2. Introduction
Adenosine 5’-triphosphate (ATP) is present intracellularly as the major energy
source for a myriad of biochemical reactions and physiological processes that are
critical to the viability and normal functions of cells in living organisms.
Conceivably, ATP release into extracellular space occurs as a sequel or an
indicator of tissue damage that causes cells to lose the integrity of the plasma
membrane (PM), and ATP acts as a danger-associated molecular pattern molecule.
There is also an extensive collection of evidence to show that many mammalian
cell types can release ATP in a non-lytic fashion under physiological and
pathological conditions [1, 2, 3, 4]. It has been well established that ATP, once
outside the cell, acts as an autocrine signal regulating multiple cell functions,
or as a paracrine signal enabling cell-to-cell communication [5, 6, 7].
Intracellular Ca is a ubiquitous second messenger that stimulates
Ca-dependent signal pathways underpinning the short-term and/or long-term
effects of numerous external stimuli or signals on a wide range of cell functions
[8]. Not surprisingly, the most common action modality of extracellular ATP as a
signalling molecule is to raise intracellular Ca concentration
([Ca]) [9]. More specifically, ATP can generate intracellular
Ca signals with spatiotemporal dynamics through a family of cell surface
receptors termed P2 purinergic receptors, which can be categorised into two
functionally and structurally distinct subfamilies, P2X and P2Y [10]. Mammalian
cells express seven P2X proteins or receptor subunits (P2X1–P2X7) [11], which
have a membrane topology composed of intracellular N- and C-termini, two
transmembrane domains and an exceptionally large extracellular domain, and can
form homo/hetero-trimeric ATP-gated Ca-permeable cation channels (Fig. 1A)
[12, 13, 14]. There are eight different P2Y receptors in humans (P2Y,
P2Y, P2Y, P2Y and P2Y-P2Y), structurally all
belonging to the seven-transmembrane domain guanosine diphosphate (GDP)/guanosine
triphosphate (GTP)-binding protein (G protein)-coupled receptor superfamily. They
display a differential sensitivity to extracellular ATP and various other
nucleotides (e.g., UTP, UDP, ADP, and UDP-galactose) and coupling with different
G-proteins and downstream signal pathways [15]. ATP preferentially activates the
P2Y, P2Y and P2Y receptors, all of which are coupled to the
G protein, with the P2Y receptor known to link alternatively with
the G protein. Activation of these G-coupled receptors stimulates
phospholipase C (PLC) to generate inositol 1,4,5-triphosphate (IP) from
membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP), and IP in
turn opens the Ca-release channel IP receptor (IPR) in the
endoplasmic reticulum (ER) (or sarcoplasmic reticulum in muscles), resulting in
Ca release from the ER (Fig. 1A). Constitutively, a majority of studies
examining ATP-induced Ca signaling and regulation of cell function have
drawn attention to the P2X and P2Y receptors. However, it is widely documented
that the reduction in the ER Ca level can trigger the so-called
store-operated Ca entry (SOCE), through the store-operated Ca (SOC)
channel, to restore intracellular Ca homeostasis, particularly the
Ca content in the ER [16, 17]. This Ca entry mechanism, while
initially and mostly reported in non-excitable cells, is also widely utilized in
excitable cells [18, 19]. For example, a variety of neurotransmitters and
neuromodulators, with ATP being one of them, activate their cognate
G-coupled receptors to induce ER Ca release and subsequent SOCE
to shape neuronal Ca signaling [18]. It is recognized nowadays that SOCE
is one of the most common Ca signalling mechanisms [16].
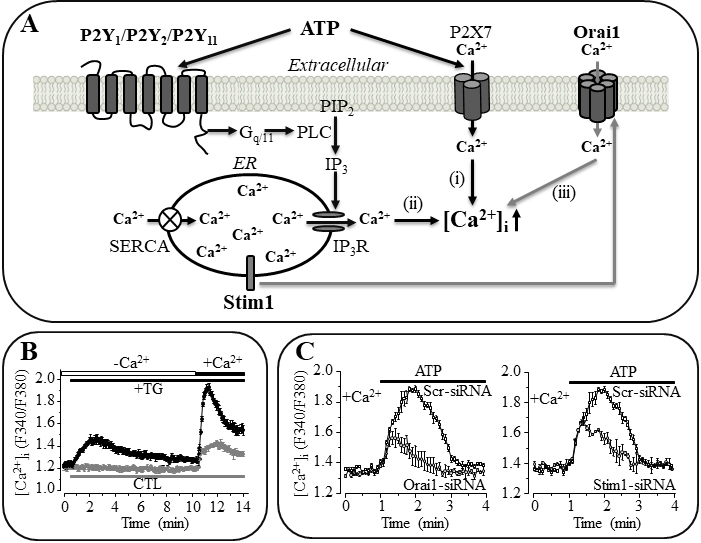 Fig. 1.
Fig. 1.
A graphic illustration of the molecular mechanisms that
participate in ATP-induced Ca signalling in mesenchymal stem cells (MSC).
(A) Extracellular ATP induces an increase in intracellular Ca
concentration via the P2X7 receptor that mediates Ca influx (i).
Alternatively, ATP activates the G-coupled P2Y receptor (P2Y,
P2Y and/or P2Y) and phospholipase C (PLC) to generate inositol
1,4,5-triphosphate (IP) from membrane lipid phosphatidylinositol
4,5-bisphosphate (PIP) and induce IP receptor (IPR)-mediated
Ca release from the endoplasmic reticulum (ER) (ii). Release of the ER
Ca subsequently triggers store-operated Ca entry (SOCE) through the
store-operated Ca (SOC) channel, particularly the
Ca-release-activated Ca (CRAC) channel (iii). Inhibition of the
sarcoplasmic/endoplasmic Ca-ATPase (SERCA) with thapsigargin (TG) to
prevent the cytosolic Ca uptake can lead to loss of the ER Ca,
which is widely used in “Ca add-back” experiments to activate the SOC
channel. (B) Example recordings using “Ca add-back” to show that
treatment of human dental pulp-derived MSC with TG induced release of the ER
Ca in the absence of extracellular Ca led to a greater Ca
response upon re-introduction of extracellular Ca. CTL, control (without
TG treatment). (C) Example recordings showing that small interference RNA
(siRNA)-mediated knockdown of the expression of Orai1 or Stim1 reduced
ATP-induced Ca response in human dental pulp MSC. Scr-siRNA, scrambled
siRNA. (B) and (C) taken and modified from Peng et al. (2016) [80].
Mesenchymal stem cells (MSC) are present in stem cell niches in many adult
tissues, like bone marrow, adipose tissue and dental pulp, and play an essential
role in the homeostasis of residing tissues [20]. They are multipotent stem cells
and able to differentiate into several cell lineages [21, 22]. Decades of studies
have demonstrated their promising applications in regenerative medicines. MSC
represent an attractive source of cells for tissue engineering to repair,
regenerate or replace damaged or lost tissues (e.g., [23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34]). There are
extensive interests in, and emerging evidence to support, the use of MSC in
cell-based therapies to treat a variety of pathological conditions (e.g.,
[35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53]). A multiplicity of extracellular stimuli or signals, physical, chemical
or biological, have been identified to regulate MSC functions and fate (e.g.,
[54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74]).
ATP represents one extracellular signal that regulates MSC differentiation,
proliferation, migration and tissue homing [75, 76, 77, 78, 79, 80, 81, 82, 83]. It is well known that MSC
exhibit a high sensitivity to diverse mechanical forces, for example, fluid
flow-induced shear stress and shockwaves, as well as the mechanical properties of
residing tissues and cell-supporting matrix. Such mechanical signals have been
shown to significantly regulate MSC functions [77, 78, 84, 85, 86, 87, 88, 89, 90, 91, 92]. Interestingly,
accumulating evidence from examining MSC and other mechanosensitive cells
suggests that ATP release and induction of P2X/P2Y receptor-mediated Ca
signalling represent an important mechanism that transduces the mechanical signals into
adaptative cell functions [93]. Multiple P2X and P2Y receptors are reported for
their expression in MSC preparations from different species and tissues, albeit
with some noticeable variations in the receptor type, expression level and role
in ATP-induced Ca signalling (reviewed by [94]). In addition to the P2X7
receptor, P2Y, P2Y and P2Y are the major receptors that
participate in mediating ATP-induced Ca signalling (Fig. 1A) [75, 76, 77, 78, 79, 80, 81, 82, 83, 95, 96, 97]. There is some evidence to show that SOCE can be induced in MSC by the
reduction in ER Ca following activation of the bespoke
G-coupled P2Y receptors. However, it remains less well understood with
respect to the contribution of SOCE in ATP-induced Ca signalling in MSC.
This min-review article aims to provide an overview of studies, particularly the
recent studies from our own and also from other groups that evolve our
understanding towards the molecular identity of the SOC channel in MSC and its
role in ATP-induced Ca signalling and, additionally, its potential role in
ATP-induced regulation of cell differentiation, proliferation and migration.
3. The SOC/CRAC channel
As already introduced above, the SOC channel is activated by the loss of ER
Ca and thus, by its unique activation mode, is distinguished from the
receptor-operated, ligand-gated or voltage-gated Ca channels.
Experimentally, SOC channel activation or SOCE can be readily induced by
depleting the ER Ca using thapsigargin (TG) to block the
sarcoplasmic/endoplasmic reticulum Ca-ATPase (SERCA) that mediates
Ca uptake from the cytosol into the ER (Fig. 1A), circumventing the need
for activating the G-PLC-IPR pathway. Thus, as illustrated in
Fig. 1B, one widely used means to demonstrate the SOCE is treating cells with TG
in the absence of extracellular Ca and measuring the [Ca]
upon addition of extracellular Ca, an experimental protocol often referred
to as “Ca add-back”.
Early electrophysiological studies show that the Ca permeability of the
SOC channels varies considerably from being modest to highly selective, depending
on the cells in which they are expressed [98]. So far, not all of the SOC
channel-forming proteins have not been molecularly identified or established, and
the Ca-release-activated Ca (CRAC) channel represents the best
understood SOC channel. The CRAC channel displays several hallmark
electrophysiological properties, including highly selective Ca
permeability (conducting Ca1000 times better than Na under
physiological conditions), tiny single channel unitary conductance (9–24 fS in
2–110 mM extracellular Ca) and strong inward rectification [16]. Several
candidates, including members of the transient receptor potential (TRP) channel
superfamily, were proposed to form or function as the CRAC channel [16]. It is
now mostly accepted that the families of Orai proteins and Ca-sensing
stromal interaction molecule (Stim) proteins, particularly Orai1 and Stim1, are
the two core components of the CRAC channel [16, 99, 100]. These two proteins
have distinctive structural features, subcellular location and role in the CRAC
channel activation (Fig. 1A). Orai1 contains intracellular N- and C-termini and
four transmembrane segments and assembles as a hexameric complex forming a
central Ca-permeating pore in the PM, whereas Stim1 is a single
membrane-spanning protein present in the ER membrane and uses an EF-hand
Ca-binding motif in the ER-facing part as the Ca sensor. The CRAC
channel is activated through the so-called diffusion trap mechanism [16]. Namely,
the reduction in ER Ca promotes Stim1 to aggregate and translocate
(diffuse) to the PM-ER junction, where Stim1 binds to Orai1 and induces the
channel to open, allowing extracellular Ca to enter the cytosol and then
re-fill the ER via the SERCA. Orai1 and Stim1 are the most common components of
the SOC channel in many cell types. Nonetheless, there is increasing evidence to
show that the other two members of the Orai family, Orai2 and Orai3, working
together with Stim1 or Stim2, can also form CRAC channels, independently of or
via heteromerizing with Orai1, that have some distinctive differences in
pharmacological properties [99, 100, 101, 102].
4. Role of the SOC/CRAC channel in ATP-induced Ca signalling in
MSC
The first experimental evidence suggesting the SOC channel being an integral
part of the ATP-induced Ca signalling mechanism in MSC was from an early
study by Kawano et al. [75] examining the molecular mechanisms
underpinning the spontaneous oscillations or periodic increases in the
[Ca] in human bone marrow-derived MSC (BM-MSC) observed under
in vitro culture conditions. Such Ca oscillations were ablated
by treatment with the generic P2 receptor antagonist PPADS or the PLC inhibitor
U73122, as well as 2-aminoethoxydiphenyl borate (2-APB) known to block the
IPR and SOC channel. The Ca oscillations and ATP in the culture
medium were also obliterated by treatment with hexokinase together with
glutamate, a combination known to consume ATP, and by treatment with octanol,
palmitoleic acid or 18α-glycyrrhetinic acid (AGA), all of which are known to block the hemi-gap junction
channel. Furthermore, the Ca oscillations were lost after treatment with
BzATP or APPS, both of which can block the P2Y receptor. Collectively,
these observations led to the proposal of a mechanism generating the spontaneous
Ca oscillations, in which ATP is spontaneously released into the
extracellular space, through the hemi-gap junction channel, and activates the
P2Y-G-PLC-IPR pathway to release the ER Ca and induce
SOCE [75]. Both ER Ca release and SOCE, albeit differing spatiotemporally,
contribute to the increase in the [Ca]. The molecular identity of
the SOC channel however was not determined in the study. Riddle and colleagues
proposed ATP release and subsequent activation of the
P2Y-G-PLC-IPR pathway to trigger ER Ca release as an
important mechanism resposnbile for the rise in the [Ca] in human
BM-MSC in response to oscillatory flow fluid-induced shear stress [54, 77]. They
demonstrated the expression of P2Y and P2Y, and also P2X7, but not
P2Y, using western blotting or immunocytochemistry, but did not examine in
detail the exact roles of these receptors and the SOC channel in fluid
flow-induced ATP-mediated Ca signalling. Interestingly, it was shown that
fluid flow-induced ATP release was insensitive to AGA but was considerably
suppressed by treatment with monensin, which is known to prevent vesicle budding
from the Golgi apparatus, or N-ethylmaleimide, which is known to block vesicle
fusion with the PM. These observations suggest that BM-MSC releases ATP in
response to fluid flow through a vesicular mechanism [77], rather than through
the hemi-gap junction channel initially proposed to mediate spontaneous ATP
release [75].
We have examined in a recent study the expression of the SOC channel as well as
the P2X and P2Y receptors and their roles in mediating ATP-induced Ca
signalling in human dental pulp derived MSC (DP-MSC) [80]. We showed using the
“Ca add-back” experimental protocols that depletion of the ER Ca
by treatment with TG induced strong SOCE (Fig. 1B). Furthermore, TG-induced SOCE
was reduced by treatment with 2-APB, or syntha 66, a SOC channel selective
inhibitor. These results clearly support the expression of the SOC channel in
human DP-MSC [80]. In human DP-MSC, exposure to exogenous ATP also induced
strong but transient Ca responses in extracellular Ca-free
solutions, indicating release of the ER Ca as a result of ATP-induced
activation of the P2Y-G-PLC-IPR pathway. In addition, we have
shown that ADP, a P2Y selective agonist, and BzATP, an agonist for the
P2Y receptor (and also for the P2X receptors), were effective in inducing
Ca responses in extracellular Ca-containing solutions [80].
ATP-induced Ca response was significantly attenuated by treatment with
2-APB or syntha 66, as well as by treatment with PPADS or AZ11634737, a P2X7
receptor specific antagonist. Consistently, the mRNA transcripts of P2X7,
P2Y and P2Y, but not P2Y, were consistently detected in human
DP-MSC, using reverse transcription-polymerase chain reaction (RT-PCR).
Furthermore, ATP-induced Ca responses were reduced after treatment with
small interference RNA (siRNA) that specifically knocked down the expression of
P2X7, P2Y or P2Y. Taken together, these results support
participation of the SOC channel, in addition to the P2X7, P2Y and
P2Y receptors, in mediating ATP-induced Ca signalling (Fig. 1A)
[80]. Two recent studies, one using human adipose tissue-derived MSC (AT-MSC)
[97] and the other using rat DP-MSC [82], have also shown that exposure to
exogenous ATP induced Ca responses in the absence, as well as in the
presence, of extracellular Ca. ATP-induced Ca response was
inhibited by treatment with TG, U73122 or 2-APB, consistently supporting a
critical role of ER Ca release following activation of the
P2Y-G-PLC-IPR pathway in ATP-induced Ca signalling. One
study has proposed, based on the pharmacological profile, P2Y as the
receptor mediating ATP-induced Ca signalling [97], and the other study did
not identify the P2Y receptor(s) involved [82]. None of these studies have
determined the role of the SOC channel, or the contribution of SOCE, which would
occur following release of ER Ca, in ATP-induced Ca signalling.
As the CRAC channel made of Orai1 and Stim1 represents the SOC channel with the
best-established protein components and activation mechanism, we have further
examined the expression of Orai1, Stim1 and Stim2, and their roles in ATP-induced
Ca signalling in human DP-MSC [80]. The mRNA expression for Orai1, Stim1
and Stim2, in human DP-MSC was detected using RT-PCR. Importantly, TG-induced
SOCE was reduced by siRNA-mediated knockdown of the expression of Orai1 or Stim1,
but not Stim2, supporting that Orai1 in pairs with Stim1 forms the CRAC channel
[80]. Moreover, consistent with the inhibition by syntha 66, which has recently
been shown as an Orai1-specific CRAC channel inhibitor [102], ATP-induced
Ca response was suppressed by siRNA-mediated reduction of the expression
of Orai1 or Stim1 (Fig. 1C). These results provide the first line of evidence to
show that Orai1 and Stim1 constitute the CRAC channel as a significant mechanism
contributing in ATP-induced Ca signalling.
In summary, accumulating evidence supports the SOC channel, particularly the
CRAC channel made of Orai1 and Stim1, as an integral part of the mechanism for
ATP-induced Ca signalling in MSC.
5. Role of the SOC/CRAC channel in ATP-induced regulation of MSC
function
Studies have shown that extracellular ATP, applied exogenously or released by
MSC, can regulate MSC differentiation, proliferation and migration. Moreover,
these studies have gathered substantial evidence to support that both P2X7 and
G-coupled P2Y receptors and their downstream Ca-dependent signal
pathways play a signficant role in such ATP-induced regulation of MSC functions
(reviewed by [103]). In contrast, the role of the SOC or CRAC channel in
ATP-induced regulation of MSC function, despite being implied, still remains
elusive.
In the study examining the molecular mechanisms underlying the spontaneous
Ca oscillations in human BM-MSC, Kawano et al. [75] noticed that
the spontaneous Ca oscillations disappeared after induction of
differentiation to adipocytes. They also showed that such Ca signaling was
critical for the translation from the cytosol to the nucleus of nuclear factor of
activated T-cells (NFAT), a vital transcription factor driving the expression of many genes. However, it is still unknown
regarding the mechanisms underlying the contribution of such spontaneously
occurring Ca signalling, with SOCE being part of it, in NFAT activation
and, furthermore, in adipogenesis. The recent study by Stovall et al.
[82] has shown that exposure of rat DP-MSC to exogenous ATP stimulated osteoblast
formation and the expression of multiple osteogenic genes. As discussed above,
the study has proposed the G-coupled P2Y receptor as the major ATP
receptor in rat DP-MSC, leading to the conclusion that ATP enhances osteogenic
differentiation via G-coupled P2Y receptor-dependent Ca
signalling. However, the role of the SOC channel-mediated Ca signalling in
such ATP-induced regulation of osteogenesis remains unknown. At this point, it is
worth mentioning that several other recent studies using human MSC preparations
from several tissues provide evidence to show that the P2X7 receptor also plays a
significant role in ATP-induced regulation of osteogenic differentiation [68, 69, 79, 81].
In the above-discussed studies revealing that fluid flow evoked Ca
signalling through ATP release and activation of the
P2Y/P2Y-G-PLC-IPR pathway to cause ER Ca
release in human BM-MSC, Riddle et al. [54, 77] also demonstrated that
fluid flow enhanced cell proliferation. Furthermore, they showed that fluid flow
stimulated the activity of protein kinase C (PKC) and downstream signalling
molecules, MEK and ERK1/2 mitogen-activated protein kinases, as well as
calcineurin, a Ca/calmodulin-dependent phosphatase. Consistent with the
well-established roles of these Ca-dependent signal pathways in the
regulation of cell proliferation, fluid flow-induced stimulation of cell
proliferation was inhibited by treatment with the MEK/ERK inhibitor U-0126 or the
calcineurin inhibitor cyclosporine A [54]. Moreover, fluid flow-induced
activation of calcineurin and stimulation of cell proliferation, as well as fluid
flow-induced increase in the [Ca], were inhibited by treatment with
apyrase, supporting a critical role of ATP release and induction of intracellular
Ca signalling and activation of downstream Ca-dependent signal pathways [77].
Like ATP released by fluid flow, exposure to exogenous ATP, but not ADP, AMP and
adenosine, the major ATP metabolites, significantly stimulated cell
proliferation. Taken together, these results provide clear evidence to show that
fluid flow stimulates MSC proliferation via inducing ATP release and activation
of the G-coupled P2Y receptors, leading to ER Ca release and
activation of the downstream Ca-dependent signal pathways. As pointed
above, it was anticipated that SOCE occurred following ER Ca release under
these conditions. It is interesting to investigate the role of the SOC channel,
particularly the Orai1/Stim1 CRAC channel, in participating in fluid flow-induced
ATP-mediated Ca signalling and regulation of cell proliferation.
In our recent study we have shown that exposure to exogenous ATP stimulated
human DP-MSC migration and provided evidence to support a significant role of the
Orai1/Stim1 CRAC channel, in addition to the P2Y, P2Y and P2X7
receptors, in mediating ATP-induced stimulation of cell migration [80].
ATP-induced stimulation of cell migration was not affected by treatment with
CGS1593, an adenosine receptor antagonist, consistent with no critical
involvement of ATP metabolites in ATP-induced cell migration, as discussed above
in fluid flow/ATP-induced cell proliferation. ATP-induced stimulation of cell
migration was suppressed by treatment with 2-APB and also ablated by
siRNA-mediated knockdown of the expression of Orai1 or Stim1, as well as
knockdown of the expression of P2Y, P2Y or P2X7. Moreover, in a
more recent study, we have shown that ATP-induced cell migration was largely
inhibited by treatment with PF431396, an inhibitor of PYK2, a Ca-sensitive
tyrosine kinase which is a member of the focal adhesion kinase family, or
treatment with U0126 to inhibit MEK/ERK, which is known to be activated
downstream of PYK2 [83]. Collectively, our studies support that intracellular
Ca signalling, generated via the G-coupled P2Y/P2Y
receptors and Ora1/Stim1 CRAC channel, as well as the P2X7 receptor, and
subsequent activation of downstream Ca-dependent signal pathways are
important in driving ATP-induced stimulation of MSC migration. Furthermore,
consistent with human MSC releasing ATP in response to mechanical signals, we
have presented evidence to show that the mechanosensitive Piezo1 channel is
expressed in human DP-MSC, and its activation promotes cell migration that
critically depends on ATP release and activation of the P2 receptor, PYK2 and
MEK/ERK [83]. These results have led us to propose that ATP as an extracellular
signal can induce Ca signalling to stimulate MSC migration, through
activation of the P2Y/P2Y-G-PLC-IPR pathway that
results in ER Ca release and subsequent Orai1/Stim1 CRAC channel-mediated
SOCE, in addition to Ca influx through the P2X7 receptor.
In summary, emerging evidence supports the SOC/CRAC channel in MSC to be
important in ATP-induced regulation of cell migration, but more investigations
are required to understand the role of the SOC/CRAC channel in ATP-induced
regulation of cell proliferation and differentiation (Fig. 2).
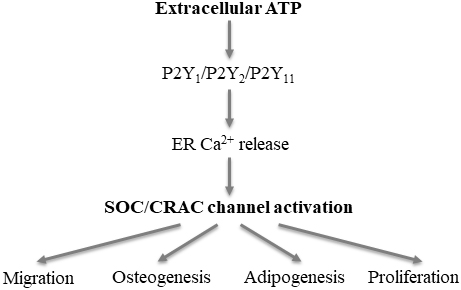 Fig. 2.
Fig. 2.
Proposed roles of the SOC/CRAC channel in ATP-induced regulation
of mesenchymal stem cell (MSC) function. Extracellular ATP activates the
P2Y, P2Y and/or P2Y receptor that leads to Ca release
from the endoplasmic reticulum (ER), which in turn activates the store-operated
Ca (SOC)/Ca-release-activated Ca (CRAC) channel and results
in extracellular Ca entry (illustrated in Fig.1A). Such a mechanism in MSC
has been shown to play a signficant role in ATP-induced regulation of cell
migration or implied in ATP-induced ostogenesis, adipogenesis and proliferation
(see text for further details).
6. Concluding remarks
Extracellular ATP has been shown as an autocrine/paracrine signal that induces
Ca signaling in MSC via the P2X receptors that mediate Ca influx
and/or the G-coupled P2Y receptors that lead to ER Ca release to
stimulate Ca-dependent downstream signal pathways and thereby regulates
cell proliferation, migration and differentiation. The reduction of ER Ca
further activates the SOC channel, a distinctive Ca influx mechanism that
is widely documented in mammalian cells. Emerging evidence supports the SOC
channel, or more specifically, the Orai1/Stim1 CRAC channel, as an important
mechanism that participates in ATP-induced Ca signalling in MSC and
ATP-induced regulation of cell function. Nonetheless, compared to the P2X and P2Y
receptors, the SOC/CRAC channel in terms of its contribution to ATP-induced
Ca signalling and regulation in MSC function remains less well understood.
As discussed above, MSC exhibit a high sensitivity to diverse mechanical signals
that regulate multiple MSC functions. This attribute is of particular importance
to the translational applications of MSC, considering mechanically different
scaffolds used in tissue engineering that may affect cell viability,
proliferation, migration and differentiation. The interactions of MSC with
extracellular matrix and recipient tissues may also influence their ability of
migration and tissue homing, a well-recognised factor limiting the efficacy of
MSC-based therapies. Interestingly, increasing evidence supports ATP release and
activation of the P2 receptors, particularly the G-coupled P2Y
receptors, as a mechanism converting mechanical signals to Ca signals in
the regulation of cell functions [93]. More research efforts are clearly required
to better understand the role of the SOC/CRAC channel in ATP-induced Ca
signalling in MSC and regulation of cell function by physical, chemical and
biological stimuli or signals known to induce ATP release and activation of the
P2Y-G-PLC-IPR pathway. Such information is useful not only to the
utilisation of MSC in regenerative medicines but also to the improvement of our
knowledge about basic MSC biology.
7. Author contributions
LHJ initiated the discussion and drafted the manuscript. LW, SR and XBY
contributed to the discussion and revised the manuscript. All authors approved
the manuscript.
8. Ethics approval and consent to participate
Not applicable.
9. Acknowledgment
Not applicable.
10. Funding
LW is supported by the Natural Science Foundation of Henan Province
(202300410316) and the Key Scientific Research Projects of Universities of Henan
Province (21A310016).
11. Conflict of interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2.