Implantation in humans is a multistep process that involves apposition, adhesion, and invasion of the developing blastocyst into the receptive maternal endometrium. Though significant volume of research in this direction has identified important players orchestrating this delicate process, there are still gaps in our understanding of all the sequence of events during embryo implantation. Also, the early pregnancy-related complications that lead to fetal loss and miscarriage often occur in this critical window of implantation, which is primarily defined as the time when the maternal endometrium is supposed to be most receptive to the free blastocyst that emerges out from the zona pellucida. Studies in humans and rodents have identified several mediators like folliculin, LIF, IL11Rα, splicing factor SC35, etc. to be essential for early implantation. Trophoblasts, that form the outer epithelial layer of the blastocyst, participate in the formation of the placenta. During placentation, invasive extravillous trophoblasts (EVTs), migrate into the endometrium, and a transient epithelial to mesenchymal transition (EMT) and remodel the uterine arteries for blood and nutrient exchange.
Trophoblast cells are derived from the outer trophoectodermal layer of the developing blastocysts as a part of the outer cell mass that eventually forms the placenta (1). These cells help in establishing cell to cell interactions that eventually secure and anchor the blastocysts into the uterus (2)
In humans, the blastocyst “hatches” out of the protective zona pellucida approximately after five days, which facilitates the blastocyst adhesion to the uterine endometrium, thereby gaining access to the maternal cytokine and nutrient milieu (3). Through a process called zona hatching, the embryo is released from the blastocyst. The cells on the outer side of blastocyst become trophectoderm, which differentiates into chorion, forming the future placenta while the inner cell mass becomes the embryo. This process is the first step towards embryogenesis and often subjected to precision control both by the maternal as well as embryonic factors. This process is followed by a strictly controlled differentiation program of the trophoblast that gives rise to multiple lineages of cells with diverse functions (4). An inherent capacity of pseudo-malignant trophoblast cells is to invade the maternal endometrium, during which they seem to manifest EMT. However, what is spectacular is that unlike cancer, these invasive maneuvers are mostly transient and self–limiting (5). It is, therefore, safe to conclude that EMT forms an essential component of the trophoblast invasion program lack of which could result in serious complications. In this review, we use the word “EMTiness” to denote transient EMT behavior of the trophoblast cells.
First-trimester mononucleated cytotrophoblast (CTB) with stem cell-like properties undergo lineage commitment through differentiation into different trophoblast cell types (6). While the STB of the floating villi are engaged in nutrient and gas exchange, villi that comes in contact and attach to the maternal decidua the anchoring villi forms the cytotrophobast cell coloumn. While the proximal cells of these coloumn can proliferative, they loose their mitotic activity when they differentiate distally. These are the cells that eventually leave the cell column , assume invasive nature and invade the endometrium forming iCTB( instersitial cytrophoblast). This population seems to arise from an anchorage-dependent epithelial phenotype of trophoblast cells of the trophoblast cell columns into a mesenchymal-like invasive extravillous trophoblasts (8). These events are crucial for the development of the feto-maternal interface, which in turn, promotes fetal survival. Numerous studies have shown that differentiation of the first-trimester cytotrophoblast to extravillous trophoblast requires an EMT like process that transforms these cells into highly invasive interstitial trophoblasts (9). In this review, we compared the epithelial-mesenchymal characteristics of the CTBs and EVTs to the malignant cancers and addressed the overlapping denominators between the two invasive systems. Besides, this review also focused on multiple facets of this multifunctional system, along with any associated pathology.
Research in this area is primarily driven by the information gathered from rodent models. Though there are some functional similarities between the human and mice placenta; anatomically, they have several species-specific differences (6). For example, in mice, we see a discoid placenta with a single cotyledon, whereas human placenta has multiple cotyledons, consolidated into a single cluster (6). Further, the trophoblast layer covering the placental villi is different in humans and mice. While in mice, three trophoblast cell layers (tri-chorial containing two syncytial layers) comes in direct contact with maternal blood-forming hemochorial placenta, in humans, we just have one syncytial layer covering the cytotrophoblast (6). Trophoblast cells undergo differentiation along different lineages to take over the varied function of the placenta. Abnormal trophoblast differentiation seems to be responsible for several placenta related pregnancy complications like fetal growth restriction, pre-eclampsia etc.
Trophoblasts are the first of the few cells that initiate an elaborate differentiation program. Mononucleated CTBs with stem cell-like properties spontaneously fuse to form multinucleated syncytiotrophoblasts (4). At about seven days post-fertilization, the trophoblasts interact with the maternal endometrium for implantation, leading to the formation of anchoring chorionic villi. Subsequently, the unique structure of the feto-maternal interface is established by the differentiation of these stem cell-like CTBs into the anchoring villi (10). An in-depth electron microscopic study by Enders et al. (2001) of the anchoring villi from the first trimester placentae of species such as macaque and humans showed that while anchoring villi of macaque were elongated cell columns, e those from the humans showed ECM components between the CTB cells (Enders et al., 2001). It was observed and later confirmed that maternal fibrillin from the ECM helps to strengthen the anchoring villi in addition to preventing the maternal cells from migrating into the trophoblastic shell (11).
Peripheral trophoblasts cells that form the tips of anchoring villi help to attach the placenta to the uterine endometrium (12,13). The formation of anchoring villi is the prerequisite for several subsequent events. These anchoring villi give rise to a specialized trophoblast population that displays migratory properties enabling them to invade and colonize into the maternal interstitium of the decidua. Furthermore, an additional differentiated lineage of the trophoblast emerges out of the anchoring villi during the first few weeks of pregnancy (14). While few differentiated trophoblast lineages migrate towards the uterine stroma from the placental base, yet another class of trophoblast undergoes a process wherein single cells of CTB fuses to form multinucleated syncytiotrophoblasts (14). The CTB fusion with the overlying syncytiotrophoblast is a continuous process during the lifetime of the placenta (15-17). It is an effective mechanism that replenishes the syncytiotrophoblast with the essential nutrients from the fusing CTBs. A dynamic equilibrium is established to maintain this delicate stoichiometry, which is based on the release of apoptotic fragments from these fused syncytiotrophoblasts into maternal blood. Interestingly, trophoblast fusion is orchestrated by numerous autocrine, paracrine, endocrine, and juxtracrine factors, such as fusion proteins, ECM components, hormones, cytokines, and caspases (18-22) derived both from maternal as well as fetal poles. Mi et al. reported a retroviral envelope captured protein, known as syncytin, expressed in the placenta; it directs the trophoblast fusion (23). Recent studies showed that RNA binding proteins, LIN28A/B, are associated with this fusion process and syncytium formation (24,25). Another review by Finley et al. (25) pointed out that cellular stress in the form of reactive oxygen species (ROS) generation, nutrient deprivation, or stress hormones trigger the transposable elements through AMPK activation, which in turn, propagate the trophoblast differentiation process (26,27). Further intracellular mediators, such as Kruppel-like factor 6 (KLF6), a transcription factor expressed in the human placenta, were also found to be associated with this fusion process (27-29). siRNA-mediated knockdown of KLF6 hindered the cell-cell fusion in the human placenta-derived BeWo cell line along with a decreased expression of the fusogenic protein syncytin-1. Liempi et al. proposed that Typasnsoma cruzi, which causes Chagas disease, is associated with trophoblast fusion. T. cruzi induces trophoblast differentiation that elevates the trophoblast turnover (29). This phenomenon might have evolved as a protective antiparasitic mechanism. In addition to an elaborate differentiation process, trophoblasts exhibit a complex cross-talk with the surrounding environment that could provide critical developmental cues (30-31).
Trophoblast differentiation is under a complex regulatory circuitry. Several factors that regulate trophoblast differentiation are derived from both fetal as well as maternal sides. Trophoblast differentiation leading to cell fusion generates the multinucleated syncytium, as well as invasive trophoblast cell clusters migrating into the maternal endometrium. These invasive populations, known as extravillous cytotrophoblast (EVT are in turn derived from the trophoblast cell columns as part of the anchoring villi. The villous counterpart (vCTB) is essentially non-invasive and forms a part of floating villi that are primarily engaged in feto-maternal gas and nutrient exchange (10). Throughout pregnancy, the primary villi change into secondary villi that are transformed into tertiary villi. This ramification of the villus system is crucial for effective feto-maternal dialog (32). The steps in trophoblast differentiation involve making contact with and invading the endometrium. The components of ECM effectuate this process through adhesion, migration, and differentiation speculated to be regulated by cell surface receptors, called integrins (33,34). The cell adhesion integrins are heterodimeric transmembrane glycoproteins composed of α and β subunits that act as receptors for the ECM and participate in cell-cell and cell-substratum interactions (35). Mammalian genomes encode about 18 α and 8 β subunit genes, resulting in 24 α-β combinations (Figure 1) (36). Integrins are also involved in cell signaling and regulation of cell invasion, differentiation, migration, survival, and growth (37). The activation of these signaling pathways modulates the cell morphology, motility, proliferation, survival, and cell-type-specific gene expression (38-41). vCTB and EVT express different integrin classes depending on their functions (41-43). vCTB predominantly expresses a6ß4 integrin that plays an important role in anchoring the trophoblast to the basement membrane. It is speculated that its gradual loss may play a role in facilitating the trophoblast cells to migrate into the maternal compartment (37). Damsky et al (45) reported that a switch in cell surface integrin repertoire is necessary to execute the trophoblast invasive programme (37). This involves a downregulation in alpha 6 integrins in invasive cytotrophoblast (CTB) with an upregulation of alpha 5 beta 1 and alpha 1 beta 1 integrins (45). However, post-invasion, the decidua reverses the integrin repertoire from α6β4-positive and α5β1-negative (45).
 Figure 1
Figure 1Possible combination of the subunits α and β, their specific ligands on different cell types. RGD: Arg-Gly-Asp.
Trophoblast cells display a physiologically regulated invasive behavior (31,32) in a temporospatial fashion that draws parallel to cancer invasion. However, this pseudo-malignant behavior is transient and restricted to first-trimester pregnancy and uterine endometrium (14). Thus, many similarities exist between blastocyst implantation and cancer cells. Though all the factors that contribute to the transient autoregulated tumor-like pseudo-malignant phenotype of human trophoblast cells are yet to be identified (44), still a considerable amount of information in this direction is available derived both from human as well as rodent models. This physiological in-build check-in system is often underrated. It becomes relevant when trophoblast incursion into the endometrium becomes unregulated, leading to life-threatening complications affecting both the mother and child (50). Thus, human trophoblast cells display extreme phenotypes with an unparalleled capacity to proliferate, migrate, and invade (Figure 2). On the other hand, these cells also slow down the tumor-like attributes by the end of the second trimester into a non-motile, non-invasive state, thereby rendering it a pseudo-malignant tumor (51). EMTness of the trophoblast is thus a transient phenomenon.
 Figure 2
Figure 2Invading trophoblast front of into maternal endometrium.
Besides invading the maternal decidua, trophoblasts also display multiple invasive routes. EVT that invades the endometrium in the first trimester is essentially the interstitial trophoblast (iCTB) and is believed to be a lineage-committed stage (52). It ensures adequate blood and nutrient supply to the growing fetus. Any disruptions in this process result in a plethora of pregnancy-related complications, such as preeclampsia, intrauterine growth restriction (IUGR), or recurrent abortion (53). After the invasion into the decidual bed (54,55) i.e., the endometrium, the migrating EVT cell populations are influenced by growth factors cytokines and hormones secreted by the decidual stromal cells and the decidual immune cells (macrophages, uterine NK (uNK) cells ) (53-55) thereby establishing a regulated cross-talk between immune components and the trophoblast cells (Figure 3). Apart from the paracrine influence by the cytokines on trophoblast invasion, oxygen saturation in the placental bed seems to be another crucial determinant orchestrating this process in vivo (56). The role of oxygen during embryo implantation is not completely understood., however it is accepted that maintainance of a strict oxygen dynamics in the feto-placenta milieu is a critical determinant for successful pregnancy. Hence a delicate titration of oxygen is the key for a uneventful pregnancy outcome. The endovascular subpopulation of the cytorophoblast seems to orchestrate uterine blood flow through the remodeling of maternal spiral arteries (57). This part of the process involves the regulation of oxygen levels at the feto-maternal interface via trophoblast-mediated plugging of uterine arteries upto 8-10 weeks of gestation (56, 153). This is essentially to maintain a low oxygen tension (2-3%) . This is followed by endovascular trophoblast mediated remodeling of maternal vessels (57) to enable a rapid uninterrupted blood flow when these trophoblast plugs are dissolved. This raises the oxygen tensin upto 6-8% after 12 weeks. Defects in these processes can cause severe pregnancy-related complications like preeclampsia and intrauterine growth retardation (IUGR), thereby severely compromising with the growth and development of the fetus (58). Poor perfusion resulting from incomplete uterine artery remodeling also results in inadequate nutrition for the developing fetus (57). This in turn trigger a stress response that seems to have a profound influence in the postnatal life. Studies also found that babies delivered from females with elevated stress markers pose a high risk of developing adult-onset hypertension, cardiovascular complications, and type 2 diabetes mellitus (18,22,59,60). Due to the close association between maternal stress with poor pregnancy outcome and fetal complications that are arising due to this stress (59) an in-depth understanding of mechanisms regulating trophoblast invasion need to be elucidated.
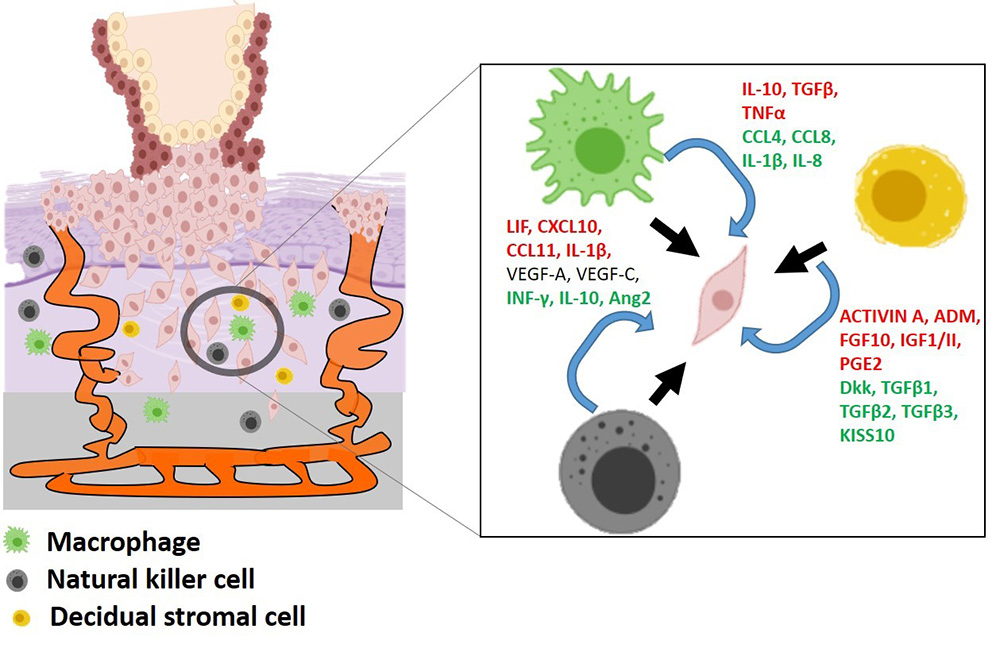 Figure 3
Figure 3Model of trophoblast invasion. PV = Placental villi, FV = Floating villi, AV = Anchoring villi, evCTBP= Endovascular cytotrophoblast, (Image reproduced with permission from Biorender : https://biorender.com)
Early embryo mostly develops under hypoxia (low oxygen tension) with about 2-3% oxygen tension upto first 10 week . Under such conditions, the embryo implants and the trophectoderm, the outer layer of blastocyst, proliferates, forming the cytotrophoblastic shell, the early placenta (153)
Trophoblast cells derived from the human placenta are programmed to proliferate, migrate, and invade the endometrium (16). In addition, these cells also modify and remodel the maternal vasculature, similar to that as been observed by tumors on blood vessels (Figure 4). Another differentiated type of trophoblast that originates from the trophoblast cell columns of the anchoring villi are the endovascular trophoblasts that originates from the CTB stem cell core (60). This endovascular trophoblast population mediates maternal spiral artery invasion. The vascular invasion begins during the first month of pregnancy with moderate to massive degenerative changes in the spiral vessel walls leading to endothelial hypertrophy, muscular regression, and the appearance of swollen cells (61-64).
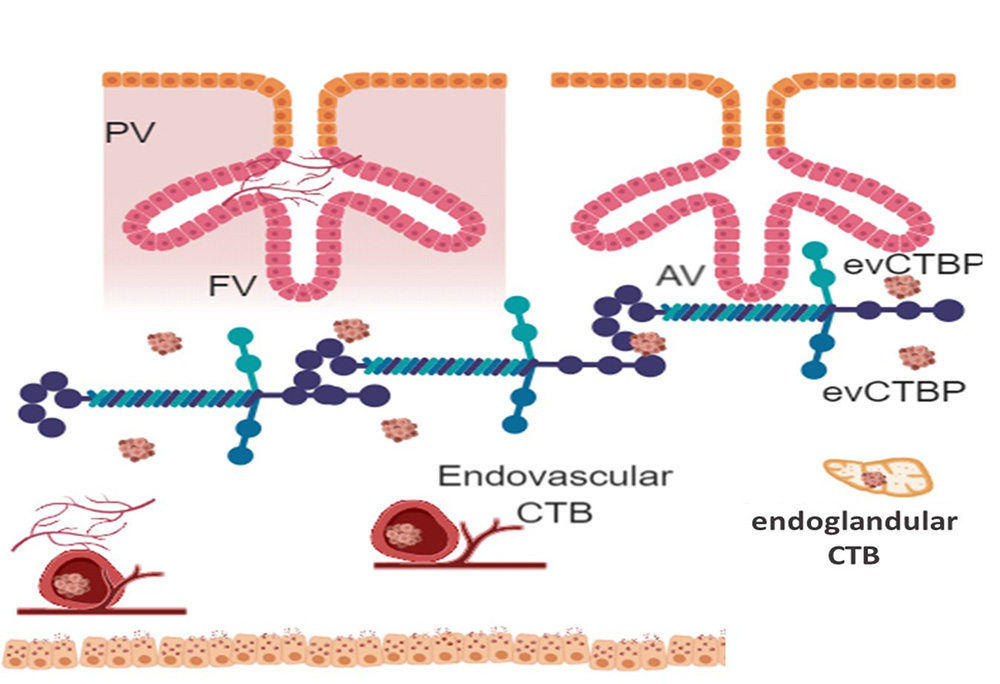 Figure 4
Figure 4Hypoxia mediated activation of multiple signaling cascasdes in cancer and trophoblast cells.
During the first trimester, the maternal uteroplacental arteries undergo a series of pregnancy-specific changes which are as follows: 1) apparent replacement of endothelium and media smooth muscle cells by invasive trophoblast, 2) loss of elasticity, 3) dilation of in-contractile tubes, 4) loss of vasomotor control and 5) free blood-flow.
It is well-established that fetal trophoblast cells ensure their vascular connectivity to maternal blood vessels through vascular invasion. Incomplete or suboptimal vascular invasion is associated with common pathologies, such as pre-eclampsia and fetal growth retardation, and other pregnancy-related complications (65,66). Trophoblast cells that form the primary villi gradually mature into secondary and tertiary villi. This, in turn, leads to villous branching and vascularization. Villi are connected to the basal plate of the placenta. This gives rise to trophoblast cell columns. It is known that migratory subpopulations of trophoblast (Extravillous CTB) originate from these cell columns. Another differentiated trophoblast lineage that originates from the stem cell population is Endovascular trophoblast. Vascular invasion by tumor-like trophoblast cells is the outcome of cell migration and invasion. Trophoblast emerging out of trophoblast cell columns (these helps to anchor the floating villi to endometrium) can migrate collectively in a mesenchymal-like crawl expressing EMT markers. The endovascular trophoblast intravasate the blood or lymph vasculature. However, intravasation by the interaction of trophoblast cells with the vascular endothelium is yet poorly understood. It is speculated that endovascular trophoblast cells invade the lumen of uterine spiral arteries, eventually replacing the vascular endothelial ends of the blood vessels (intramural trophoblast) (60). This leads to the replacement of arterial elastic material with an extracellular matrix called the fibrinoid (67). The uniqueness of endovascular trophoblast invasion is the capacity of trophoblast cells to replace the arterial vascular endothelial cells to facilitate adequate uninterrupted blood flow towards the developing embryo (67).
Maternal factors and fetal-derived trophoblast factors release cell adhesion molecules, such as integrins and matrix-degrading proteases (MMPs, secretory mediators like cytokines, growth factors, and hormones) (68). Eventually, enzymes released by the trophoblast cells degrade the ECM proteins, such as collagen IV, vitronectin, and fibronectin, to promote cell migration and invasion (69). Trophoblast cells express a variety of transcription factors related to development, maturation, and differentiation (70): Hand1, AP-2y, ETs-2, Mash2, Gcm1, Ascl2, and GATA2. Gcm1 inhibits the expression of trophoblasts differentiation-specific, helix-loop-helix (bHLH) transcription factor genes, while Hand1 and Mash2 are essential for placental development in mice (71-74). Hand1 promotes the differentiation of trophoblast giant cells in mice, which is essential for the maintenance of these giant cell precursors. Mash2 is downregulated as the cells differentiate along the invasive pathway (70). Thus, both EVT and as well as endovascular trophoblasts, display an extensive migratory and invasive phenotype similar to metastatic cancer. Furthermore, like cancer cells, the trophoblasts seem to co-opt tumor feeding vascular network to ensure that adequacy of blood can be maintained towards the feto-maternal pole. This phenomenon is supported by the initial hypothesis by Thompson et al. (75) that tumors acquire vasculature by incorporation of host tissue capillaries. The morphological evidence by Holash et al. suggested that the co-option of pre-existing blood vessels in human malignancies is a standard event during tumor growth (76).
Oxygen tension is crucial for embryo development. Accumulating evidence suggested that the early placental (and embryonic) development occurs in a hypoxic uterus. Jauniaux et al. investigated 25 pregnancies, wherein the partial pressure of oxygen in the placenta was 2.5-fold lower than that in the decidua before 11 weeks of gestation (77). The trophoblast cells from early pregnancy that had formed plugs sealing the maternal artery were gradually loosened, thereby perfusing the developing placenta to maternal blood flow (77-79). However, even with mounting evidence of trophoblast occluding the arteries, few preliminary studies reported that all the arteries are not blocked, and the blood flow is uninterrupted (81-83). Nevertheless, it is typical for the placenta and fetus to develop in a hypoxic environment in the first trimester, and that the invading CTBs proliferate in vitro under this low oxygen tension. As the interstitial trophoblast emerging from the trophoblast cell columns invades into the uterus, they encounter an increased oxygen level, which in turn, triggers the proliferation and facilitates the exit from the cell cycle towards a differentiated phenotype thereby slowing down the invasion (10).
Early-stage pregnant human uteri showed embryos surrounded by a pool of trophoblast cells by microscopy. Hypoxia stimulates CTB proliferation, thereby differentiating in size between the embryo and the placenta. Although the oxygen-detection mechanism of the trophoblasts is not yet clarified, it is speculated that ROS might play a role (84-88). Under chemical-induced hypoxia, studies using trophoblast explants showed the involvement of several pathways with upregulated expression of hypoxia-inducible factor (HIF), resulting in EVT proliferation and outgrowth. Interestingly, in the presence of mitochondrial poison rotenone, explants showed inhibited EVT outgrow, implying that trophoblasts use mitochondria as oxygen sensors during pregnancy (84).
HIF-deficient Placenta has numerous defects, including faulty lineage commitment, lack of fetal angiogenesis, and shallow invasion into maternal tissue (89). Similarly, hypoxia alters cancer cell metabolism and contributes to drug resistance (Figure 5). Hypoxia activates cell signaling networks in cancer cells, including the HIF, PI3K, MAPK, and NF-κB pathways (90,91). The response of cancer cells to hypoxia is complicated and depends on several key determinants primarily been the time of exposure, which determines cell fate: death or survival. While acute short-term hypoxia activates autophagy, cycling or even suboptimal hypoxia elevates the production of ROS, thereby promoting tumor cell survival and progression (92,93). In addition, hypoxia enhances tumor resistance to radiotherapy treatments (94-97) and activate EMT (98).
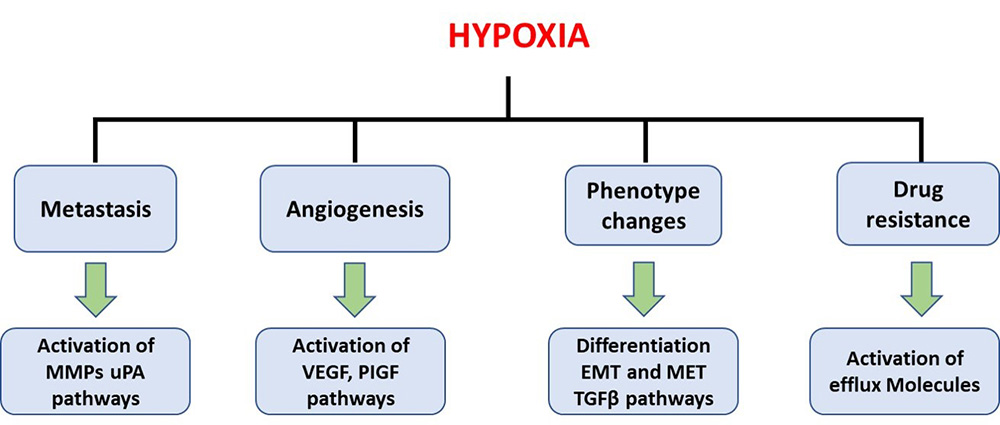 Figure 5
Figure 5Cross-talk between invading trophoblast and maternal immune system through soluble and cell surface mediators.
HIF1 plays a dominant role in the transcriptional regulation of hypoxia genes to facilitate the hypoxic adaptation of cancer cells (99). As shown previously, hypoxia has a profound influence on trophoblast function, orchestrating migration, invasion, apoptosis, gene expression, and proliferation (62,88,89,91). Recently, Zhu et al. investigated the role of ten-eleven translocation methylcytosine dioxygenase 1 (TET1) enzyme activity in hypoxic cancer cells and their effect on trophoblast function under 3% oxygen mimicking events in early placentation (100). Some studies showed that TET1 knockdown reduced the migration, invasion, and proliferation of JEG3 cells exposed to 3% oxygen into trophoblasts. This observation reinforces the fact that trophoblast invasion behaves like a cancer invasion and is possibly under epigenetic control. Furthermore, Reza et al. reviewed recent developments in this area and summarized (Table 1) that the epigenetic machinery, including histone modifications, DNA methylation, and ncRNAs, orchestrate multiple aspects of trophoblast differentiation, migration, and invasion during placental development (101).
| Epigenetic marker; |
Target (s) | Model (s) | Functional effect | ||
|---|---|---|---|---|---|
| Species | Type | Differentiation | Migration/invasion | ||
| Acetylation |
H2A/H2B | Ms | TS | ||
| H3K3 (Maspin) | Hu | PT,CL | - | ||
| H3K9/14 | Hu | CL | - | ||
| Methylation |
H3K27me3 | Hu | Plc | - | |
| H4K20me3 | Hu | Plc | - | ||
| H3K9me3 | Ms | ES | - | ||
| Ribosylation | Global | Ms | ES | ||
An epithelial-mesenchymal transition (EMT) is the process during which a polarized epithelial cell, regularly interacting via its basal surface with the basement membrane, undergoes several morphological changes, thereby assuming a mesenchymal-like phenotype (102). This involves an enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and a greatly increased production of ECM components (103). During EMT, polarized epithelial cells assemble to form elaborate cell-cell adhesions, including adherens junctions and tight junctions, and are transformed into mesenchymal cell types (104). Three physiological types of EMT are observed: (1) EMT during embryonic development, (2) EMT during wound healing, and (3) EMT during cancer.
There are, however, significant differences between physiological (developmental and wound healing) EMT and pathological EMT as seen in cancer. Developmental EMT involves not just cell motility. It is an orchestrated and coordinated process in response to physiological requirements guided by cell-cell and cell-ECM interactions as well as several soluble molecular factors of autocrine and paracrine in nature. Pathological EMT, on the other hand, is uncoordinated and unregulated, leading to loss of epithelial integrity, as seen in cancer(105).
The significant aspect of EMT is its clinical application in cancer metastasis. EMT empowers tumor cells to avoid chemo and radiotherapy and develop resistance (106). Epithelial cells can lose their cell-cell adhesion under the effect of aberrant signaling pathways or reactivation of oncogenes and transform into motile and invasive mesenchymal cells. These cells then invade the ECM basement membrane and migrate to distant sites (107,108). A critical determining force during embryonic development and differentiation is the transition of cells from static to migratory behavior. During this event, the cells oscillate between static and transient phenotypes (109). As mentioned before, these cell migrations are regulated processes. Aberrant and inappropriate activation of EMT, however, can lead to several tumor formations (102). An exciting feature of EMT is to investigate its execution and its delicate orchestration, mostly mediated by several soluble as well as ECM factors.
Cells undergoing EMT can either move as a single cell or as cell clusters (110). The latter is a complex assembly of cells with a mosaic phenotype, few of which are epithelial and few as mesenchymal. Studies by Jolly et al. pointed out that cells can also attain a hybrid epithelial/mesenchymal (i.e., partial or intermediate EMT) phenotype, i.e., mixed epithelial (for example, adhesion) and mesenchymal (for instance, migration) properties, allowing the movement of the clusters (111,112). These cells can be found as circulating tumor cells (CTCs) in circulation, that avoid anoikis (anchorage-dependent apoptosis). This small cell clusters can metastasize efficiently than single cells (110,115). This finding indicates that the mesenchymal phenotype can protect from anoikis. Following intravasation and extravasation, cancer cells migrate to distant sites to colonize. Conversely, trophoblasts colonize in the uterine matrix (interstitial trophoblast), maternal blood vessels (endovascular trophoblasts), and maternal glands (endo-glandular invasion) (4).
Epithelial-mesenchymal transition (EMT) is a physiological process necessary to normal embryologic development EMT is a normal process necessary to the development of the body plan as part of organogenesis. Epithelial tissues are characterized by a strict apical-basal polarity, cell-cell junctions that allow forming sheet-like structures. EMT in wound healing and EMT during embryogenesis are physiological EMT that are important for maintaining homeostasis. Embryological example of EMT is seen during mouse embryo gastrulation that exhibits a downregulation of epithelial maker notably E-cadherin. Wound healing associated EMT is mediated through inflammatory cells and fibroblasts, which secretes inflammatory molecules. TGFβ is a master regulator of EMT and in the wound healing process. Moreover, other cytokines like IL6, IL8 also contribute to the EMT process (112,113).
EMT can also be seen as a reversible transdifferentiation program whereby non-motile epithelial cells transform into migratory mesenchymal cells with enhanced cell survival attributes. This transformation is recognized by a loss of epithelial nature, followed by an increase in mesenchymal markers such as N-cadherin and vimentin (112-114).
A battery of critical transcriptional factors like SNAI1, SNAI2, ZEB1, ZEB2, Twist, as well as several lncRNA (long non-coding RNAs) through the complex chromatin remodeling process, brings about epigenetic modifications that drive the EMT process. Signaling from most of these pathways converges to repress the expression of epithelial marker E-cadherin, regarded as a ‘master’ regulator of EMT. Loss of E-cadherin gene somatically is found in several cancers such as breast cancer, gastric carcinoma. This is often associated with loss of epithelial characteristics and the acquisition of a highly invasive mesenchymal phenotype (112-114).
Migratory EVT invades the uterine endometrium, loses the epithelial phenotype, and transit into a migratory and invasive mesenchymal phenotype (116). Unlike cancer, where cellular pathways are distorted from their normal state, trophoblast cells are non-cancerous (117). Although several studies were conducted on EMT from cancer, the cellular mechanisms regulating EMT in trophoblasts are poorly understood. Trophoblast cells are pseudo-malignant and share common features, such as hyper-proliferation, metastasis, and invasion, in addition to the expression of several typical receptors for growth factors, cytokines, hormones, and receptors (118,120).
As emphasized before, although the placenta is just another healthy tissue, the trophoblastic cells, behave like pseudo-malignant cells. With the high proliferation index and lack of contact inhibition, these cells are the epitome of “physiological metastasis” (121). Numerous studies have elucidated that several oncogenes and proto-oncogenes that transmit proliferative signals through cytokine and growth factor receptors are expressed by trophoblast cells. EGF-receptor and c-fos products may serve as prognostic factors for the aggressiveness of squamous cell carcinomas of the lung (122). Similarly, the expression of the proto-oncogene-encoded proteins (c-erbB1, c-erb B2, c-myc, c-fos) belonging to the c-erbB1 (HER1, ERBB1 or EGFR) proto-oncogene is detected by the CTB in 4- to 5-week Placenta (123,124). Furthermore, receptor tyrosine EGFR, erbB family of RTKs, linked to breast and lung cancers (125), is strongly detected in placental cells. Proto-oncogenes, encoding c-fms (CSF1R), c-met (MET), erbB2 (HER2/neu, ERBB2), and c-kit (KIT), and RTKs are expressed by both normal trophoblasts and cancer cells (Table 2). vCTBs express the stem cell factor (SCF) receptor (126) critical for cell proliferation and migration. Kim et al recently proposed that calcitriol exploits an ERK signaling pathway to promote EMT induction and MMP expression of EVTs. The detrimental role of cigarette smoke extract (CSE) on lung, head, and neck cancer is well-established (127). It also affects cell proliferation and migration with a reduction in E-cadherin and upregulation of N-cadherin. In addition to events related to invasion, EVT differentiation into different lineages also involves EMT (128).
| Populations | erB-2 | C myc | P53 | p21 | RB | References |
|---|---|---|---|---|---|---|
| VCTB | No | No | Faint | Strong | Moderate | 119,120,122 |
| CTB cell column | Faint | No | No | Moderate | Moderate | 119,121 |
| Anchoring villi | Moderate | Faint | Faint | Moderate | Moderate | 121 |
| Interstitial trophoblast | Moderate | Moderate | No | Faint | Moderate | 119,121,123 |
| Endovascular trophoblast | Moderate | Moderate | No | Faint | Moderate | 123 |
| STB | Moderate | Strong | No | Faint | Moderate | 119,120 |
Interestingly, EVT differentiation and trophoblast EMTiness seems to share molecular events with those of EMT observed during embryonic development, wound healing, and cancer metastasis (128), except been highly regulated and well-orchestrated, unlike cancer. The EMT markers that are tightly associated with cancer are also found to be omnipresent in trophoblasts. Da Silva et al. reported that lentiviral-mediated overexpression of ZEB2 in trophoblast-derived cell lines resulted in an epithelial-mesenchymal shift in gene expression accompanied by a substantial increase in the invasive capacity of human trophoblast cells (129). ZEB1/2 involvement in trophoblast EMT demonstrated that miR-431 targets ZEB1 and inhibits the migration and invasion of trophoblasts. This phenomenon could have severe implications in preeclampsia, wherein trophoblastic invasion is compromised (129).
The unregulated proliferation of cells accompanied by angiogenesis initiates cancer growth (130,131). In most cases, this is followed by the acquisition of an invasive phenotype leading to the breach in the basement membrane, followed by tumor dissemination and distant metastasis. The biochemical mechanisms that trigger this induction are under intensive research.
EMT inducers include cytokines, growth factors, hormones, chemokines integrins, or others. This, in turn, stimulates the mitogen-activated protein kinases (MAPKs), focal adhesion kinase (FAK), and the phosphoinositide 3-kinase (PI3K)-Akt pathways that promotes hyperproliferation, differentiation, migration, and apoptosis (132,133). Members of transforming growth factor β (TGFβ) family regulate cell growth and proliferation in a dose- and time-dependent manner. Also, TGFβ is a known inducer of EMT (134,135) and is believed to play an essential role in regulating trophoblast proliferation, differentiation, migration, and invasion (136-139). TGFβ exists in three isoforms; of these, TGFβ1 and TGFβ2 are important in implantation. Results from our laboratory showed that TGFβ1 plays a crucial role in regulating trophoblast invasion by downregulating the MMPs and upregulating the protease inhibitors (TIMPs) (140,141). Besides, maternal decidua-derived TGFβ is a critical growth factor that plays a role in controlling trophoblast invasion. It has been shown that TGFβ1 activity was mediated via transcriptional factors SNAIL and TWIST (142).
In addition to TGFβ1, epidermal growth factor (EGF) belongs to the human epidermal growth factor receptor (HER) family (143) also induces EMT and is associated with the proliferation of several human cancers via multiple signal transduction pathways (Figure 6) (158,159). The family constitutes HER-1, HER-2, HER-3, and HER-4, also called ErbB1, ErbB2, ErbB3, and ErbB4, respectively. Secreted by maternal decidua as well by trophoblast cells, EGF and EGFR1 are abundantly detected in the early invasive placenta. Also, during EVT formation, cells downregulate EGFR1 and induce ERBB2 (also termed HER-2/neu) similarly to tumor cells (144). Previous studies demonstrated EGF-induced invasion and cell migration in villous explant cultures and primary CTBs (145).
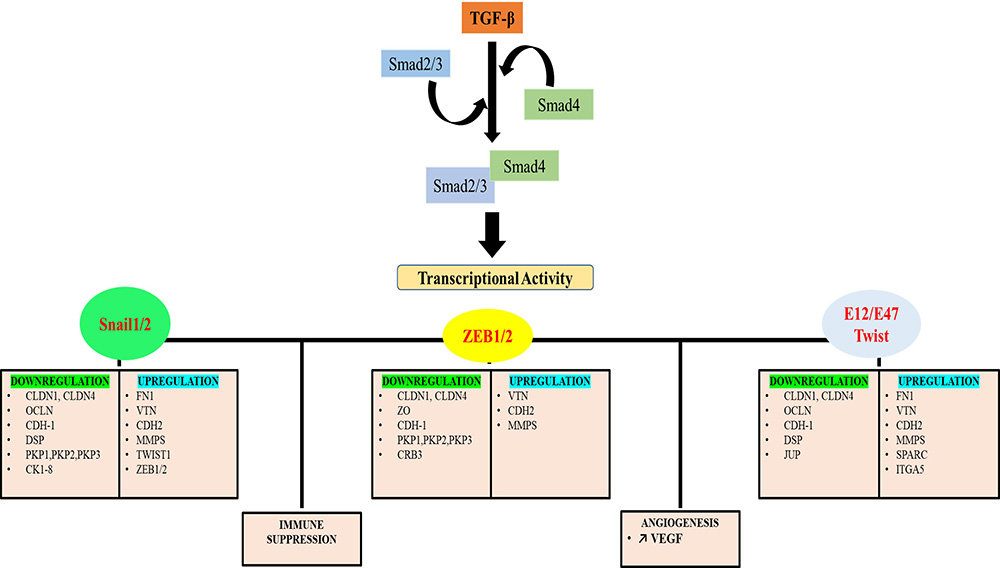 Figure 6
Figure 6Pleiotropic function of TGF-beta on EMT.
MMPs and TIMPs coordinate the breakdown and remodeling of the basement membrane and ECM in the physiological and pathological situations. Intriguingly, a balanced stoichiometry of MMP-TIMP system plays an essential role in regulating the EVT invasion as well as subsequent placentation (146-148).
The regulation of trophoblast invasion is multidimensional regulated by cytokines, hormones, local acting substances, and growth factors secreted by decidua. Also, trophoblasts modulate the MMP secretion of CTB (140-149). Bischof et al. reported that MMP-9 secretion by CTB is a prerequisite for Matrigel invasion and that putative AP1 sites are present within MMP9 promoters (151).
Maternal uterus and uterine-derived factors, as well as the microenvironment, also regulate trophoblast invasion by preventing the excessive breakdown of basement membrane components by trophoblast-derived MMP2/9 and serine protease (uPA) (Figure 7). The decidua-derived TGFβ induces TIMP both in the decidua and the trophoblast, thereby controlling the invasion of trophoblasts (140) both spatially and temporally throughout pregnancy. Thus, a gradual but definite decline is observed in the invasiveness of the trophoblasts with increasing gestational age.
 Figure 7
Figure 7Tempo-spatial regulation of trophoblast invasion by MMPs and their inhibitors.
A successful pregnancy is the outcome of a delicate balance, and synergistic dialogue between maternal and fetal compartments Placental trophoblasts are pseudo-malignant in nature with a temporospatial dynamics. During placentation, these trophoblast cells undergo an elaborate and complex differentiation program that leads to lineage differentiation into cells of different functions and destiny. EVT invasion leads to a phenotypic and biochemical transition, thereby acquiring an EMT phenotype before these invasive cells enter the uterine endometrium. Endovascular trophoblast invades and remodels the maternal blood vessels, whereas the endoglandular trophoblast invades the maternal endocrine glands (151). All these different modes of trophoblast invasion closely mimic cancer invasion and metastasis, thereby providing a platform to compare between the two systems.
The similarity between cancer EMT and trophoblastic EMT is fascinating. However, an in-depth understanding of the signaling pathways underlying the trophoblast EMT and to decipher their mechanistic details will give researchers an additional clue to understanding the mechanism of cancer invasion and metastasis in greater detail. Here we have an excellent self-regulating self-limiting system that is mechanistically so similar to a malignant tumor yet the elements of regulation which is missing in a tumor. Apart from this, understanding trophoblast invasion and EMT may offer novel strategies for targeted therapy in medical emergencies arising out of different placental pathologies like preeclampsia and FGR.
Further interestingly in contrast to the tumor microenvironment, the maternal uterine endometrium seems to play a crucial role in restricting the trophoblast invasion with a spatiotemporal dynamic, a feat that is not observed in tumors. It is speculated that placental bed giant cells contribute to the lineage commitment and terminal differentiation of the migrating trophoblasts, thereby acting as a natural barricade (152-154). Trophoblast giant cells either contain two diploid nuclei (BNCs) or multiple nuclei (human placental bed GCs) or single nuclei with amplified DNA content (rodent and rabbit GCs) as part of the endo-duplication process. GC/BNCs exhibit reduced migration or invasion capacity while upregulating the production of steroid hormones and other synthetic activities (154). In mice models, homozygous mutations in trophoblast-specific transcription factors, such as Hand1, Mash-2, I-mfa, or GCM1 regulate the induction, maintenance, or differentiation of distinct placental trophoblasts (152).
Further invading trophoblasts encounter a gradual -increase in oxygen tension as it inches close to the maternal vasculature within the endometrium (153). This increase in oxygen is believed to play an important role in EVT differentiation that occurs during the first trimester. This involves several signaling pathways, including those acting through hypoxia (HIF), the nutrient sensor (mTOR), and the endoplasmic reticulum stress-induced unfolded protein response pathway. A fine-tune regulation of this process is fundamental for a successful pregnancy outcome. Even a slight disruption in this delicate balance contributes to several pregnancy-associated pathologies (155-157). For example, the shallow invasion is a characteristic feature of preeclampsia and FGR, while abnormal deep EVT invasion is associated with placenta accreta/increta/percreta, and uncontrolled invasion by EVT is associated with choriocarcinoma (58-65).
New insights on trophoblast invasion, its EMT switch as well as pseudo-malignant behavior has kindred interest for a better understanding of such a crucial and a unique system. A thorough understanding of the transient pseudo-malignant trophoblast invasion process along with its finely controlled EMT program is crucial in our understanding of cancer invasion and metastasis besides addressing its essentialities in relation to embryo implantation and pregnancy. Given the fact that several pregnancy-associated pathologies result from an outcome of defective feto-maternal cross-talks, it is of prime importance to understand this process such that clinical intervention can be initiated during a crisis, thereby improving pregnancy outcome and reducing fetal mortality.
SK acknowledges RSS and ICMR INDIA for financial assistance. Department of Obstetrics and Gynecology at All India Institute of Medical Sciences, New Delhi, India for patient studies and providing tissue materials. SK and RD conceptualized and wrote this manuscript and SS assisted in formatting the manuscript, help to make the figures, and to arrange the references. RSS ,IM, SS, KP and SG, assisted in a critical review of this manuscript. We express our regards to Dr JBS and PD for providing clinical input. PD provided us with tissue histology consultation.
EMT
Epithelial to mesenchymal Transition
Cytotrophoblast
Extra villous cytotrophoblast
Villous cytotrophoblast
Interstitial CTB
Endovascular trophoblast
Natural killer cell
Uterine NK
Matrix Metalloproteinases
Tissue inhibitor of metalloproteinase
Extra cellular matrix
Hypoxia Inducible factor
Giant Cell
Circulating Tumor cells
Intra-uterine growth retardation
Fetal growth restriction
