Academic Editor: Marina Ivanišević
Background: The DENN (differentially expressed in neoplastic versus normal cells) domain containing 1A (Dennd1a), a guanine nucleotide exchange factor (GEF) for the small GTPase Rab35, is essential for mouse embryogenesis. Disruption of Dennd1a impairs the migration and differentiation of fetal germ cells. In the present study, we further elucidated the role of Dennd1a in oogenesis and meiosis in the fetal ovary. Results: Ablation of Dennd1a disrupted the mRNA expression of Sohlh2, Figla, Stra8, and Rec8 in the ovary of Dennd1a-/- mutants at E13.5. Using ex vivo culture of E12.5 female gonads and adenoviral Dennd1a shRNA infection, we demonstrated that transcription of Sohlh2, Figla, Stra8 and Rec8 were not activated in the fetal ovary lacking Dennd1a. Dennd1a in the somatic cells might stimulate Sohlh2 expression at early stage of oocyte differentiation via regulating Wnt5a synthesis. On the other hand, meiotic initiation of the fetal germ cells required Dennd1a-mediated RA production from the somatic cells, which induced the expression of Stra8 and Rec8. Conclusions: Dennd1a could be involved in multiple signal pathways in the somatic cells that are critical for various processes of oogenesis and meiosis in the fetal ovary.
The DENN (differentially expressed in neoplastic versus normal cells) domain containing 1A (Dennd1a) functions as a guanine nucleotide exchange factor (GEF) for the small GTPase Rab35. It mediates the switch from GDP-bound Rab35 to GTP-bound form, to promote endocytic recycling of various cargoes on clathrin-coated vesicles (CCVs) after internalization [1, 2, 3]. Dennd1a has been identified as a susceptible gene in patients with polycystic ovary syndrome (PCOS), a complex endocrine disorder causing infertility in women of reproductive age [4, 5]. Dennd1a protein was found increased in the theca cells of PCOS patients, and knockdown of a Dennd1a isoform in isolated PCOS theca cell cultures reduced the androgen biosynthesis, indicating that Dennd1a plays a role in the hyperandrogenemia associated with PCOS [6]. However, the role of Dennd1a in the ovaries at either developmental or adult stage is largely unknown.
Previous study revealed that Dennd1a is essential for mouse embryogenesis, and homozygous deletion of Dennd1a causes embryonic mouse lethality. Moreover, disruption of Dennd1a impairs the migration and differentiation of fetal germ cells [7]. In mouse, the population of primordial germ cells (PGCs) arise during gastrulation in the extra-embryonic mesoderm of the posterior amniotic fold, distinguished by high alkaline phosphatase (AP) activity [8]. PGCs at embryonic day 7.5 (E7.5) migrate from the base of allantois, then through the hindgut endoderm and mesentery before colonizing the genital ridge by E11.5 [9]. Dennd1a deficiency affects the migration of PGCs and results in a significant reduction in gonadal germ cell numbers [7].
Following migration of PGCs to the genital ridge, the long and narrow gonadal primordium becomes thicker and undergoes fate determination into testis or ovary by the action of sex-determining gene Sry [10]. In female mice, expression of spermatogenesis and oogenesis bHLH transcription factor 2 (Sohlh2) begins in the germLine as early as E12.5, which is an essential regulator of oocyte differentiation, independent of meiosis [11]. Since Sohlh2 mRNA expression is significantly low in the fetal ovary of Dennd1a-null embryo at E13.5, it suggests that Dennd1a may be involved in the regulation of Sohlh2 during fetal ovary development [7].
Meiosis is another big event in the fetal ovary, which begins approximately at E13.5. Retinoic acid (RA) produced by the somatic cells of the gonad and mesonephros is required for female germ cells to initiate meiotic prophase [12]. RA is derived from retinol (vitamin A), and members of aldehyde dehydrogenase family 1 subfamily A (Aldh1a1-3) catalyze the last step of RA synthesis [13]. In the fetal ovary, Stra8 (Stimulated by Retinoic acid gene 8), a gene required for meiotic initiation, and Rec8, a gene required for meiotic progression, are induced by RA [14].
Wnt5a and its receptor, Ror2, have been implicated in migration and
proliferation of PGCs. Wnt5a-null and Ror2-null embryos display similar
phenotypes, resulting in a diminished number of PGC in the embryonic gonad due to
abnormal PGC migration prior to gonadal colonization [15, 16]. In specific
somatic niches, Wnt5a-Ror2 pathway suppresses canonical Wnt/
In this study, we revealed that Dennd1a plays a role in Wnt5a and RA synthesis in the somatic cells of the fetal ovary and mesonephros. Dennd1a deficiency impairs the expression of genes associated with oocyte differentiation and initiation of meiosis in the fetal ovary, due to insufficient production of Wnt5a and RA respectively.
The Dennd1a mutant strain used in this study was reported previously [7]. Wild-type Kunming mice were purchased from the Laboratory Animal Center of Shandong University. The sex was determined by PCR analysis using Sry primers (Table 1) [20]. Care and use of experimental animals described in this work comply with the guidelines and policies of the University Committee on Animal Resources at Shandong University.
| Gene | Primer sequence (5′ to 3′) |
| Sry-forward | TTGTCTAGAGAGCATGGAGGGCCATGTCAA |
| Sry-reverse | CCACTCCTCTGTGACACTTTAGCCCTCCGA |
| Aldh1a1-forward | CCTCCTGGCGTGGTAAACAT |
| Aldh1a1-reverse | TTGATCCAGTGAAGGCCACC |
| Aldh1a2-forward | AGCCACAGGAGAGCAAGTGT |
| Aldh1a2-reverse | GTCTGCAAGCTTGTCCAACA |
| Axin2-forward | CTTCCAGATCCCAGCAGCAG |
| Axin2-reverse | AACGGGCATAGGTTTGGTGG |
| Dennd1a-forward | ACATTGACGGCCTGCATCCA |
| Dennd1a-reverse | AGCACAGCAGTAGTCCAGCA |
| Figla-forward | ACAGAGCAGGAAGCCCAGTA |
| Figla-reverse | CAGCTGGTAGGTTGGGTAGC |
| Rec8-forward | CCCGCTTCTCCCTCTATCTC |
| Rec8-reverse | TGGGAAGAAGCAAGCTAGGT |
| Stra8-forward | GCTTTTGACGTGGCAAGTTT |
| Stra8-reverse | AACACAGCCAAGGCTTTTGA |
| Wnt5a-forward | AAGTATGATAGCGCGGCGGC |
| Wnt5a-reverse | TTGCGCACACAGTAGTCCGG |
| Sohlh2-forward | ATGGCCCAGGTTACAGAAGC |
| Sohlh2-reverse | CTCCTGCAGCAGTCATGGAA |
| Gapdh-forward | AACTTTGGCATTGTGGAAGG |
| Gapdh-reverse | ACACATTGGGGGTAGGAACA |
Mouse tissues were dissected and fixed in 4% paraformaldehyde at 4
For immunohistochemistry, the sections of E11.5 embryos were incubated with Dennd1a antibodies which were detected with horseradish peroxidase (HRP)-conjugated secondary antibodies (ZSGB-BIO, PV-9001), followed by enzymatic color reaction (Vector Laboratories, SK-4100). Images were taken using OLYMPUS BX53F microscope and analyzed by OLYMPUS cellSens Imaging 1.15 software (OLYMPUS, Japan).
The fetal gonads were obtained from E12.5 embryos, and divided into 2 pieces and
cultured on 24-well plates (corning) with 1 mL of growth medium in each well at
37
The somatic cells were obtained from E12.5 wild-type female mesonephros, which
were cultured in each well of a 6-well plate with 2 mL of Dulbecco’s modified
Eagle’s medium (Gibco, 11995065) containing 10% (v/v) fetal bovine serum (FBS)
(BI, 04-001-1ACS) and 1% Penicillin/Streptomycin (Gibco, 10378016) in a
humidified incubator at 37
The adenoviral Dennd1a shRNA (Vigene Biosciences) containing GFP labeling was
added into medium of cultured tissues. The adenovirus containing no inserted gene
was used as control. The gonad tissues were successfully transfected with
adenovirus at the dose of 1
Total RNA kit I (OMEGA, R6834-02) was used to extract total RNA from gonads and
mesonephros. The total RNA was subject to the first strand cDNA synthesis using
the PrimeScript RT reagent kit with gDNA Eraser (Takara, Japan, RR047A). RNeasy
Mini Kit (QIAGEN,74104) was used to extract RNA from the fetal germ cells and
somatic cells of the gonads of E11.5 or E13.5 embryos. The first strand cDNA
synthesis was performed using the Evo M-MLV RT for PCR Kit (Accurate Biology,
AG11603). The cDNAs were then amplified by real-time PCR with TB Green Premix Ex
Taq (Takara, RR420A) in Light Cycler real-time PCR instrument (Roche, Basel,
Switzerland, LC480) according to the manufacture’s specification. Gene expression
changes were normalized to Gapdh and analyzed by the
2
Proteins were extracted from the fetal gonads using Minute™ Total
Protein Extraction Kit for Animal Cultured Cells/Tissues (Invent, SD-001/SN-002).
Proteins from the tissue or cell cultures were extracted using
T-PER™ Tissue Protein Extraction Reagent (Thermo, 78510).
Complete™ Protease Inhibitor Cocktail (Roche, 04693116001) were
used to inhibit the degradation of a broad spectrum proteases. The extracted
samples were separated, then transferred onto polyvinylidene fluoride membranes
(Millipore, Billerica, MA, PFL00010). The membranes were probed with Rabbit
anti-Dennd1a (abcam, ab125347), Rabbit anti-ALDH1A1(cell signaling techonogy,
12035, Rabbit anti-ALDH1A2 (cell signaling technology, 83805S), Rabbit anti-Wnt5a
(cell signaling technology, 2392S), Rabbit anti-non-phospho
Supernatant culture medium of gonads or somatic cells was collected to quantify concentrations of RA using Mouse Retinoic Acid ELISA kit (CUSBIO, CSB-EQ028019MO) according to the manufacture’s specification, using a microplate reader (Molecular devices; SPECTRA MAX 384plus) set to 450 nm. The software used was SoftMax Pro7 (Molecular Devices, USA). Each standard and sample were in duplicates, calibrated with blank well without any solution, and a standard curve was set up by using software Curve Expert. The data was linearized by plotting the log of the Retinoic acid concentrations versus the log of the O.D., and the best fit line was determined by regression analysis.
Graphpad PRISM8 analysis software was used for plotting, and SPSS (version 21.0,
IBM Corp., Chicago, IL, USA) analysis software was used for statistical analysis.
p
At E11.5 during mouse embryo development, Dennd1a protein was found in both the hindgut mesentery and genital ridge of mouse embryos (Fig. 1A), suggesting Dennd1a may play a role when PGCs are migrating through the mesentery and colonized in the genital ridge. In fetal ovaries, the levels of Dennd1a mRNA were gradually increased on E12.5, E13.5, and E15.5, during early stages of the oocyte differentiation and meiosis (Fig. 1B). The findings suggest that Dennd1a-mediated signaling pathway may be involved in the regulation of fetal ovary development.
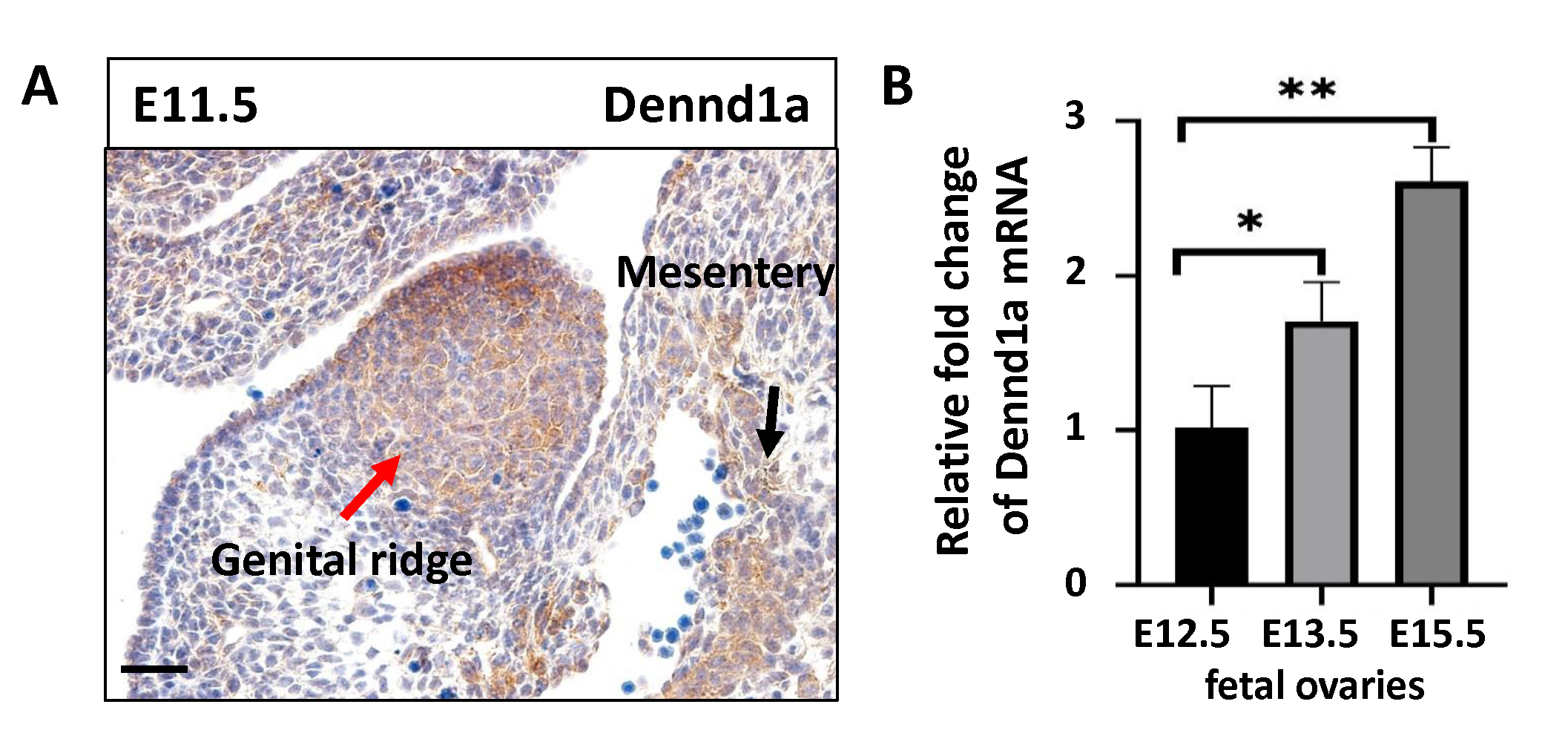 Fig. 1.
Fig. 1.Expression of Dennd1a in the fetal ovary. (A)
Immunohistochemistry shows the localization of Dennd1a in the genital ridge and
posterior mesentery of E11.5 mouse embryo. The red arrow indicates genital ridge.
The black arrow indicates posterior mesentery. Scale bar = 20
To assess the oocyte differentiation and initiation of meiosis during embryonic
development, we examined mRNA expression of Sohlh2, Figla
(Factor in the germLine
 Fig. 2.
Fig. 2.Dennd1a deficiency impairs the initiation of oogenesis and
meiosis of the fetal germ cells. (A) Real-time RT-PCR analysis shows the mRNA
expression levels of Sohlh2, Figla, Stra8 and Rec8 in wild-type
fetal ovaries of E12.5, E13.5 and E15.5 mouse embryos (n = 5). (B)
Real-time RT-PCR analysis of the mRNA levels of Sohlh2, Figla,
Stra8, and Rec8 in the fetal ovaries of E13.5 Dennd1a+/- and
Dennd1a-/- embryos (n = 3). (C) (i–iv) Morphological changes of E12.5, E13.5,
E14.5, E15.5 wild-type fetal ovaries, scale bar = 50
Since homozygous Dennd1a-/- mutants die circa E14.5, they are not suitable to determine whether loss of Dennd1a eventually disrupts the expression of genes associated with oocyte differentiation and meiotic initiation. Moreover, due to limited availability of Dennd1a-/- gonads, we therefore established an ex vivo gonad culture by incubating genital ridges isolated from E12.5 wild-type female embryos. The long and narrow E12.5 gonads ex vivo became short and thick after being cultured for 24, 48, and 72 h (Fig. 2C, v–viii), recapitulating the morphological changes of developing gonads in vivo from E13.5 to E15.5 (Fig. 2C, i–iv). The mRNA expression of Sohlh2, Figla, Stra8 and Rec8 in wild-type gonads ex vivo were activated after being incubated for 48 h (Fig. 2D), showing a similar expression pattern as that in gonads in vivo at E13.5 (Fig. 2A). To knockdown the expression of Dennd1a, the ex vivo E12.5 gonads were infected with adenoviral Dennd1a shRNA carrying a GFP fluorescent tag for 24, 48, 72, or 96 h (Fig. 2C, ix–xii). After 48 h or longer of infection, the expression of Dennd1a mRNA and protein were decreased to approximately half the wild-type level (Fig. 2E). In gonads with Dennd1a knockdown, the mRNA expression of Sohlh2, Figla, Stra8 and Rec8 were not fully activated, as shown in representative samples treated for 72 h (Fig. 2E). These results suggest that Dennd1a deficiency disrupted the proper activation of genes associated with oocyte differentiation and initiation of meiosis in fetal ovaries.
We suspected that Dennd1a deficiency disturbed somatic components which are important for inducing fetal oocyte differentiation and meiosis. It has been reported that Wnt5a was predominantly expressed in the somatic niches and necessary for gonadal differentiation [15]. Furthermore, Wnt5a was involved in oocyte maturation and meiotic resumption during oocyte development [19]. Therefore, we examined whether loss of Dennd1a disrupts Wnt5a production in the somatic cells. In E12.5 female gonad culture infected with adenoviral Dennd1a shRNA for 48, 72 or 96 h, the expression of Dennd1a mRNA and protein was disrupted (Fig. 3A,B). Knockdown of Dennd1a decreased the mRNA and protein levels of Wnt5a in the gonad culture (Fig. 3A,B).
 Fig. 3.
Fig. 3.Production of Wnt5a and RA is reduced in the somatic cells of
the fetal ovary lacking Dennd1a. (A,B) Real time PCR and Western blot show the
mRNA and protein levels of Dennd1a, Wnt5a, and Aldh1a1 in E12.5 wild-type fetal
ovaries cultured ex vivo and infected with Dennd1a shRNA adenovirus (KD)
or adenovirus containing no inserted gene as control for 72 h (n = 3). (C) ELISA
shows the concentrations of RA in the culture medium of E12.5 wild-type fetal
ovaries cultured ex vivo and infected with Dennd1a shRNA adenovirus (KD)
or adenovirus containing no inserted gene as control for 72 h (n = 5). (D) The
primordial germ cells (PGC) and the somatic cells (SC) were isolated from E11.5
female gonads. The mRNA expression of Aldh1a1 and Wnt5a in the PGC and SC
isolated from E11.5 female gonads were examined by real time PCR (n = 5). (E)
Total RNA was extracted from E13.5 wild-type fetal ovaries and mesonephros. The
mRNA expression of Dennd1a, Aldh1a1, Aldh1a2, and
Wnt5a was analyzed by real-time quantitative PCR (n = 5). (F)
Immunofluorescence shows the localization of Aldh1a1 in the ovary and Aldh1a2 in
the mesonephros of E13.5 embryos (Red). The section was counterstained with DAPI
(blue). The Dashed line indicates the boundary between the fetal ovary and
mesonephros (scale bar 40
Fetal germ cells in the ovary initiate meiosis in response to RA [12]. Deficiency of Dennd1a also reduced the mRNA and protein expression of Aldh1a1, which encodes RA-synthesizing enzyme in the fetal ovary (Fig. 3A,B), and reduced secretion of RA into the culture medium (Fig. 3C). The main source of Wnt5a and RA in the fetal ovary is the somatic cells as confirmed by quantitative analysis of the mRNA expression of Wnt5a and Aldh1a1 in isolated PGCs and somatic cells from E11.5 female gonads (Fig. 3D). It seemed that Dennd1a was involved in the production of Wnt5a and RA from the somatic cells of the fetal ovary.
An alternative source of RA and Wnt5a is the fetal mesonephros. At E13.5, unlike the ovary which expressed mainly Aldh1a1, the mesonephros expressed RA-synthesizing enzymes Aldh1a2 (Fig. 3E,F) and Aldh1a3 [12]. The mRNA expression of Dennd1a in the mesonephros was at the same level as in the ovary, while the mesonephros expressed more Wnt5a than the ovary (Fig. 3E). The mesonephros were then isolated from E12.5 wild-type female embryos, and dissociated cells were cultured in vitro and infected with adenoviral Dennd1a shRNA for 48, 72 or 96 h (Supplementary Fig. 1). Knockdown of Dennd1a resulted in decreased mRNA and protein levels of Wnt5a and Aldh1a2 in the culture (Fig. 3G,H), and decreased production of RA in the medium (Fig. 3I). Therefore, Dennd1a may play a role in regulating the production of Wnt5a and RA from the somatic cells of the fetal ovary and mesonephros.
To explore the mechanism by which Dennd1a regulates RA and Wnt5a in the somatic
cells, we used the culture of fetal mesonephros which produce RA and Wnt5a, and
also contribute to the somatic niche for germ cells, in order to avoid the
complexity associated with the mixture of germ cells and somatic cells in gonadal
culture. As a major GEF for Rab35 to regulate endosomal membrane trafficking,
Dennd1a may be involved in Rab35-mediated PI3K/Akt pathway [21]. To determine
whether Dennd1a deficiency may compromise PI3K/Akt pathway, the E12.5 fetal
mesonephros infected with or without adenoviral Dennd1a shRNA for 48 or 72 h were
assessed. Dennd1a knockdown led to decreased phosphorylation of Akt in the
somatic cells (Fig. 4A,D). Dennd1a deficiency also caused reduced accumulation of
non-phosphorylated
 Fig. 4.
Fig. 4.Knockdown of Dennd1a decreases the phosphorylation level of Akt
and accumulation of active
To provide evidence of a direct causal link between PI3K/Akt axis and Wnt5a or
RA production in the mesonephros culture, we treated the somatic cells with
LY294002 to inhibit PI3K activity, the efficacy of which was tested by measuring
the phosphorylation levels of the PI3K target protein Akt. Treatment with
LY294002 (10
 Fig. 5.
Fig. 5.Inhibition of PI3K/Akt pathway downregulates
The role of Wnt/
 Fig. 6.
Fig. 6.Wnt3a/
To determine the effect of Wnt5a and RA on the regulation of genes associated
with oocyte differentiation and meiotic initiation, we treated the fetal gonad
culture from E12.5 female wild-type mice with recombinant Wnt5a (350 ng/mL) or RA
(1
 Fig. 7.
Fig. 7.Exogenous WNT5A and RA stimulate the expression of genes
associated with initiation of oogenesis and meiosis in the fetal germ cells. (A)
The fetal gonad culture of E12.5 female wild-type mouse embryos was treated with
recombinant Wnt5a (350 ng/mL) or RA (1
Next, we investigated whether insufficient Wnt5a and RA was responsible for
dysregulation of genes associated with oocyte differentiation and meiosis
initiation in the fetal ovary lacking Dennd1a. In the E12.5 fetal ovary culture
infected with adenoviral Dennd1a shRNA for 48 h or 72 h, Dennd1a knockdown
impaired the expression of Sohlh2. Treatment with exogenous Wnt5a (350 ng/mL)
could stimulate the expression of Sohlh2 in Dennd1a knockdown fetal ovaries to
the level comparable to that in wild-type control (Fig. 7B showing representative
samples treated for 48 h). However, exogenous Wnt5a could not rescue the
decreased mRNA expression of Figla, Stra8, or Rec8 in the fetal ovaries affected
by Dennd1a knockdown (Fig. 7B). On the other hand, RA treatment (1
The importance of Dennd1a in human reproduction is highlighted by its involvement in PCOS, an endocrine disorder causing female infertility [4, 5, 6]. The well-studied function of Dennd1a is to mediate the switch from GDP-Rab35 to GTP-bound form and regulate endocytic recycling of cellular components on clathrin-coated vesicles [21, 22]. During embryogenesis, Dennd1a is essential for the development of fetal organs such as brain, liver, and ovary [7]. In the present study, we further elucidated the role of Dennd1a in oogenesis and meiosis in the fetal ovary.
We revealed that Dennd1a is mainly expressed in the somatic cells of the fetal ovary. Ablation of Dennd1a disrupted the mRNA expression of Sohlh2, Figla, Stra8, and Rec8 in the ovary of Dennd1a-/- mutants at E13.5. Using ex vivo culture of E12.5 female gonads and adenoviral Dennd1a shRNA infection, we further demonstrated that the transcription of Sohlh2, Figla, Stra8 and Rec8 were not activated in deficiency of Dennd1a. In mouse embryo, oogenesis begins with the differentiation of PGCs into oogonia following sexual differentiation at approximately E12.5 [11]. Meanwhile, PGCs receives RA signals from the somatic cells of the ovary and mesonephros and gains the capacity for meiotic initiation [12]. Sohlh2 protein is expressed in the fetal germ cells as early as E12.5, while Sohlh1 protein expression occurs circa E15.5. Sohlh2 is among the early oocyte-specific transcription factors that regulates oocyte differentiation without affecting meiosis [11, 23]. Figla is another basic helix-loop-helix transcription factor that is primarily expressed in female germ cells and plays a critical role in oocyte differentiation. Moreover, Figla deficiency impairs oocyte meiotic progression [24, 25]. RA signal activates transcription of Stra8 and Rec8 in ovarian germ cells. Stra8 is required for meiotic initiation including meiotic DNA replication as well as the subsequent processes of meiotic prophase. Rec8 encodes a component of the cohesin complex that accumulates during meiotic S phase, and is essential for chromosome synapsis and segregation. These two factors are independently activated by RA and precede the expression of other meiotic markers [12, 14]. Thus, Dennd1a in the somatic cells seems necessary for activation of these genes in the fetal germ cells, which are critical for oocyte differentiation and meiotic initiation.
Dennd1a in the somatic cells may be involved in regulating the production of Wnt5a and RA because Dennd1a deficiency impaired the expression of both Wnt5a and genes that encoding RA-synthesizing enzymes in the fetal ovary and mesonephros. Wnt5a has been identified as an important cytokine for oocyte maturation and meiotic resumption [18]. We therefore focused our study on the effect of Wnt5a on fetal oocyte differentiation and meiosis. Wnt5a stimulated Sohlh2 during early stage of oocyte differentiation in fetal ovary, though it had no effect on the expression of Figla, Stra8 or Rec8. Moreover, the compromised activation of Sohlh2 in the fetal ovary in deficiency of Dennd1a could be rescued by exogenous Wnt5a. These findings suggest that Dennd1a in the somatic cells might stimulate Sohlh2 expression at early stage of oocyte differentiation via regulating Wnt5a synthesis. On the other hand, Dennd1a-mediated expression of Figla, Stra8 and Rec8 seems independent of Wnt5a. Although the concentrations of RA detected in the culture medium of both gonads and mesonephros by ELISA are lower than the average concentrations in the tissues of mouse embryos [26], our data is consistent with previous reports about the source of RA during germ cell development [12]. Knockdown of Dennd1a in gonad and mesonephros culture resulted in a reduced release of RA in the culture medium, suggesting Dennd1a is implicated in RA synthesis. Furthermore, RA treatment could rescue the compromised activation of Stra8 and Rec8 due to Dennd1a deficiency, indicating that meiotic initiation of the fetal germ cells depends on Dennd1a-mediated RA production. Taken together, Dennd1a could be involved in multiple signal pathways in the somatic cells that are critical for various processes and stages of oocyte differentiation and meiosis.
Dennd1a is a GEF for the small GTPase Rab35, which plays a regulatory role in
PI3K/Akt pathway [21, 27]. In our study, Dennd1a deficiency could be responsible
for reduced phosphorylation of Akt by compromising Rab35 activity. Reduced
phosphorylation of Akt could result in downregulation of
We discovered that the ablation of Dennd1a in the somatic cells disrupts transcription of Sohlh2, Figla, Stra8, and Rec8 in the fetal germ cells of developing ovary. Dennd1a could be involved in multiple signal pathways in the somatic cells and mediate Wnt5a and RA synthesis, which are necessary for the initiation of oocyte differentiation and meiosis during embryonic ovary development.
JF and JM conceived and designed the experiments; JS and QN performed the experiments; JF and QG analyzed the data; JF, QG and JM contributed reagents and materials.
All procedures performed in studies were in accordance with the ethical standards for the care and use of laboratory animals. Approval (#20160704) was obtained from the Institutional Review Board of Shandong University.
Thanks to all the peer reviewers for their opinions and suggestions
This work was supported by the Basic Science Center Program, China (Grant number: 31988101), the National Key Research and Development Program of China (Grant number: 2016YFC1000601), the Natural Science Foundation of Shandong Province, China (Grant numbers: ZR2016HM79 and ZR2014HM017), and Shandong Provincial Key Research and Development Program, China (Grant number: 2020ZLYS02).
The authors declare no conflict of interest.
