Academic Editor: Alessandro Poggi
Importance: Statins have been linked to an increased risk for insomnia,
but the literature is controversial. Moreover, it is unknown, if the potential
effects are directly related to the inhibition of the statin target HMGCR, the
subsequently lowered cholesterol levels, or other off-target effects of statins.
Aims: To investigate the association of statin treatment and genetic
proxies of cholesterol lowering drugs with the risk for insomnia and chronotype
in a large population-based cohort. Methods: A cross-sectional cohort
study based on baseline data collected between 2006–2010 in UK biobank cohort
was conducted. European participants without any history of
psychiatric/neurological disorders or of stroke and with available genetic data
as well as information on statin use were included in the present study.
Self-reported measures of insomnia and chronotype were analysed (a) in statin
users versus control subjects, (b) subjects carrying single nucleotide
polymorphisms (SNPs) in the HMGCR gene, which are associated with
reduced enzymatic function and lower cholesterol levels (rs17238484 and rs12916)
and (c) subjects carrying a SNP in the PCSK9 gene (rs1159147), which
leads to lower cholesterol levels independent of HMGCR. The main analysis were
performed using multivariable regression models. Statin treatment and SNPs in
HMGCR and PCSK9 genes were used as exposures and main outcomes
were insomnia and chronotype. Results: A total of 206,801participants
(mean [SD] age, 57.5 [7.9] years; 56% women; 20% statin users) were included in
the present study. Statin users had an increased risk of insomnia compared to
controls (odds ratio [95% CI], 1.07 [1.03 to 1.11]; p = 1.42
Hypercholesterolemia is a major causative risk factor for cardiovascular diseases (CVDs) [1], which in turn are the primary cause of death around the world (WHO). The main drug target to counteract hypercholesterolemia is the enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), which catalyses the rate-limiting step in cholesterol biosynthesis [2]. Statin drugs inhibits HMGCR enzyme activity, which leads to a reduction of cholesterol synthesis in the liver by increasing low-density lipoprotein (LDL) receptor expression at the surface of the liver cells, resulting in increased uptake of LDL from the blood and ultimately decreased plasma concentrations of LDL- and other apolipoprotein B-containing lipoproteins. This, in turn, leads to an effective reduction in primary and secondary CVDs [2, 3]. As a result of increased prevalence of CVDs and higher efficiency of treatment, statin drugs have become one of the most widely prescribed medication worldwide. In the US, every fourth adult aged over 40 years is receiving statins, and an estimated 56 million people are eligible for statin treatment based on new guidelines [4].
A second class of effective drugs for CVD protection are the so-called PCSK9 inhibitors (i.e., alirocumab and evolocumab) [5, 6]. PCSK9 inhibitors lower plasma cholesterol by preventing the protein Proprotein convertase subtilisin/kexin type 9 (PCSK9) from attaching to LDL receptors [7, 8]. This enables the recycling of LDL receptors, increases the uptake of LDL particles into cells and thereby reduces circulating LDL particles.
In the existing literature, sleep disturbances, including symptoms of insomnia, have been linked to the treatment with cholesterol-lowering drugs in general and with statin treatment in particular [9, 10, 11]. Insomnia is a frequent complaint in the general practice, and epidemiological studies show that the prevalence of insomnia symptoms is about 30‒35% in the global population [12]. Depending on the severity, insomnia can be a serious condition with large impact on quality of life [12, 13, 14]. There are many predisposing factors for insomnia, including genetic factors and the use of medication [12], and insomnia symptoms experienced as side effects of drug treatment may significantly affect the probability of a patient adhering to treatment [15]. These clinical considerations underscore the urgency to identify the association between insomnia and a given drug.
However, with regard to statin treatment and sleep disturbances, literature remains inconclusive as the results of previous studies have been highly inconsistent [10]. These inconsistent findings might relate to a range of different factors. First, major side effects are difficult to identify in randomized controlled trials (RCTs) due to the usually relatively small size of the study population [11, 16, 17]. In addition, only few studies have investigated sleep disturbances as a primary outcome [18, 19, 20]. Furthermore, there is a range of factors that tend to vary across studies, including statin type [21], patient age [22], study type and statistical analysis [23] which might all influence the study outcome. Finally, it is known that statins not only interact with their primary target HMGCR (on target), but also directly interact with other proteins as well as membranes (off target effects) [24, 25, 26]. Little is known so far about the contribution of on-target versus off-target effects of statin treatment, which would aid in understanding the drugs’ side effects as well as controversial study outcomes.
A way to overcome many of the aforementioned confounding factors and gain better insight into actual on-target effects is to perform genetic association studies that evaluate the effect of different functional gene variants on specific phenotypes. Using this approach, a long-suspected link between both statins and PCSK9 inhibitors on diabetes measures (e.g., plasma cholesterol, body weight, waist circumference, plasma insulin and glucose concentrations) has been confirmed by studying the association of single-nucleotide polymorphisms (SNPs) in the two respective target genes, HMGCR and PCSK9 [27, 28]. Thus, by using the SNPs as proxies for lowering cholesterol levels directly related to the primary targets of these two drug classes, potential on-target side effects of drug treatment can be identified by exploring their molecular inhibition.
Following this rationale, our work aimed to shed light on the controversial connection of statins and sleep disturbances by investigating a possible relationship between statin treatment, on the one hand, and SNPs in the HMGCR and PCSK9 genes, on the other hand, and the risk for insomnia as well as the individual chronotype in a large European population.
This study used the UK Biobank resource (http://www.ukbiobank.ac.uk/) to analyse data that has previously been collected. The UK Biobank study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants in the UK Biobank study have given informed consent for data collection, data storage and subsequent data analysis. Ethical permission for our study was given by the responsible local authorities, Regionala Etikprövningsnämden (now Etikprövningsmyndigheten, Swedish Ethical Review Authority, EPN), Uppsala, under registration number (Diarienummer, Dnr) 2017/198.
UK Biobank is an open access resource with a collection of longitudinal health-related data and biological samples [29]. It constitutes a prospective study of more than 500,000 participants aged between 40 and 69 years when attending the assessment centre, and living in the United Kingdom. Present study is based on baseline data, collected between 2006 and 2010. Generally, detailed information regarding all variables included in the study can be found on the UK Biobank website (http://www.ukbiobank.ac.uk/). Specifically, we extracted data on the following parameters at baseline: insomnia (UK Biobank Field ID (FID): 1200), chronotype (FID: 1180), age (FID: 21003), sex (FID: 31), total cholesterol level and LDL-cholesterol level (FID: 30690), body mass index (BMI) (FID 21001), education (FID: 6138), alcohol intake frequency (FID: 1558), sleep duration (FID: 1160), smoking status (FID: 20116), physical activity (FID: 884), diagnosis of diabetes (FID: 2443), diagnosis of hypertension, angina and heart attack (ID: 6150), use of hypertensive medications, statins and insulin (FID: 20003) and Townsend deprivation index (TDI) reflecting socio-economic status of the participant based on area of residence at the time of recruitment (FID: 189).
In the current analyses, participants with non-white ethnical background (n = 26,916), genetic relatedness and quality-control failure of the genotyped data (n = 138,617) were excluded (Fig. 1). Moreover, all the participants with neurological (n = 19,916) or psychiatric disorders (n = 3882), or persons who have had a stroke (n = 5197) were excluded to minimize confounding due to these factors. Following these exclusions, 206,801 participants with genetic data as well as information on medication use were included in the present study (Fig. 1). The study participants were then subjected to two independent analyses, i.e., non-genetic and genetic. In non-genetic analyses, statin medication was used as exposure while in genetic analyses, genetic variants were used as proxies for statin use.
 Fig. 1.
Fig. 1.Study population.
We analysed three SNPs (two in the HMGCR gene (rs17238484 and rs12916) and one in the PCSK9 gene (rs11591147)) that are known to be linked to cholesterol lowering and can be used as proxies for statin use and PCSK9 inhibition respectively [28, 30]. We confirmed that all SNPs were in Hardy-Weinberg equilibrium.
Use of statin medication was self-reported, and only concerned medication that is regularly taken. The prescribed statin drugs present in the UK Biobank cohort were simvastatin, atorvastatin, fluvastatin, rosuvastatin and pravastatin. However, majority of the statin users (93%) were taking lipophilic statins (i.e., atorvastatin, simvastatin and fluvastatin) and very few (7%) were taking hydrophilic statins (i.e., rosuvastatin, pravastatin). Moreover, of the lipophilic statin users, 79.0% used simvastatin, 20.7% used atorvastatin and only 0.3% used fluvastatin at the first assessment in the UK Biobank cohort.
Information regarding insomnia symptoms had been collected as part of a touch-screen questionnaire. The participants had to answer the question “Do you have trouble falling asleep at night, or do you wake up in the middle of the night?” by choosing one of the three semi-quantitative options: (1) “never/rarely”, (2) “sometimes” or (3) “usually”. For our study, we recoded this information into a binary variable by grouping together “never/rarely” and “sometimes” and treated them as controls as done previously [31], while participants reporting “usually” were treated as those having insomnia.
Information regarding chronotype was collected through a question at baseline by asking “Do you consider yourself to be?” and the participants can choose from the answers “definitely a ‘morning’ person”, “more a ‘morning’ than an ‘evening’ person”, “more an ‘evening’ than a ‘morning’ person” or “definitely an ‘evening’ person”.
The analysis included a set of covariates, which were chosen based on their link
to sleep behaviour and cardiovascular health [31, 32] (Supplementary
Table 1). In brief, age (years), sex (male/female), BMI (weight/(height)
The current analyses were conducted using the IBM Statistical Package for Social
Science software (SPSS) version 26 (IBM Corp., Chicago, IL, USA). The analyses
were restricted to the baseline data for self-reported insomnia and chronotype,
the above-mentioned SNPs, statin treatment and covariates from the UK Biobank.
For all the variables wherever applicable, the answers “I don’t know”
and “Prefer not to answer” were recoded as missing. The genotypes were
coded as 0, 1, and 2 (based on the number of risk alleles) and included in
regression models as predictor (independent) variable. Insomnia was treated as a
dichotomous trait (0 representing “controls” and 1 representing “insomnia
cases”) and was tested via binary logistic regression. Chronotype was treated as
categorical (1–4 for the respective four answer options) or dichotomous
(“definite morning person” set to control and “definite evening person” set
to case). The threshold for significance was further adjusted to account for
multiple testing, i.e., p
We first examined if the genetic variants of HMGCR and PCSK9 were associated with lower total cholesterol and LDL-cholesterol levels as well as lower incidence of heart attack in our study population, by using linear regression and logistic regression, respectively. Then, we compared the prevalence of insomnia and the chronotype in the whole study population by using covariate-adjusted binary and multinomial logistic regression models. Both unadjusted and adjusted analyses were conducted and the results are reported. Analyses were adjusted for age, sex, sleep duration, total cholesterol level, LDL cholesterol level, BMI, smoking, alcohol consumption, heart attack, angina, hypertension, diabetes, education, physical activity, hypertensive medication, insulin use, TDI, first ten genetic principal components and UKB test centre. The covariates were selected for their link with the sleep related outcomes and cardiovascular disease. The association of insomnia with covariates was tested by employing chi-square test for categorical variables (sex, education, alcohol intake, smoking, comorbid conditions, medications and chronotype) and linear regression for continuous variables (age, TDI, physical activity, sleep duration, BMI, total cholesterol and LDL cholesterol). Moreover, a possible interaction effect of statin treatment and the presence of one of the SNPs was investigated using a multiplicative term in the logistic regression models adjusted for the aforementioned covariates.
Lastly, as majority of the statin users (93%) used lipophilic statins, we additionally conducted the analysis for association of statin use with insomnia and chronotype after excluding the hydrophilic statin users (n = 2739). Since we observed no significant difference in the findings, the results based on entire sample (i.e., including both hydrophilic and lipophilic statin users) are presented throughout the manuscript.
Insomnia, defined as having trouble falling asleep at night or waking up in the
middle of the night on a regular basis, was reported by 30.5% of individuals in
our study population (mean age
As we described in a previous study [22], statin users differed from controls in
a range of health-related parameters (Table 1). In the current study, statin
users had 0.95 mmol/L lower LDL cholesterol levels compared to controls [95% CI,
0.94 to 0.96] in the unadjusted analyses and after the adjustment with age and
sex, the circulating LDL cholesterol was further decreased to 1.0 mmol/L [95% CI,
0.99 to 1.01]. With respect to sleep, statin users more often reported insomnia
symptoms compared to controls, the unadjusted odds ratio (OR) was 1.15 [95% CI,
1.12 to 1.17; p = 2.89
| Variable | Non-users | Statin users | |
| N | 165,372 | 41,429 | |
| Continuous variables, mean (SD) | |||
| Age (years) | 56.50 |
61.60 | |
| Townsend deprivation index | –1.62 |
–1.31 | |
| Physical activity ( |
3.60 |
3.53 | |
| Body mass index (kg/m |
27.24 |
29.23 | |
| Sleep duration (hours/day) | 7.16 |
7.24 | |
| Total cholesterol concentration (mmol/L) | 5.91 |
4.66 | |
| LDL cholesterol (mmol/L) | 3.71 |
2.77 | |
| Categorical variables, N (%) | |||
| Sex | Male | 64,396 (38.9%) | 25,860 (62.4%) |
| Female | 100,976 (61.1%) | 15,569 (37.6%) | |
| Yes | 51,770 (31.3%) | 9660 (24.1%) | |
| No | |||
| Alcohol intake | Daily or almost daily | 34,767 (21.0%) | 9396 (22.7%) |
| 3 to 4 times a week | 38,981 (23.6%) | 9412 (22.7%) | |
| Once or twice a week | 43,611 (26.4%) | 10,122 (24.4%) | |
| One to three times a month | 19,246 (11.6%) | 4263 (10.3%) | |
| Special occasions only | 17,880 (10.8%) | 4945 (11.9%) | |
| Never | 10,782 (6.5%) | 3265 (7.9%) | |
| Smoking | Never | 91,729 (55.6%) | 18,351 (44.5%) |
| Former | 57,114 (34.6%) | 18,606 (45.1%) | |
| Current | 15,994 (9.7%) | 4261 (10.3%) | |
| Comorbidity | Diabetes | 2711 (1.63%) | 8139 (19.6%) |
| Hypertension | 42,536 (25.7%) | 25,337 (61.2%) | |
| Heart attack | 964 (0.6%) | 4652 (11.2%) | |
| Angina | 1457 (0.9%) | 6126 (14.8%) | |
| Medication use | Antihypertensive | 30,542 (18.6%) | 25,678 (62.2%) |
| Insulin | 709 (0.4%) | 1790 (4.3%) | |
| Chronotype | Definitely morning | 38,930 (26.2%) | 10,324 (28.1%) |
| Mild morning | 53,933 (36.3%) | 13,152 (35.7%) | |
| Mild evening | 42,734 (28.7%) | 10,234 (27.8%) | |
| Definitely evening | 13,086 (8.8%) | 3081 (8.4%) | |
| Insomnia symptoms | No | 115,831 (70.1%) | 27,840 (67.3%) |
| Yes | 49,441 (29.9%) | 13,555 (32.7%) | |
We first validated that the HMGCR and PCSK9 gene variants were associated with lower circulating cholesterol as well as a lower incidence of coronary artery diseases in our study population, as shown in other studies [27, 28, 33]. The demographic characteristics of individuals carrying different HMGCR and PCSK9 gene variants are shown in Supplementary Table 2 and Supplementary Table 3.
Each additional HMGCR rs17238484-G allele was associated with 0.047
mmol/L lower LDL-cholesterol levels [95% CI, 0.040 to 0.053; p, 2.50
Similarly, the PCSK9 rs11591147-T allele was associated with 0.29
mmol/L lower LDL-cholesterol [95% CI, 0.26 to 0.31; p = 1.30
After validating our genetic instruments, we analysed the association of rs17238484, rs12916 and rs11591147 genotypes with insomnia symptoms. Interestingly, gene variants in HMGCR and PCSK9 were both associated with insomnia, but in a differential manner (Fig. 2B‒D, Table 2). We observed that each additional rs17238484-G allele was associated with 2.0% lower odds for insomnia [95% CI, 0.96 to 0.99; p = 0.014] (Fig. 2B). Similarly, the rs12916-T allele was associated with 2.0% lower odds for insomnia [95% CI, 0.96 to 0.99; p = 0.002] (Fig. 2C). In contrast, we observed that each additional PCSK9 rs11591147-T allele was associated with 8.0% higher odds for insomnia [95% CI, 1.02 to 1.14; p = 0.014] (Fig. 2D).
| SNP | Gene | Model | OR | 95% CI | p | P |
| rs17238484_G | HMGCR | Unadjusted | 0.98 | 0.96–0.99 | 0.008 | 0.150 |
| Adjusted | 0.98 | 0.96–0.99 | 0.014 | |||
| rs12916_T | HMGCR | Unadjusted | 0.98 | 0.97–0.99 | 0.004 | 0.025 |
| Adjusted | 0.98 | 0.96–0.99 | 0.002 | |||
| rs11591147_T | PCSK9 | Unadjusted | 1.05 | 1.00–1.11 | 0.047 | 0.163 |
| Adjusted | 1.08 | 1.02–1.14 | 0.014 | |||
| Tested with logistic regression on self-reported insomnia symptoms. SNP, single
nucleotide polymorphism; OR, odds ratio; CI, confidence interval;
P | ||||||
 Fig. 2.
Fig. 2.Association of statin use, HMGCR rs17238484 and rs12916 as well
as PCSK9 rs1159147 alleles with self-reported insomnia symptoms. Odds
ratios of insomnia with (A) statin use and (B‒D) different copies of respective
gene variants of HMGCR (B: rs17238484, C: rs12916 and D: rs1159147) were analysed
via binary logistic regression, adjusted for age, sex, sleep duration, plasma
lipid concentration, BMI, smoking, alcohol consumption, heart attack, angina,
hypertension, diabetes, education, physical activity, hypertension medication,
insulin medication, Townsend deprivation index, first ten genetic principal
components and UK Biobank test centre. The threshold for significance after
correction for multiple testing was p
We did not observe any association of either statin treatment or HMGCR gene variants with chronotype (Fig. 3A‒C). In contrast, each additional copy of the PCSK9 rs11591147-T allele was associated with 15.9% greater odds for evening preference [95% CI, 1.06 to 1.30; p = 0.001] (Fig. 3D). Moreover, when the analyses were restricted to compare only definitely evening preference with definitely morning preference, PCSK9 rs11591147-T allele was associated with 17% greater odds for evening preference [95% CI, 1.05 to 1.29; p = 0.003] (Table 3).
| SNP | Gene | Model | OR | 95% CI | p |
| rs17238484_G | HMGCR | Unadjusted | 1.02 | 0.99–1.06 | 0.13 |
| Adjusted | 1.02 | 0.99–1.05 | 0.24 | ||
| rs12916_T | HMGCR | Unadjusted | 1.02 | 0.99–1.04 | 0.20 |
| Adjusted | 1.01 | 0.98–1.04 | 0.39 | ||
| rs11591147_T | PCSK9 | Unadjusted | 1.15 | 1.04–1.26 | 0.004 |
| Adjusted | 1.17 | 1.05–1.29 | 0.003 | ||
| Tested with logistic regression on extreme chronotypes (definite morning vs
definite evening type). SNP, single nucleotide polymorphism; OR, odds ratio; CI,
confidence interval. Analysis adjusted for age, sex, first ten genetic principal
components, Townsend deprivation index, UK Biobank test centre, sleep duration,
chronotype, body mass index, type 2 diabetes, total plasma cholesterol, LDL
cholesterol, statin use, alcohol intake frequency, smoking status, heart attack,
angina, hypertension, physical activity, education, antihypertensive medication
and insulin medication. Note: increased OR indicates greater evening preference.
The threshold for significance after correction for multiple testing was
p | |||||
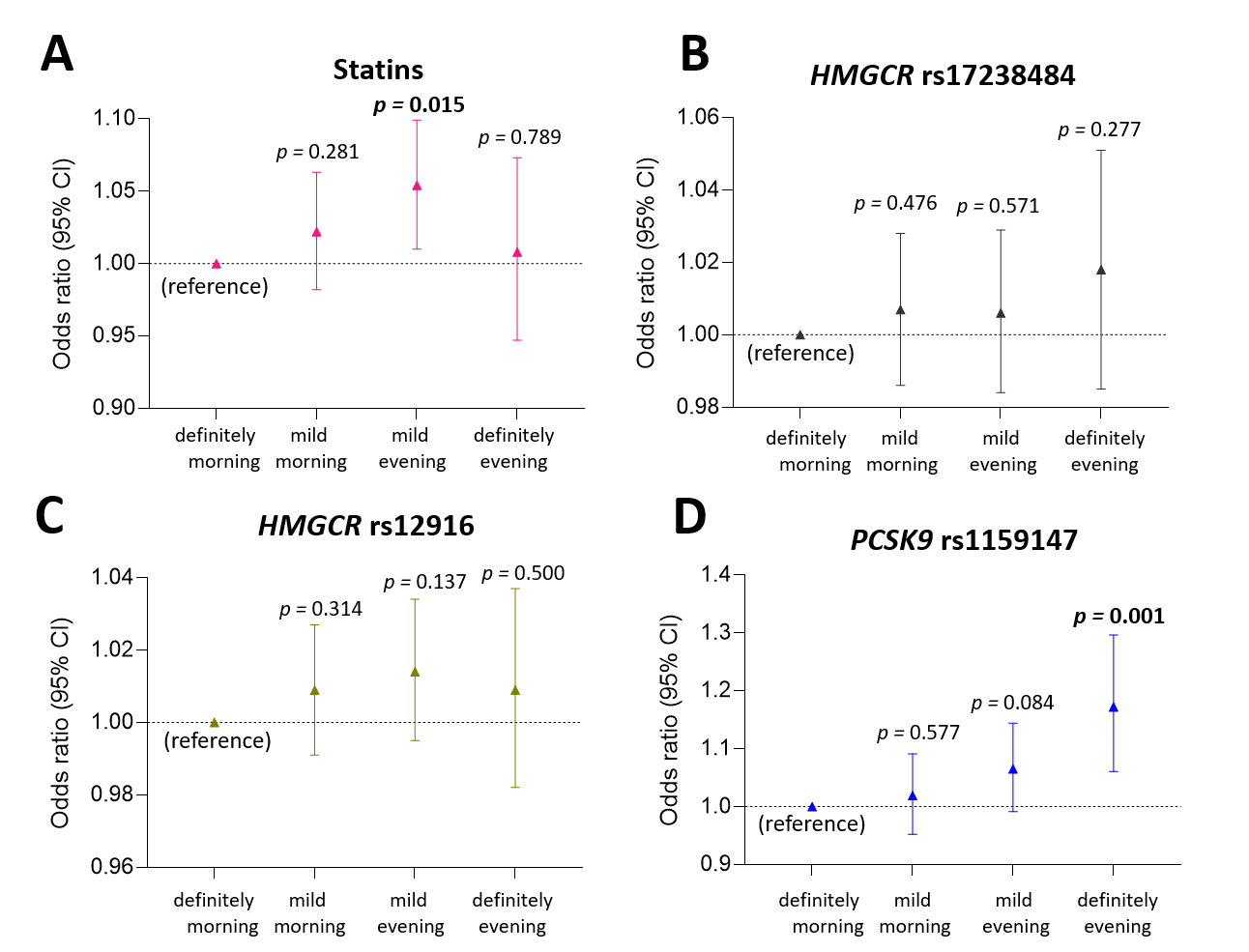 Fig. 3.
Fig. 3.Association of statin use, HMGCR rs17238484 and rs12916 as well
as PCSK9 rs1159147 alleles with self-reported chronotype. Odds ratios of
the strength of self-reported morning or evening preference (mild or definite)
with (A) statin use and (B‒D) different copies of respective gene variants of
HMGCR (B: rs17238484, C: rs12916) and PCSK9 (D: rs1159147) were analysed via
multinomial logistic regression, adjusted for age, sex, sleep duration, total
plasma cholesterol, LDL cholesterol, BMI, smoking, alcohol consumption, heart
attack, angina, hypertension, type 2 diabetes, education, physical activity,
hypertension medication, insulin medication and Townsend deprivation index, first
ten genetic principal components, UK Biobank test centre. The threshold for
significance after correction for multiple testing was p
No significant interaction was observed between statin treatment and genetic
variation in HMGCR or PCSK9 genetic variants on the risk of
having insomnia. Only a nominal significant interaction effect was observed
between statin treatment and HMGCR rs12916-T (the allele with the
greater positive effect on insomnia symptoms) on insomnia symptoms
[P
In the present study, the possible relationship between cholesterol-lowering treatment and sleep disturbances was investigated by using genetic variants in the HMGCR and PCSK9 genes that mimic the effects of cholesterol-lowering drugs (statins and PCSK9 inhibitors), in a large population-based cohort from the UK. Treatment with statins as well as genetic variation mimicking PCSK9 inhibition were found to associate with a greater risk for insomnia, while genetic variants mimicking HMGCR inhibition were found to be protective against insomnia. Moreover, the PCSK9 genetic variant significantly associated with chronotype, while no effect on chronotype was observed in relation to statin use or genetic variation in HMGCR.
We found that the association of statin treatment, as well as its genetic
proxies in the HMGCR gene (the intended drug target), with insomnia are
not directionally consistent. HMGCR genetic variants in the UK
population were not associated with a higher risk of insomnia, but instead had
protective effects. Statin treatment, on the other hand, was associated with an
increased risk of insomnia, suggesting that these effects are not a consequence
of HMGCR inhibition. First, the effect of HMGCR on sleep is interesting, as to
our knowledge no specific role has so far emerged for HMGCR in the
regulation of sleep behaviour. Further studies investigating the cellular and
molecular basis of this involvement will be interesting. Secondly, two previously
identified off targets of statins might instead be related to the unfavourable
effects of statin treatment on sleep, the peroxisome proliferator-activated
receptor alpha (PPAR
While HMGCR and PCSK9 seem to play a similar role in the development of type-2 diabetes [27, 28], the current results indicate that they act differently on sleep behaviour. This seems to indicate that cholesterol lowering is not the mechanism underlying the development of insomnia symptoms. It is possible that different roles of the two proteins in different tissues (e.g., liver versus brain) are responsible for their opposing effect on sleep. Following this hypothesis, individual statins with different tissue disposition properties may exert different effects on sleep behaviour, contributing to different study outcomes. The majority of statin users in our study had prescriptions for simvastatin, a lipophilic statin with high tissue penetration (and brain permeability) [40, 41], while hydrophilic statins were only used by a minor fraction (1.3%) of our study cohort.
There are several strengths and limitations of this study that need to be acknowledged. The biggest strengths are that the study used an extraordinarily large sample size and included both genetic and phenotypic data. Moreover, the comprehensive dataset allowed for the adjustment for several potential confounding factors to minimize bias. Still, some limitations should also be considered. First data on sleep was self-reported, and there are indications that some self-reported side effects of statins might underlie a nocebo effect, meaning that statin users might report negative side effects to a greater extent than control persons due to higher awareness or negative expectations [15, 42]. Thus, we cannot rule out that a nocebo effect also played a role in this study. Second, there is no sufficient data on dose and duration of statin treatment available in the UK Biobank, and most participants on statin treatment have been using simvastatin, which limits the conclusions that can be drawn with respect to statin pharmacology. Third, due to unavailability of data on use of PCSK9 inhibitors, it was impossible to compare the effect of drug use versus genetic variant on the studied sleep parameters as it was possible for statins. Finally, we restricted the analysis to the European ancestry to reduce specific bias in our genetic analyses, such as population stratification, limiting the translatability to other ancestries.
In summary, our study revealed interesting new results on the effect of cholesterol-lowering therapy on sleep behaviour, which contributes to our understanding of the multifaceted, seemingly controversial outcomes of statin effects in literature. Further investigations may address for instance more or different sleep parameters, the effect of statin type, and effects in non-European study cohorts. Our results also encourage further mechanistic studies to decipher the mechanism behind the differential effects of statins, HMGCR and PCSK9 and the potential role of HMGCR and PCSK9 in sleep and other circadian rhythms in cell or animal models.
AMA, XT, DC, MJW, CB and HBS conceived and designed the study. AMA, GR, DC, XT and MHA performed the statistical analysis. AMA, GR, LEC, CB and HBS wrote the manuscript and interpreted the data; All authors revised the paper.
All participants in the UK Biobank study have given informed consent for data collection, data storage and subsequent data analysis. Ethical permission for our study was given by the responsible local authorities, Regionala Etikprövningsnämden (now Etikprövningsmyndigheten, Swedish Ethical Review Authority, EPN), Uppsala, under registration number (Diarienummer, Dnr) 2017/198.
Thanks to all the UK biobank participants for providing all the data and consenting to participate in research studies.
HBS is supported by the Swedish Research Foundation. AMA is supported by the King Abdualaziz University, Faculty of Medicine, Saudi Arabia. LEC is supported by Tore Nilsons Stiftelse. GR is supported by grant from Svenska Sällskapet för Medicinsk Forskning (SSMF).
The authors declare no conflict of interest.
CVD, cardiovascular disease; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; LDL, low-density lipoprotein; PCSK9, proprotein convertase subtilisin/kexin type 9; angiopoietin; TDI, townsend deprivation index.
