUNEL staining was used to quantify the apoptosis of cardiomyocytes with the
TUNEL kit. Cardiomyocytes were rinsed with PBS after H/R treatment and fixed with
the blocking solution for 5–15 min at the room temperature followed by
permeabilisation with 0.3–0.5% by volume of triton-X-100 for 5–10 min at 4
NP40 lysis Buffer was used to extract the total proteins from the cultured
cardiomyocytes. Protein sample were separated on 6–15% SDS PAGE gels and
transferred to the NC or PVDF membranes for 90 min followed by blocking for 2 h
in the TBST with 5% non-fat dry milk. The membranes were incubated overnight at
4
RIPA lysis buffer was used to extract the total proteins from
the cardiomyocytes, 1000
The grey value of the results of Western blot assay was analysed by Image J (National Institutes of Health, USA). GraphPad Prism6 was used for statistical analysis (Inc., San Diego, CA, USA).
To determine the effects of H/R on BAG3 induction, NRCMs were cultured under H/R or normoxia conditions as a control. q-PCR and Western blot analysis revealed that both mRNA and protein levels of BAG3 in the H/R group were decreased compared with that in the normoxia group (Fig. 1A,B). Similar results pertaining to BAG3 protein levels in ARCMs are shown in Fig. 1C.
 Fig. 1.
Fig. 1.H/R decreased both mRNA and protein levels of BAG3 in NRCMs (A,
B) and ARCMs (C). (A) H/R decreased the mRNA levels of BAG3 compared to normoxia
conditions (***P
To investigate the protective effect of over-expression of BAG3, PI and TUNEL staining were used to detect the apoptosis of rat cardiomyocytes induced by hypoxia-reoxygenation respectively. As expected, over-expression of BAG3 by adenovirus vector containing rat BAG3 was evinced by western blot assay shown in Fig. 2A,B. TUNEL assay showed that the TUNEL positive nuclei cell counts were significantly enhanced by H/R stress, over-expression of BAG3 reduced the positive rate in H/R compared with the vehicle group in NRCMs (Fig. 2C,D). PI assay indicated that over-expression of BAG3 suppressed the death rate in H/R compared with vehicle group in NRCMs as shown in Fig. 2E.
 Fig. 2.
Fig. 2.Over-expression of BAG3 protects the cellular apoptosis induced
by H/R in NRCMs. (A) A representative image. Cell lysates were immunoblotted for
BAG3. GAPDH served as a protein loading control. (B) Transfected with Ad-BAG3
increased protein levels of BAG3 compared to GFP, control groups (***P
In order to identify the effects of decreased expression of BAG3, ARCMs were transfected with adenovirus vector containing sh-BAG3 adenovirus or GFP (negative adenovirus) as a control. Western blot analysis indicated that knocking down BAG3 in cardiomyocytes changed the level of apoptosis proteins BAX and Bcl-2 as shown in Fig. 3.
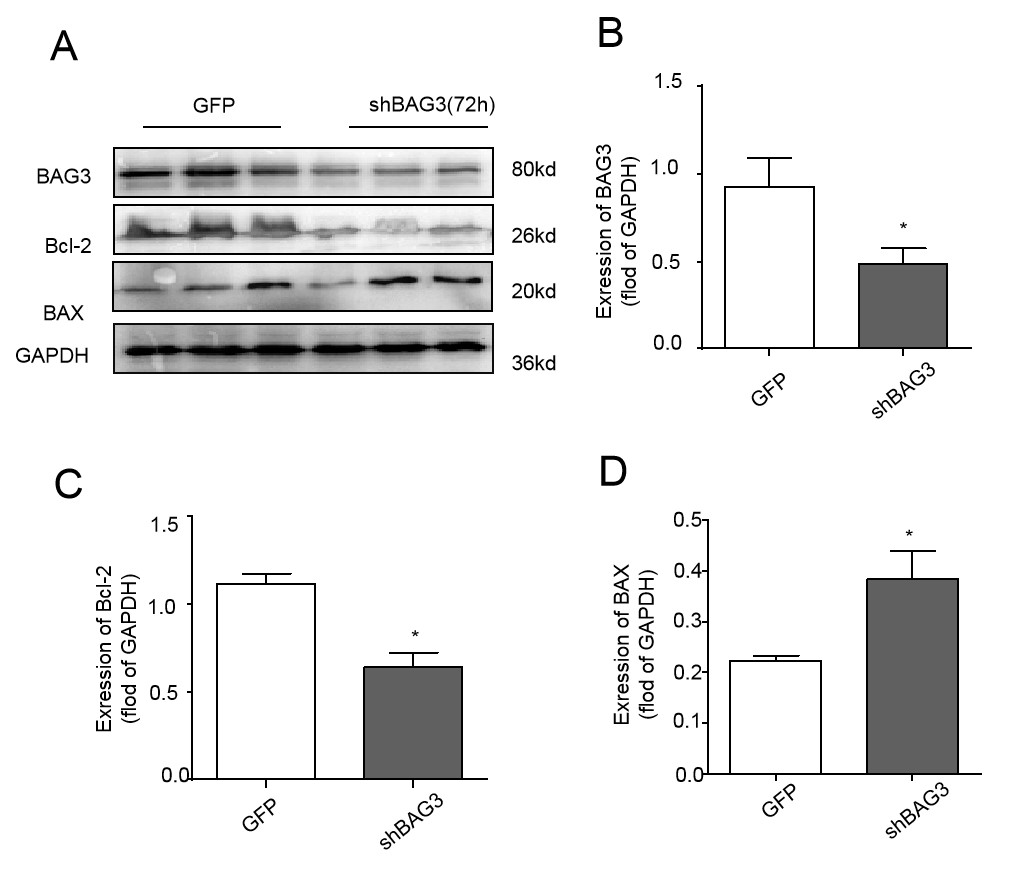 Fig. 3.
Fig. 3.Transfection of shBAG3 changes levels of both
apoptosis-associated proteins in ARCMs. (A) A representative image. Cell lysates
were immunoblotted for BAG3, Bcl-2, and BAX. GAPDH served as a protein loading
control. (B) Cells transfected with shBAG3 decreased protein levels of BAG3
compared to GFP (*P
The full-length BAG3 contains multiple functional domains (PXXP and BAG) as
shown in Fig. 4A. To determine role of a certain domain in cellular protection,
either BAG3-WT, BAG3-
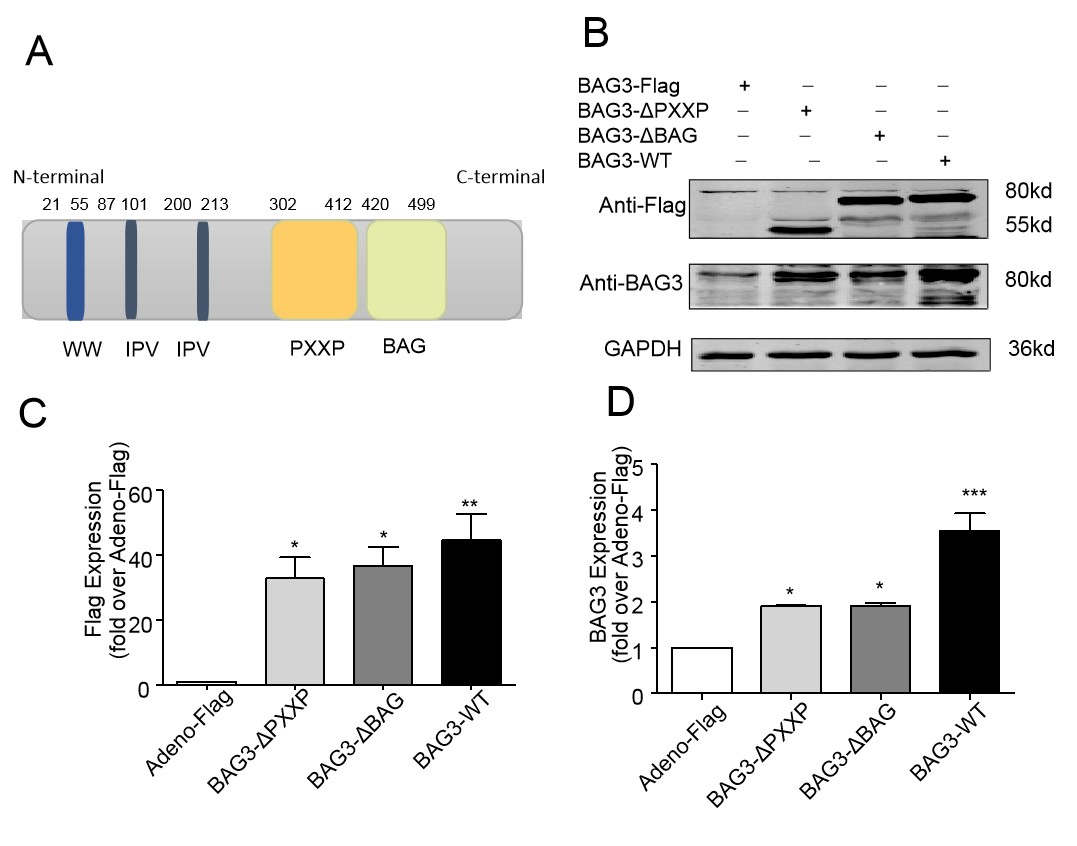 Fig. 4.
Fig. 4.Enforced expression of BAG3-WT or its four mutants in NRCMs.
(A) The full length of human BAG3. (B) A representative image. Cell lysates were
immunoblotted for BAG3, FLAG-tag. GAPDH served as a protein loading control. (C)
Cells transfected with BAG3-
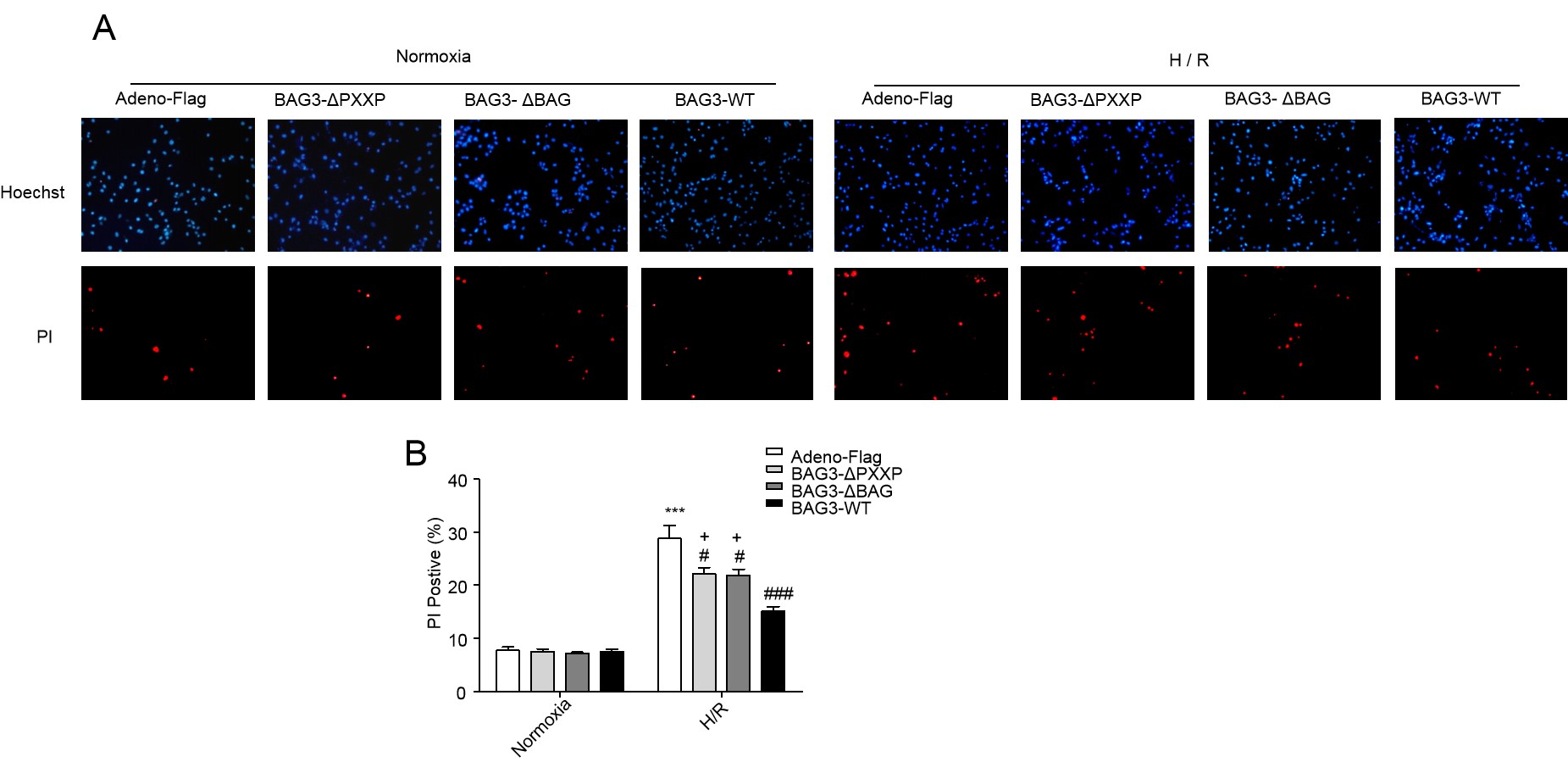 Fig. 5.
Fig. 5.Either PXXP or the BAG domain of BAG3 is necessary for cell
protection. (A) Representative confocal microscopic images. (B) H/R stress
enhanced PI positive cells compared to normoxia conditions (***P
 Fig. 6.
Fig. 6.Over-expression of BAG3-WT and its mutants attenuates the cleaved Caspase-3 induced by H/R in NRCMs. (A) Representative
images. (B) H/R stress increased the expression of cleaved-caspase3 compared to
normoxia conditions (***P
 Fig. 7.
Fig. 7.PXXP or the BAG domain of BAG3 is responsible for BAG3
attenuating cardiomyocytes apoptosis induced by H/R in NRCMs. (A) Representative
confocal microscopic images. (B) H/R stress enhanced the TUNEL positive cells
compared to normoxia conditions (***P
To define whether, or not PXXP and BAG domains of BAG3 protect cardiomyocytes
apoptosis induced by H/R and had any correlation with the activity of MAPK
members, levels of JNK, ERK, and p38 phosphorylation were evaluated by Western
blot assay. While there was no significant difference of ERK or p38 in H/R stress
compared to normoxia groups, the phosphorylation of JNK under H/R group was
significantly higher than that in the normoxia group. Although there was no
significant difference between BAG3-WT, BAG3-
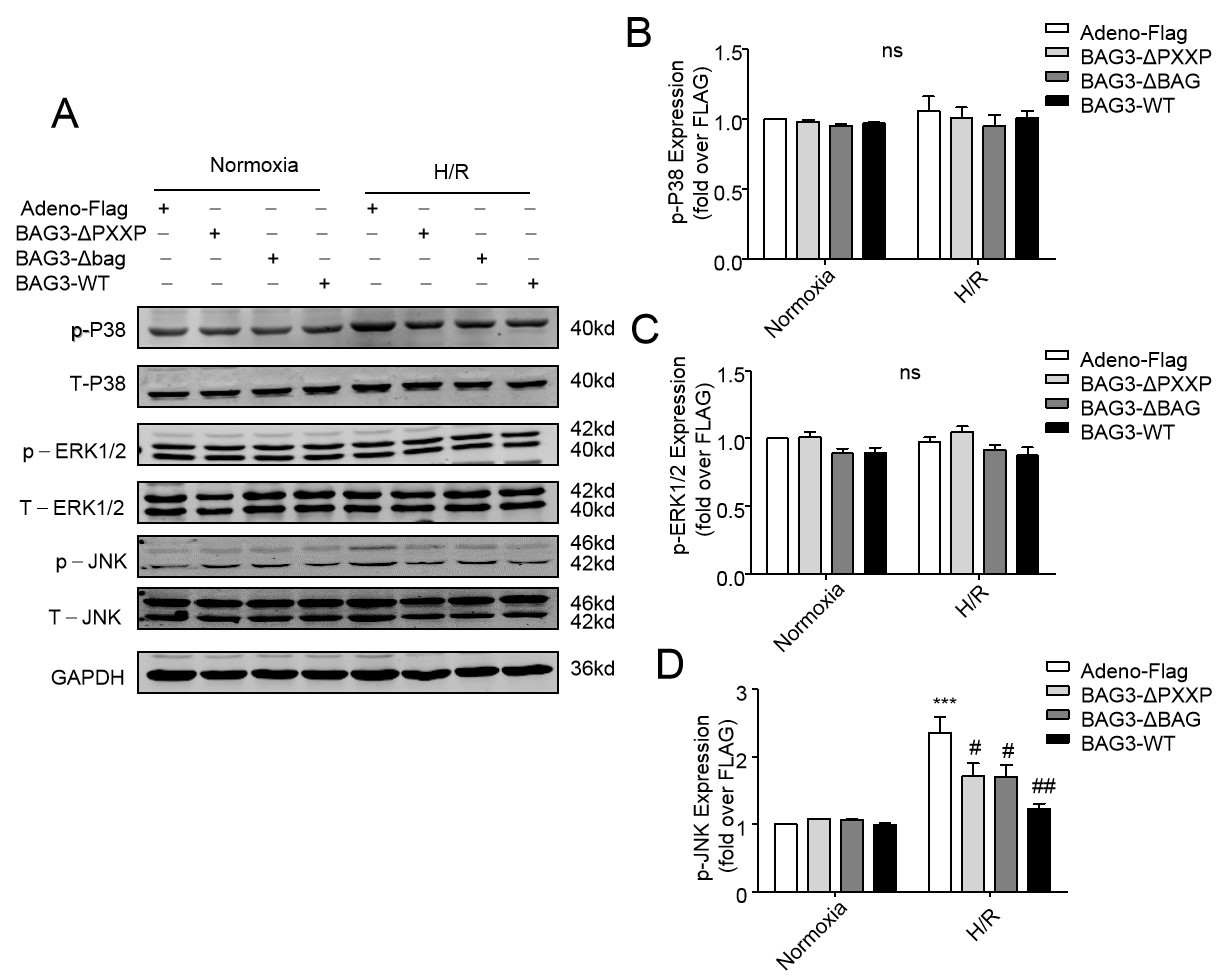 Fig. 8.
Fig. 8.Over-expression of BAG3-WT and its mutants inhibited H/R-induced
phosphorylated JNK (p-JNK) levels in NRCs. (A) A representative image. Cell
lysates were immunoblotted for p-P38, T-P38, p-ERK1/2, T-ERK1/2, p-JNK, and
T-JNK. GAPDH served as a protein loading control. (B–D) H/R stress increased the
expression of p-JNK compared to normoxia conditions (***P
To define how BAG3 and its mutants attenuate NRCs apoptosis induced by H/R through the JNK signalling pathway, Co-IP of proteins was used to explore the interaction of BAG3 with JNK. First, as shown in Fig. 9A, BAG3-WT having a high capacity for binding HSC70 and H/R stress made no significant difference to the binding capacity (Fig. 9A, lanes 2 v. 4 in the third panel). Second, the BAG3-HSC70-JNK complex was found (Fig. 9C, lanes 2 v. 4 in the second panel). There was very slight binding of BAG3 to Sek1/MKK4 and HSP70 in the precipitated complex (Fig. 9C, third panel). Finally, the structural domain PXXP or BAG knock-down suppressed the binding of BAG3-HSC70-JNK, indicating that the structural domain PXXP or BAG of BAG3 is critical for the interaction among BAG3, HSC70, and JNK (Fig. 9E, lanes 5 and 6 v. 4 in the second panel, respectively).
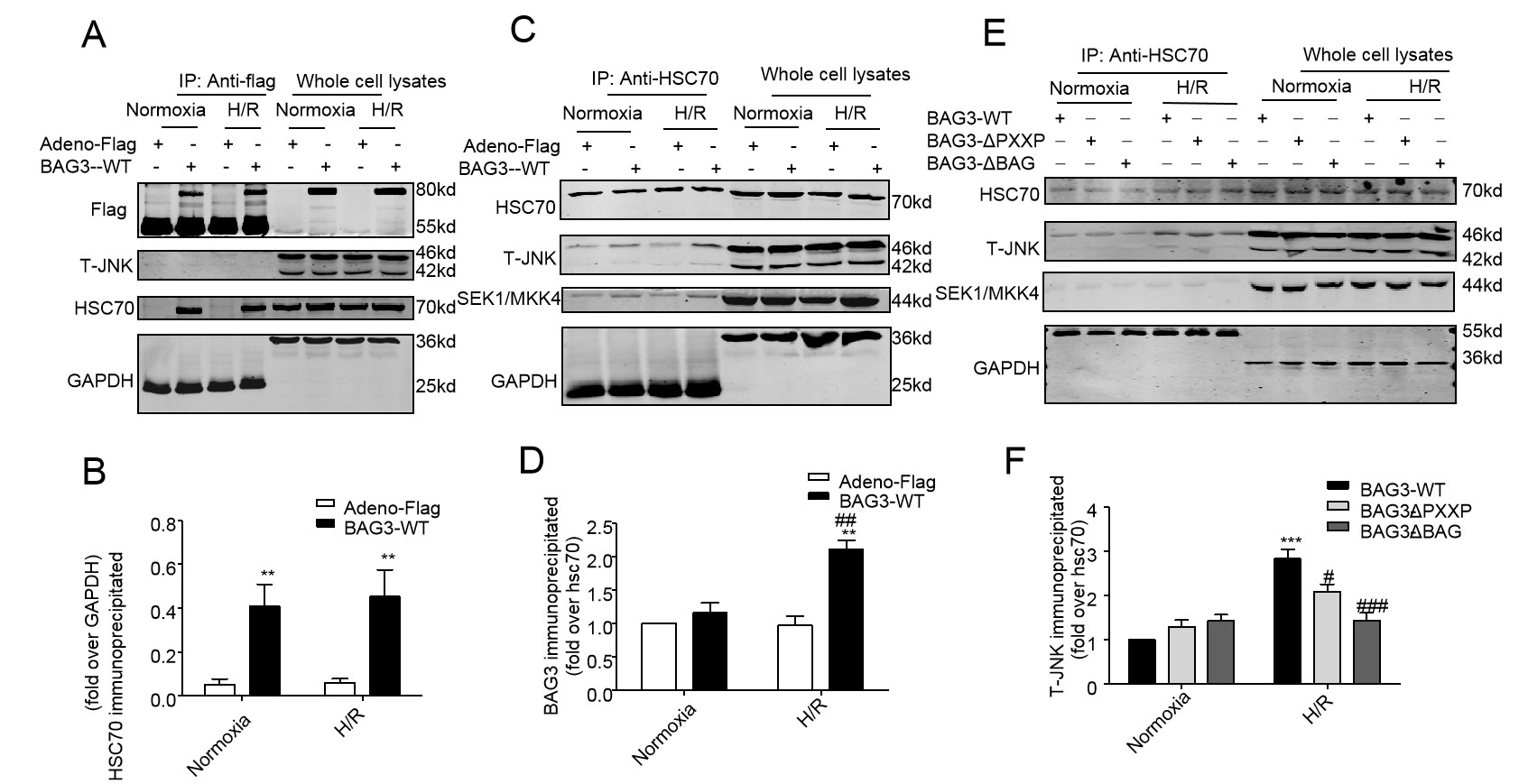 Fig. 9.
Fig. 9.BAG3 binds to HSC70 protein to form BAG3-HSC70-JNK complex. (A)
A representative image. HSC70 in immunoprecipitated complexes pulled down by
anti-Flag antibody displays the binding amount of HSC70 with Flag-BAG3. GAPDH
serves as a loading control. (B) Over-expression of BAG3 increased binding
capacity of BAG3-HSC70 compared to that in the Adeno-flag group (**P
The expression of BAG3 is regulated by multiple stressful stimuli to adapt to environmental conditions and is induced by HSF1 (heart shock factor 1), a transcription factor involved in many types of cancer [31]. The anti-apoptotic efficacy of BAG3 in cardiomyocytes attracted attention recently with researchers finding that the level of BAG3 increased in patients with hypertension and diabetes [32]. In this study, we found that the expression of full-length BAG3 in NRCMs was down-regulated after H/R stimulation, which may due to the adaptive response of BAG3 against the stimulation induced by H/R. The existing literature and the results of our laboratory showed that BAG3 may become a new therapeutic target for heart and skeletal muscle patients [33].
The role of BAG3 in protecting the myocardium, and the following clues, proved that BAG3 plays a critical role in the study of cardiac pathology: (1) Knocking down of BAG3 in ARCMs induced the change in the expression of apoptosis-associated proteins BAX and Bcl-2, and inhibited the expression of autophagy-associated protein LC3 A/B; (2) Over-expression of BAG3 in rat cardiomyocytes reduced the injury suffering from H/R stress, as demonstrated by PI staining and TUNEL staining. Previous experimental results in our laboratory have also confirmed the protective effect of BAG3 in the cardiac cell lines (H9C2 cell line). We believe that it may be a feasible treatment to restore the level of BAG3 to a normal level in patients with cardiomyopathy whose expression of BAG3 is lower than the normal level [34]. It is necessary to investigate whether the PXXP and BAG domains of BAG3 are involved in autophagy, apoptosis, and other cellular functions by changing the cytoskeleton assembly and signal transduction, however, it is unknown whether the PXXP and BAG domains alone can protect cardiomyocytes from apoptosis. In addition, the experimental data are from cells in vitro, therefore, these results should be carefully extended to physiological conditions pertaining in vivo.
Present and previous data have shown that the protection against cardiac injury might be related to its binding to chaperone protein HSP70 [35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45]. Beyond binding to HSP70 to evoke the damaged cellular organ and unfolded protein into the cellular lysosome to conserve energy for cellular survival, other signalling molecules might be involved in cellular protection. Since JNK signalling has been displayed in cellular injuries induced by hypoxia-reperfusion [22, 23, 24] and participated in BAG3 cellular function in cell death and cell proliferation [25, 26, 27], it is worthwhile to define the domain of BAG3 responsible for binding to JNK. Hypoxia-reperfusion-induced the phosphorylation of p38 mitogen-activated protein kinase (p38MAPK) and cJun N-terminal kinase (JNK) in H9C2 cardiomyocytes and in cultured rat cerebellar granule neurons [22] by MKK4 signalling. In our present study, the complex of BAG3-HSC70-JNK provides the evidence that BAG3 could be involved in JNK signalling in stressed cardiomyocytes. Thus, it is reasonable to conclude that cellular protection of BAG3 might inhibit the JNK signalling pathway, as evinced by a previous study [27]. Furthermore, PXXP or the BAG domain of BAG3 is critical for BAG3 binding to JNK in its signalling pathway.
BAG3 can inhibit JNK signal-mediated ischemia-reperfusion injury. The JNK signalling pathway is closely related to cell injury induced by hypoxia/reperfusion, and is involved in the function of BAG3 in cell death and cell proliferation [45]. Studies have found that JNK activation can lead to an increase in the level of BAG3, and then promote selective autophagy as a protective measure against stressors, however, some studies have found that when the level of BAG3 is high, there may be a feedback circuit reducing the activation of JNK in the heart, and a low level of BAG3 inducing the activation of JNK. In previous studies, we found that the mRNA and protein expression of BAG3 in H9C2 cell line increased after H/R stress [35]. However, in primary ARCMs and NRCMs, we found that the level of BAG3 protein was down-regulated under H/R stress. These results suggested that the physiological function of BAG3 protein changes differently in response to H/R stress, it may vary with cell type. However, the interaction between BAG3 and JNK is complicated, and further research is needed to ascertain the relationship between BAG3 and JNK in the heart.
We recently screened 141 differentially expressed proteins and five related signal pathways through proteomics-based high-throughput screening, and our laboratory will further explore the mechanism and biological significance of the differentially expressed proteins and signalling pathways in protecting cardiac myocytes related with BAG3. We believe that proteomics can provide a material basis for activities, and also provide a theoretical basis and solutions for the interpretation of disease mechanisms.
In conclusion, the PXXP and BAG domains of BAG3 are necessary for the complexes of BAG3 with HSC70 and JNK to attenuate H/R-induced apoptosis in NRCMs. After knock-out of PXXP and BAG domains, the binding of BAG3 to JNK and HSC70 was lost, which was closely related to the cellular protection provided by BAG3. In view of the protective effect afforded by BAG3 during I/R injury, BAG3 may represent a therapeutic target for patients receiving reperfusion therapy after myocardial infarction. These results provide theoretical support for the occurrence and development of cardiomyopathy and provide new ideas for the treatment of cardiomyopathy.
LZ, JG, and WZ contributed to the conception of the work, interpretation of the data and critical revision of the manuscript. LZ, XZ, HC, HS, BG, YW, HQ, YW, and NY contributed to the data acquisition. LZ, XZ, and WZ drafted and finalised the manuscript. All authors contributed to the final approval of the manuscript.
The study was approved by The Board of Nantong University Animal Care and Use.
We warmly appreciate Dr. Huaqin Wang for gifting both pcDNA3-FLAG-BAG3-WT and pcDNA3-FLAG-BAG3-ΔPXXP.
The study was supported by The National Natural Science Foundation of China (Grant No 81541008 and 817790400 to WZ), funding from Nantong University Co-Innovation Programme (NTU 2016-1), and Nantong Municipal Science and Technology fund (Grant No JCZ18131 & JC2019133 to HS).
All authors declare that they have no conflicts of interest.
