Academic Editor: Graham Pawelec
Background: Fat Embolism Syndrome (FES) is a clinical condition characterized by neurological, respiratory, hematological and cutaneous manifestations. Fatal FES has been described as a rare complication during or after spinal elective surgery. The investigation of the cause of death in fatalities related with spine surgery should be mandatory to exclude or confirm fat embolism; a detailed methodological approach to the body in these cases suggests to provide a cautious dissection of surgical site and collection of samples to detect embolized fat globules in vessels. Methods: Two fatal cases of fat embolism syndrome after posterior spinal fusion are presented. Conclusions: A complete post mortem examination by means of histochemical and immunohistochemical analysis explained the cause of death and prevented medical malpractice litigation.
Patients with spinal instability caused by different spine disorders (traumatic fractures, metastatic tumors, infections and degenerative conditions) often require spinal fusion surgery. Although spinal fusion surgery can be associated with serious complications, perioperative mortality is generally considered rare, with a death-rate of 0.13 [1, 2]. Fat embolism syndrome (FES) is a clinical life-threatening condition characterized by neurological, respiratory, hematological and cutaneous manifestations. Fatal FES has been described as a rare complication during or after spinal elective surgery. In the last 50 years, cases of FES-related death after spine surgery were episodic. In these cases, a full autopsy is mandatory to fully disclose the cause of death and to prevent medical malpractice litigation. Two fatal fat embolism syndrome after posterior spinal fusion are presented. In all two cases, legal claims were prevented after a complete post-mortem examination.
Case #1. A 64-year-old woman affected from degenerative scoliosis with symptomatic stenosis of the lumbar canal was admitted to elective posterior spinal fusion surgery procedure (D4–S1). In the history, chronic pulmonary obstructive disease, atrial fibrillation and chronic kidney disease were reported. General anesthesia with propofol, sevoflurane and remifentanil was performed and airways were secured with orotracheal intubation. Surgical incision of the dorsal skin from D3 to S2 preceded the position of screws and injection of polymethylmethacrylate (PMMA) into all of the interested vertebral bodies. Finally, side bars over the vertebral screws were provided, tightening the screw nuts at the cemented vertebrae. Three hours after surgery, the patient complained a sudden decrease in oxygen saturation (from 98% up to 50%), hypocapnia and severe bradycardia. When cardiac arrest occurred, resuscitation maneuvers were attempted, unsuccessfully. A complete postmortem examination was performed two days after death to investigate the rightness of surgical procedure. The patient was 184 cm length and 77 kg weighed. The surgical incision was dried and underlying soft deep tissues mildly haemorrhagic. Pulmonary oedema was described at gross examination of lungs, enlarged in volume, with white foam on the main bronchi. Pulmonary embolism was excluded. Gross examination of other organs was negative. Polivisceral stasis was also detected. Routine staining of paraffin embedded sections with hematoxylin and eosin were performed for histological and immunohistochemical investigations. In brain, lungs and kidneys samples, multiple optically empty vacuoles filled the lumen of arterioles (Fig. 1a–b). Positive reaction to anti-CD61 antibodies revealed the presence of aggregating platelets on the edge of the vacuoles while anti-fibrinogen antibodies were used to show a strong reactivity in the outer layers of the optically empty vacuoles (Fig. 1c–d). Massive fat embolism was indicated as the cause of death and medical malpractice was excluded.
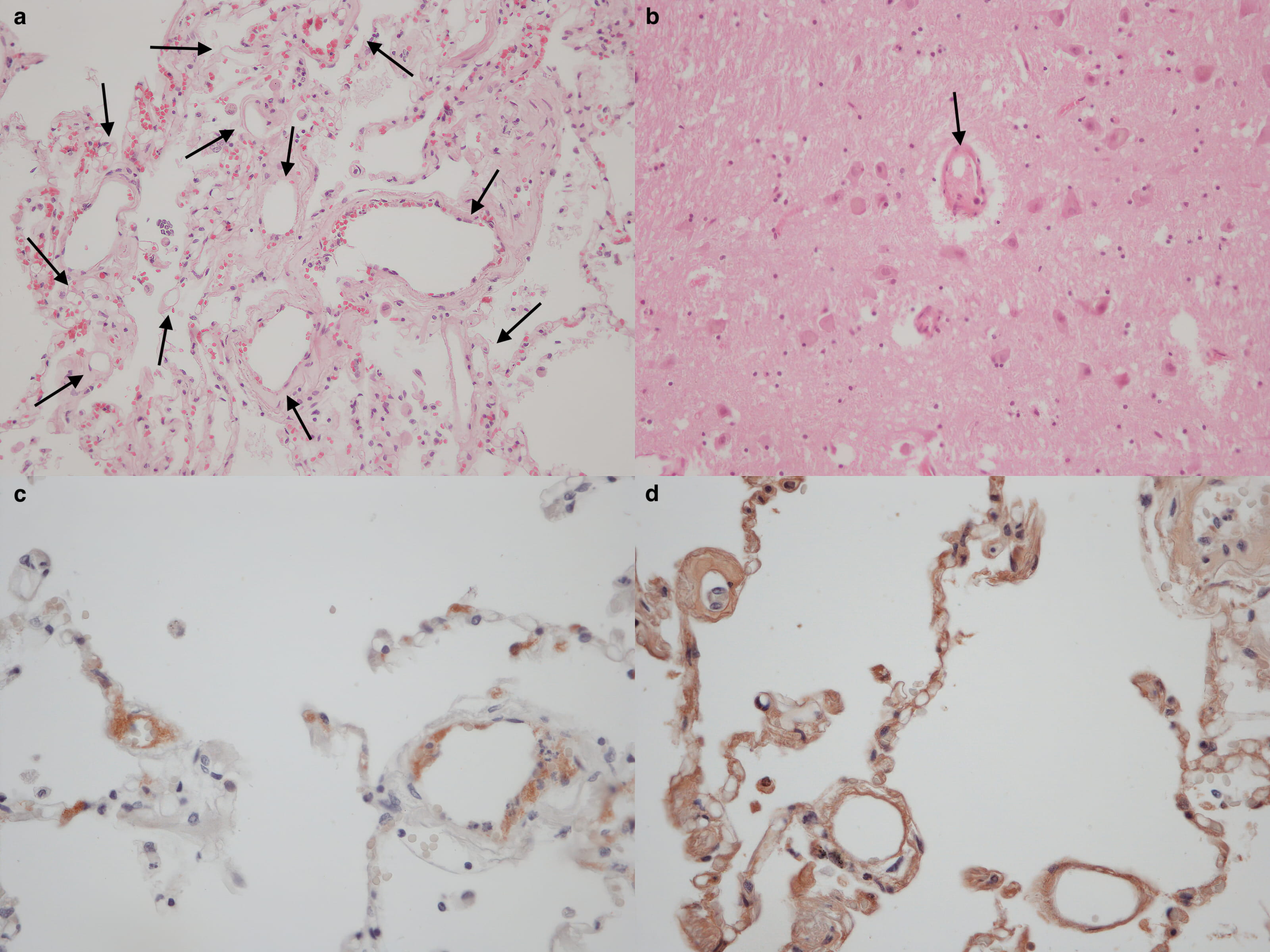 Fig. 1.
Fig. 1.Multiple optically empty vacuoles filled the lumen of arterioles of lungs (a black arrows) and brain (b, black arrow) samples. Immunohistochemistry positive reaction to anti-CD61 (c) and anti-fibrinogen (d) antibodies.
Case #2. A 51-years-old woman affected suffering from progressive and symptomatic right dorsal idiopathic scoliosis was admitted to posterior spinal fusion surgery (T9–L5) under general anesthesia. After surgical incision, titanium trans-peduncolar fixation, from T9 to L5, with screws was provided. The osteotomy of the thoraco-lumbar hinge was then performed and, finally, the stabilization bars were applied. At the same time, the vertebral laminae were decorticated with the addition of bone taken from the removed spinous apophysis. The surgery was concluded with the placement of two drainages and stitches upon the surgical wound. After surgery, the patient was discharged to the surgical ward in good clinical conditions. Two hours later, the patient was found unconscious with cardiac arrest was diagnosed. Advanced resuscitations efforts were unsuccessfully provided. A complete postmortem examination was performed. Mild basal pulmonary oedema was observed at gross examination as well as polyvisceral congestion. 400 mL of free fluid blood collection was observed in the abdomen due to the surfacing of the screws of fixation in the retroperitoneal space through the intercostal muscles. The evisceration of abdominal organ en-bloc according to modified Letulle technique excluded injuries to major vessels (Fig. 2a–b).
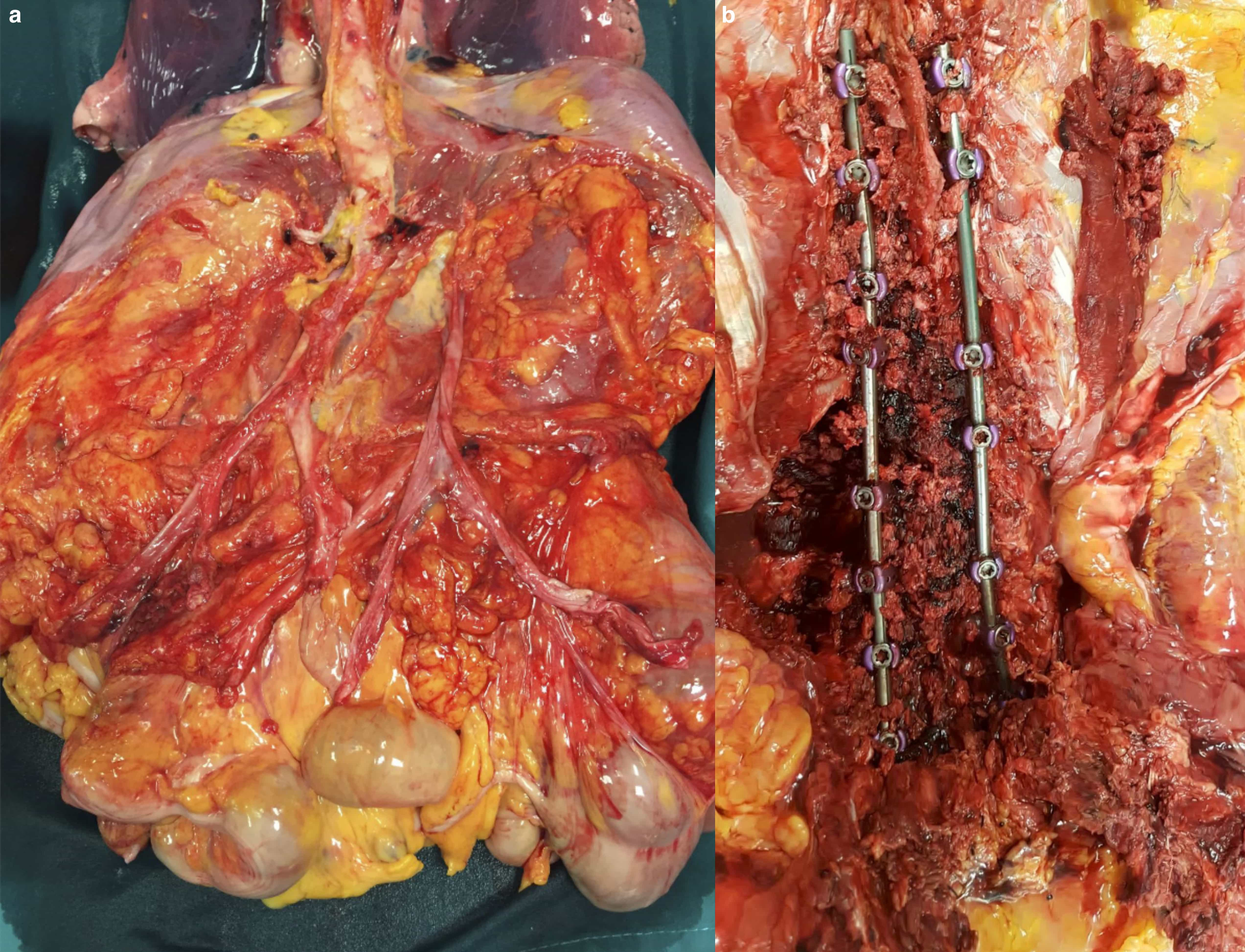 Fig. 2.
Fig. 2.(a) Removal of abdominal organ en-bloc and dissection of main vessels to exclude iatrogenic injuries. (b) Posterior approach to dorso-lumbar region and dissection of the site of surgery with instrumentation.
Histological examination showed the presence of multiple optically empty vacuoles in the pulmonary arterioles in all the lung samples displacing erythrocytes peripherally (Fig. 3a–b). Stain with red oil O confirmed the fatty nature of micro-vacuoles (Fig. 3c–d).
 Fig. 3.
Fig. 3.(a,b) H&E-stained section of lung. Extensive intravascular fat emboli (black arrows). (c,d) Oil red O-stained section of frozen lung tissue. Intravascular fat emboli and multiple fat orange-stained droplets within several vessels.
A grade III fat embolism was provided according to Falzi scale after a quantitative analysis. Post-surgical massive fat embolism syndrome was indicated as the cause of death and medical malpractice was excluded.
Neurosurgery is amongst the medical specialties subject to the highest number of claims with an average payout of $440.000 [3]. In particular medical malpractice litigation in neurosurgery is associated with spinal procedures, as six out of the seven most claimed pathologies involved the spinal column [4, 5]. In most cases, malpractice claims are motivated by three main factors: ascertaining the facts that led to a negative outcome in order to understand what happened, securing financial compensation and defining the alleged responsibility of the physician or the system. Curiously, even when poor outcome, poor performance or supposed negligence during procedure were supporting the claims, a bad physician-patient relationship represents the first risk factor of claims, no matter the outcome [4]. In a recent review about informed consent in neurosurgery, it emerged that the lack of informed consent is the basis for a large portion of malpractice. The review outlines that the consent is rarely fully informed, either because of the patient limited understanding or because of negligent medical advice, where the physician failed to help the patient make an informed decision [6]. So far, many attempts have been tried out to facilitate and improve the exchange of information provided during the informed consent process.
The risks associated with surgical procedures need to be clearly and thoroughly explained and fully understood by the patient [6]. An improved physician/medical system-patient relationship is therefore necessary to reduce the number of disputes, with the additional benefits of lowering daily medical practice stress levels and likely enhancing the overall performance. Even if during the last two decades the total number of malpractice claims has decreased, the payout per claim has increased significantly, especially in tragic cases where the patient deceased during or immediately after the procedure [7].
Fat embolism syndrome (FES) is a clinical condition that occurs when fat goblets reach the bloodstream obstructing the capillary flow. Respiratory distress, neurologic manifestation, and petechial rash represent the classic clinical triad. The pulmonary function is generally primarily involved, followed by other symptoms, when pulmonary filter is outdated. Patients with FES may complain hypoxemia, neurological abnormalities and petechiae 12 to 72 hours after orthopedic trauma or surgical procedures. Dyspnea, tachypnea and respiratory failure with hypoxemia are the main respiratory symptoms. Neurological manifestations include focal deficit, confusion, loss of consciousness up to coma [8].
When massive fat embolism occurs, clinical onset of symptoms is fulminant and patients early complain right ventricular dysfunction, cardiac failure, acute respiratory distress syndrome, cardiovascular shock and death. The pathophysiologic mechanism underlying FES clinical manifestation is still poorly understood. A mechanical and a biochemical theory are mainly provided [9]. Mechanical theory supposes that fat embolization is caused by the fracture of a long bone since trauma tears intraosseous blood vessels causing venous aspiration of disrupted fat globules; once they have entered inside circulation and reached lung (or systemic) vessels, fat goblets could interrupt blood flow causing symptomatology. Fat globules act with proinflammatory and prothrombotic effects triggering the aggregation of platelets and accelerating fibrin generation. As a direct consequence of pulmonary capillaries obstruction, interstitial hemorrhage, alveolar edema, alveolar collapse and hypoxemic vasoconstriction occur [10]. Biochemical theory was formulated trying to explain both the delay of the onset of symptomatology and the atraumatic FES. It involves fat goblet properties such as endothelial toxicity of fatty acids released by the activation of lipase with induction and release of proinflammatory factors (like IL-1 and 6) and the prothrombotic activity of free circulating fat goblet which could cause platelets covering and the setting of coagulation cascade [1, 9, 11]. Elevated levels of plasma phospholipase A2, proinflammatory cytokines (TNF-alpha, IL-1, IL-6) have been observed in patients with fat embolism syndrome [12]. In most cases, both mechanical and biochemical mechanisms are involved. Fat embolism syndrome is generally associated with orthopedic trauma and surgery, in cases of long bone fractures of the lower extremities, multiple bones fractures, ribs fractures, pelvic fractures. Vertebral body fractures are rarely associated with fat embolism despite they are often observed in post menopausal women [13]. A high risk of fat embolism syndrome up to 30% of cases has been reported in orthopedic trauma in the young population (10–40 years) [14]. Pancreatitis, alcoholic liver disease, bone marrow transplant or liposuction are the main (rare) causes of fat embolism syndrome in non-orthopedic cases. Diagnostic criteria of FES are not standardized or validated. Most cited clinical diagnostic criteria were proposed by Gurd and Wilson [15], Schonfeld et al. [16] and Lindeque et al. [17] who linked diagnosis of FES to the recurrence of major and minor criteria (Table 1, Ref. [15, 16, 17]).
| Gurd and Wilson [15] | Schonfeld et al. [16] | Lindeque et al. [17] |
| Major criteria | Petechiae (score 5) | Sustained pO2 |
| Petechial rash | X-ray chest diffuse infiltrates (score 4) | Sustained pCO2 |
| Respiratory symptoms with radiographic changes | Hypoxemia (score 3) | Sustained respiratory rate |
| Central Nervous System signs unrelated to trauma or other condition | Fever (score 1) | Increased work of breathing, dyspnea, tachycardia, anxiety |
| Minor criteria | Tachycardia (score 1) | |
| Tachycardia | Tachypnea (score 1) | |
| Pyrexia | Confusion (score 1) | |
| Retinal changes | ||
| Renal abnormalities (oliguria, anuria, lipiduria) | ||
| Acute thrombocytopenia | ||
| Acute decrease in hemoglobin | ||
| High erythrocyte sedimentation rate (ESR) | ||
| Fat globules in sputum | ||
| Diagnosis | Diagnosis | Diagnosis |
| Two major criteria or | Score |
At least one |
| One major criteria plus four minor criteria |
Despite its well-known clinical characterization, post mortem diagnosis of Fat Embolism Syndrome is still debated, especially in cases in which circumstances and clinical information are not completely available. The risk of underestimation of fat embolism syndrome in postmortem examination is real. Eriksson et al. [5] defined fat embolism syndrome as the “undiagnosed epidemic” because of the findings of fat emboli in the pulmonary circulation in 82% of trauma patients and 88% of patients who received cardiopulmonary resuscitation. In other studies, autopsy findings of fat embolism were described in 80% of trauma patients even if scarcely symptomatic [18, 19].
In most cases, definitive diagnosis of Fat Embolism Syndrome is demanded to autopsy and histopathological examination. Cleared-out circular and oval spaces into pulmonary vessels identify fat droplets deposition. Fat can be specifically stained in froze tissues with oil red O showing multiple red droplets. Fat droplets stain brown or orange when stained with Sudan black or Sudan III [20]. Post mortem diagnostic criteria of FES were proposed combining clinical, radiographic and autopsy findings by some authors [21, 22]. Miller et al. [23] in 2011 authors observed that when collapse occurs suddenly, before the onset of clinical symptoms, and no other appropriate cause of death could be ascertained at autopsy, Fat Embolism Syndrome should be suspected if fatty globlets are observed in pulmonary and cerebral vessels. Finally, multiple grading systems are available to evaluate the severity of fat embolism (Table 2) [24, 25, 26, 27, 28, 29].
| Authors | Grade | Shape | Staining |
| Falzi et al. [24] | 0 | None | NR |
| 1 | Dome | ||
| 2 | Sausage/round | ||
| 3 | Antler | ||
| Sevitt [25] | Slight (1) | NR | |
| Moderate (2) | 1–3 per field | ||
| Gross (3) | |||
| Emson [26] | Mild | 20/unit area | Oil red O |
| Moderate | 21–60/unit area | ||
| Severe | |||
| Bunai et al. [27] | Quantitative | Osmium tetroxide | |
| Busuttil et al. [28] | Fat Embolism Index | Quantitative | Osmium tetroxide |
| Turillazzi et al. [29] | 0 (A) | No emboli | H&E, Sudan III |
| 1 (A) | Sporadic presence | ||
| 2 (B) | Slight embolism | ||
| 3 (C) | Moderate embolism | ||
| 4 (D) | Massive embolism |
Determining the severity of fat embolism found at autopsy is mandatory to provide a reliable cause of death and the involvement of fat emboli. Quantitative analysis of the size and location of fat embolism may provide useful indications as well as comparing the total percentage of embolized tissue area compared with total tissue area of all collected samples, but it should be difficult to perform in daily practice.
Elective spinal fusion is considered a quite safe procedure, whose mortality rate is historically considered to be low. Poorman GW et al. [12] evaluated the mortality rate of 803.949 patients who underwent to lumbar spinal surgery, stratifying death by procedure (simple or complex fusion and decompression). An overall mortality rate of 0.13% and 0.321% was provided for spine surgery and complex spinal fusion, respectively.
The role of fat embolism syndrome related to spine surgery is emerging in the last few years and much more effort need to be provided in the future to a better awareness of the phenomenon and its real incidence in neurosurgical settings [30].
A study supported by transesophageal echocardiography during spine surgery, found that fat embolization occurs in 80% of instrumented patients but in no one of the 20 non-instrumented patients [31]. Patients undergoing vertebroplasty on multiple levels appear to be at greater risk and the involvement of methylmethacrylate has been supposed causing an increase in the intramedullary pressure dislodging the bone marrow and forcing it into the venous bloodstream [32]. Pressurization of cement in association with intramedullary placement of implants was indicated as a possible risky association for fat embolism [33].
In the last 50 years, only eight single reports of fatal Fat Embolism Syndrome related with spine surgery (with and without instrumentation) were reported and underestimation of the phenomenon need to be considered in surgical practice (Table 3) [32, 33, 34, 35, 36, 37, 38, 39].
| Authors | N° | Age | Sex | Surgery | Onset of symptoms after surgery/death | Stain |
| Temple JD, 2002 [32] | 1 | 61 | F | Revision posterior spinal arthrodesis (T2–L2) and pedicle screw augmentation | Intraoperatively | Oil red-O |
| Chen HL, 2002 [33] | 1 | 75 | F | L2, L4 percutaneous vertebroplasty | Immediate | Autopsy not performed |
| Syed MI, 2002 [34] | 1 | 69 | F | Percutaneous vertebroplasty (T10–T12) | 5 hours | Oil red-O |
| Takahashi Y, 2006 [35] | 1 | 57 | M | Revision posterior spinal fusion, removal of Luque rod and wire system (T1/2–T5/6), transpedicular screw fixation of T1,T2,T5, T6 | 5 hours | Oil red-O |
| Brandt SE, 1998 [36] | 1 | 56 | F | Instrumented dorsolateral spinal fusion and transpedicular fixation L4/L5/S1 | 6 hours | Oil red-O |
| Gittman JE, 1983 [37] | 1 | 17 | F | Posterior final fusion T5–L4 with Harrington rod insertion | Immediately | Oil red-O |
| Joffe D, 2006 [38] | 1 | 11 | M | Posterior instrumentation for scoliosis | 7 hours | Not reported |
| Stroud MH, 2006 [39] | 1 | 17 | F | Posterior spinal arthrodesis of T1 4 days after anterior spinal release followed by posterior spinal fusion with instrumentation (T10–L3) | 24 hours | Oil red-O |
Augmentation of several vertebral bodies is considered a risky procedure as well as vertebral body manipulation during surgery and insertion of pedicle screws [35, 39]. In contrast, Temple et al. [32] excluded a possible role of transpedicular instrumentation with fat embolism.
Lowering the injected dose of cement volumes was suggested to provide this complication to no more than 4 mL per thoracic vertebra and 6 mL per lumbar vertebra, anyway individual tolerance to fat and bone marrow embolization could to be considered [35]. The viscosity of the cement mixture also plays a role in that lower viscosity cement will have an increased chance of extravasation. Patients with compromised cardiopulmonary reserve with COPD, pulmonary arterial hypertension or with deep venous thrombosis or pulmonary embolism in the medical history need to be considered at risk and stratification of risk is mandatory in these cases but fatal events still appear unpreventable. Furthermore, considering to maintain tracheal intubation for several hours after surgery is a potential life-saving procedure in patient at risk and shared with anesthesiologists [35].
When fatalities occur after neurosurgery and FES is suspected, a complete post mortem investigation should be considered mandatory to disclose the causes of the unexpected deaths, to promote a policy of transparency and to prevent legal claims [7]. On 8 march 2017 Italy enacted the law no. 24/2017 named Provisions on patient care safety and professional liability of healthcare providers. It begins recognizing that patient safety is a fundamental right of each individual within any healthcare service and it is a primary goal of the national healthcare service [40]. A central aspect of patient protection is defined in the article 4 dedicated to the issue of transparency. An important step in efforts towards transparency in the health system is the provided possibility for the next-of-kin of the deceased to come to an agreement with the hospital director to execute a post mortem examination in the presence of their physician of choice, in the case of a death in the hospital [41]. These regulations bring Italy into line in the drive towards increasing transparency within healthcare at personal, provider and structural level.
Investigating the cause of death by means of autopsy in fatalities related with spine surgery should be mandatory to exclude or confirm fat embolism and to prevent medical malpractice claims in order to the surgical practice. A cautious dissection of the surgical site will provide all the needed evidences of good surgical practice. Frozen lung and brain samples should be always collected to detect embolized fat globules in vessels for diagnosis of FES. The histological aspect of fat embolism could be highlighted using the elective stain for fat, Oil red O-on frozen tissue, and in addition is very useful perform immunohistochemistry using anti CD-61 antibody to showed platelets aggregates at the edge of fat globules and an absorbed fibrinogen layer to the interface of fat globules using anti-fibrinogen antibody [42].
Conceptualization—SD and MZ; methodology—DR and MC; validation—MN and LA, writing-original draft preparation—DR and RM; supervision—PF and LC. All authors provided critical feedback and helped shape the research, analysis and manuscript.
All procedures performed in the study were in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the relatives.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. LC is serving as one of the Editorial Board members of this journal. We declare that LC had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to GP.
The authors declare to have received written consent from relatives of all persons involved in this case report, to publish the article mentioned above in Frontiers in Bioscience-Landmark.
Please contact author for data requests.
FES, Fat Embolism Syndrome; PMMA, polymethylmethacrylate; COPD, chronic obstructive pulmonary disease.
