1 Cardiology Department, Hospital Universitario, 28041 Madrid, Spain
2 Cardiology Department Hospital General Universitario Gregorio Marañón, 28007 Madrid, Spain
3 Universidad Europea, Universidad Complutense, 28040 Madrid, Spain
Abstract
The heart, like most mammalian organs, is influenced by circadian patterns. The suprachiasmatic nucleus in the hypothalamus has a key role in this influence, via various neurohumoral factors, particularly the autonomic nervous system. In addition, a local cardiac peripheral clock might drive a circadian rhythm related to the expression of ion channels. Several myocardial functions are influenced by these circadian cycles including activity/rest, regeneration, nutrient storage, growth, and myocardial repair. Numerous circadian genes have been identified in basic studies, and both biological factors and environmental features (including epigenetic) influence the human circadian rhythm. A normal circadian rhythm is important to maintain a normal heart rhythm and circadian rhythm disturbances can predispose to the development of cardiac arrhythmias. The normal heart rate presents a daily variability with a morning peak and nocturnal bradycardization. Ventricular arrhythmias and sudden death are more likely to occur in the morning after waking, while atrial fibrillation and heart blocks most commonly occur at night. Drugs such as beta-blockers might modify the chronobiology of some of these arrhythmias. On the other hand, drugs that influence circadian rhythm, like the circadian hormone melatonin, have demonstrated pleiotropic properties and show promising results as antiarrhythmics. This review is focused on the current understanding of the basic mechanism and clinical implications of the association circadian rhythms-cardiac arrhythmias/sudden death. The close relationship between circadian patterns and arrhythmias may provide us with the possibility of novel interventions to decrease the arrhythmic risk in some patients.
Keywords
- Circadian rhythm
- Circadian clock
- Arrhythmias
- Sudden cardiac death
- Chronotherapy
Cardiovascular functions are greatly influenced by internal and external modulators, including the autonomic nervous system, hormonal factors, epigenetic, lifestyle, and others that are not yet fully understood [1]. In recent years there is a growing interest in the impact of circadian rhythms on the cardiovascular system. Humans, like all mammalians, have a biological clock. Circadian rhythms might play a role in some cardiovascular events, such as myocardial infarction or aneurism rupture. Moreover, the biological clock has a key influence in the risk of cardiac arrhythmias and sudden cardiac death [1].
A central clock is located in the suprachiasmatic nucleus of the hypothalamus [2] and most organs and tissues, including the heart, have peripheral clocks [3] (Fig. 1). The light stimuli through the retina reach the central nervous system, are integrated and transformed into output signals to peripheral clocks, aiming to regulate basic physiologic functions: sleep/wake, fasting/feeding, and inactivity/activity [3, 4]. However, there is growing evidence that some peripheral clocks could, at least partially, have autonomous activity [3]. The local cardiac clock is located at the level of the working myocardial cells and the cardiac conduction system [5, 6]. Molecular mechanisms of circadian rhythms are complex [7, 8]. Circadian specific genes (“clock genes”) [1] have been described, that influence numerous regulatory mechanisms since the intrauterine period [7]. The autonomous nervous system with the sympathetic/parasympathetic balance is also influenced by the central suprachiasmatic clock, acting as a communication link that transmits information to peripheral clocks [9, 10]. Glucocorticoids [11] and mineralocorticoids are also part of this signaling communication chain [9].
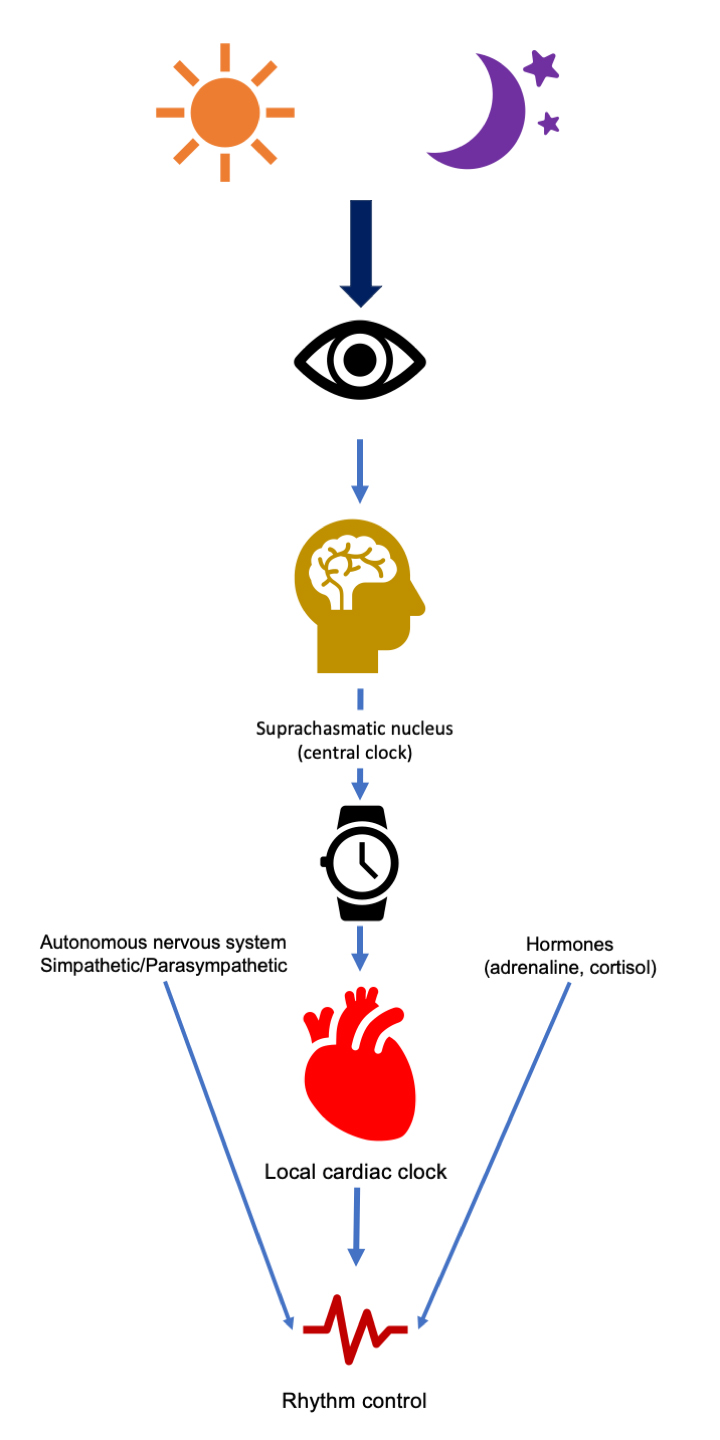 Fig. 1.
Fig. 1.Representation of the influence of circadian rhythms in cardiovascular physiology.
In healthy individuals, circadian clocks act like a pacemaker with sequential activation and inhibition cycles. These cycles are due to positive and negative feedback mechanisms that are present throughout the 24 h [1, 6]. Phases of enhanced contractile activity alternate with others of nutrient storage, growth, and myocardial repair during the resting period. The influence on cardiac electrophysiology is notorious, as circadian rhythms influence heart rate and other electrocardiographic parameters [12]. Heart rate daily variability, with the characteristic nocturnal bradycardization [13], is not entirely due to the action of the autonomic nervous system [5]. This can be seen in heart transplant recipients, that also have daily heart rate oscillations [12, 14]. Cardiac cells are influenced by the autonomic nervous system on one hand, and by the local circadian clock on the other. Circadian clocks remodel ion channel expression within cardiac conduction tissues over 24-hour cycles. Some ion channels show a circadian expression pattern that is different in healthy and failing hearts. The electrical remodeling is performed by the regulation of ion channel properties that govern excitability [15]. Natural light exposure increases resting heart rate [16], a proof that circadian pacemakers regulate heart rate.
Night rest produces lengthening of the PR and QT intervals, as well as a prolongation of QRS [12, 17]. Table 1 shows the most common electrocardiographic changes during the night. These changes are mainly explained by modifications in the electrical properties of cells located in sinus node, atrioventricular (AV) node, His-Purkinje system, and ventricular muscle. Nocturnal bradyarrhythmias are common in the general population [18] and it may be difficult to determine their clinical relevance. In patients with sinus node dysfunction, an early loss of circadian heart rate variability has been described [19]. Clinically significant bradyarrhythmias also follow a clearly circadian pattern, with an increase in frequency during the night [18] (Fig. 2). For instance, the incidence of paroxysmal atrioventricular block is highest between 2:00–4:00 AM [20]. It is important to consider that pathological nocturnal bradycardia can sometimes be caused by respiratory or systemic disorders, such as sleep-disordered breathing. An improvement in bradyarrhythmic episodes has been described in patients with sleep apnea and sinus node dysfunction when they are treated with continuous positive airway pressure treatment [21].
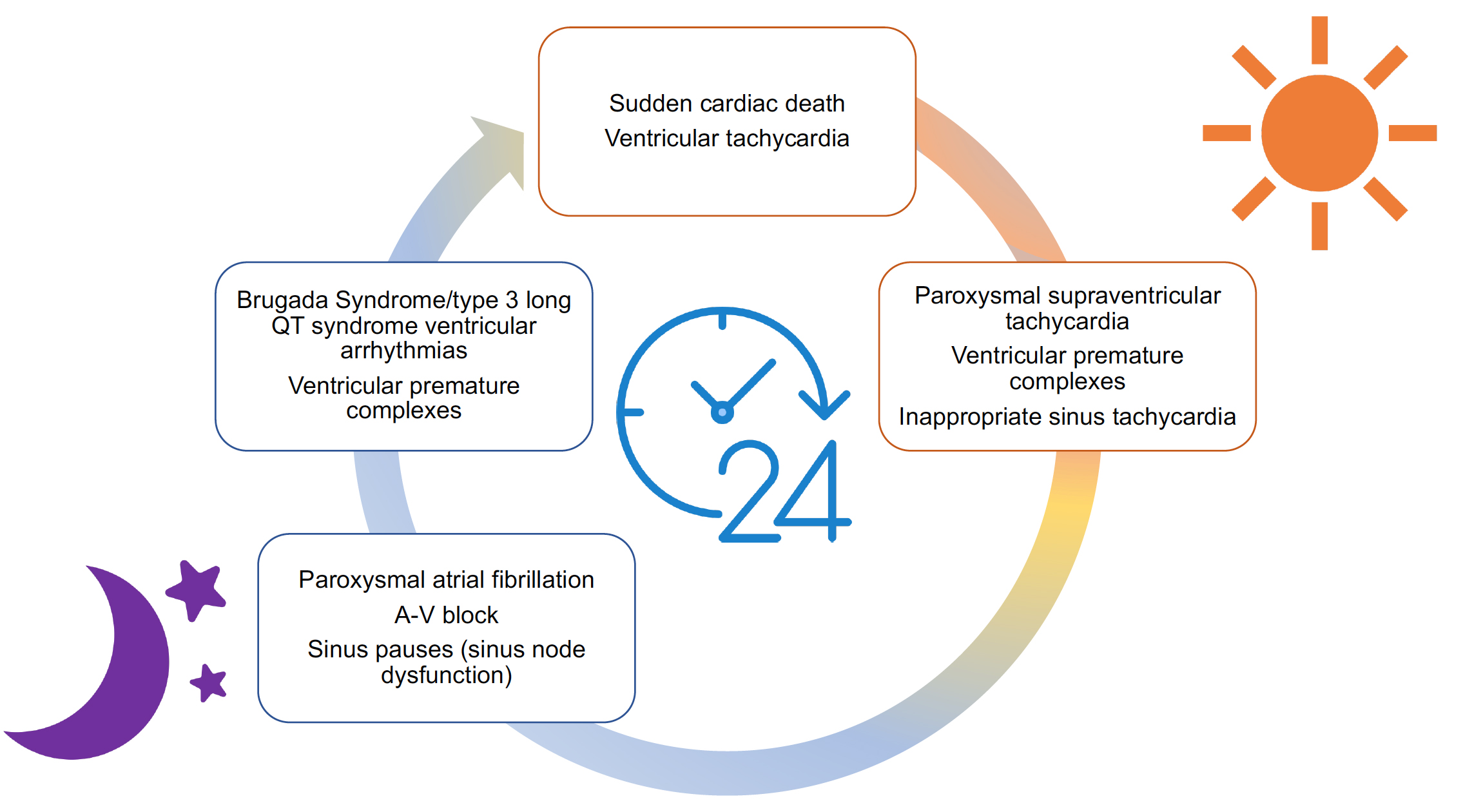 Fig. 2.
Fig. 2.Representation of the circadian pattern of the most common arrhythmias. All cardiac arrhythmias might be seen at any moment but have different peaks that are represented in this image.
Among the mechanisms underlying nocturnal bradycardization, the slowing of the
conduction velocity of the myocardial cells and a lower rate of firing of the
intrinsic pacemaker of the sinus node stand out [12]. Among the ionic channels,
special mention should be made of Hyperpolarization Activated Cyclic Nucleotide
gated potassium channel 4 (HCN4), which regulates the pacemaker current I
Supraventricular tachycardia episodes are more frequent during daytime [12], with the exception of atrial fibrillation [22]. Paroxysmal supraventricular tachycardia occurrence is more common during the afternoon [23, 24]. On the other hand, paroxysmal atrial fibrillation episodes occur more commonly at night [25]. Circadian variations in autonomic tone could act as a trigger by affecting the arrhythmogenic substrate. It has been suggested that an increased nocturnal vagal tone might explain the higher frequency of paroxysmal and persistent nocturnal atrial fibrillation episodes [25, 26]. Circadian oscillations are also observed in patients with permanent atrial fibrillation, as their ventricular response rate presents daily variability, in a similar way to subjects in sinus rhythm [27]. In addition, treatment with betablockers and some antiarrhythmic drugs changes the circadian pattern of atrial fibrillation, as patients treated with these drugs do not present the typical circadian variation of atrial fibrillation episodes [25]. In patients with inappropriate sinus tachycardia a higher average heart rate has been observed, compared to individuals with normal sinus rhythm, but without changes in the normal diurnal variation [28]. It has been hypothesized that these patients may have an intrinsic sinus node abnormality that results in a higher set-point for the 24-hour mean heart rate [28].
For supraventricular tachyarrhythmias, the molecular mechanisms are highly variable and depend on the specific type of arrhythmia [12]. In the case of atrial fibrillation, nocturnal parasympathetic activity on the IK [intermediate conductance K (+)] and acetylcholine channels causes a shortening of the refractory period and predisposes to reentry mechanisms [29].
Ventricular arrhythmias and sudden cardiac death risk increases during the early morning [12, 30] (Fig. 2). The studies that have analyzed this issue are mainly based on cardiac devices recordings. Therefore, most studied patients have structural heart disease or an underlying arrhythmic substrate. Probably several mechanisms explain this morning peak, including an increase in repolarization dispersion and in the activity of the sympathetic nervous system that promote calcium overload, early afterdepolarizations and reentry [12, 31]. L-type calcium channels exhibit circadian rhythms in both expression and function. The circadian variance in L-type calcium conductance promotes early afterdepolarizations in the morning, that may trigger fatal arrhythmias [32].
Ventricular premature complexes might be tachycardia- or bradycardia-related [33]. In addition, determining the circadian pattern of presentation of premature ventricular complexes can be useful to locate their origin, essential to the ablation procedure [34]. Tachycardia-enhanced ventricular premature complexes tend to be more frequent during the morning, and bradycardia-enhanced at night [34]. The circadian pattern has also been related with the inducibility of premature ventricular complexes during electrophysiological study and with ablation procedure outcome [35]. Ventricular tachycardia episodes follow a similar trend, with the aforementioned morning peak [36].
It has been observed that, in patients with severe ventricular systolic dysfunction, the circadian variability of ventricular arrhythmias may be absent, which has been associated with an increased activity of the sympathetic nervous system that disrupts the circadian pattern [37]. However, some neurohormonal treatments for heart failure, especially beta-blockers, can modify or attenuate the circadian pattern [38, 39]. This underscores the role of the sympathetic system as an exogenous predisposing factor for ventricular arrhythmias [39]. Observational registries of sudden cardiac death have also found a predominant morning peak [40, 41] and studies performed in patients with implantable cardioverter defibrillator have shown that appropriate shocks are more common in the early morning hours [37]. The morning peak of sudden cardiac death in the general population has been correlated with episodes of myocardial ischemia [42], but other factors such as cortisol release or the predominance of sympathetic system activity and its increase in the arrhythmic risk could have an important role [43]. In patients with structural heart disease, the presence of coronary artery disease had no impact on the circadian pattern of ventricular arrhythmias [44]. This circadian pattern is not always the same, for instance ventricular arrhythmias associated with some channelopathies such as Brugada syndrome, tend to have a nocturnal distribution peaking from midnight to early morning [45]. In the case of long QT syndrome, circadian variations are determined by genotype. In type 3, ventricular arrhythmias are more frequent at rest or during sleep, and therefore occur mainly at night [46]. For types 1 and 2, arrhythmic events most commonly occur during sympathetic stimulation, predominantly in the morning [46]. All these data confirm that the activity of the sympathetic/parasympathetic nervous system exerts an important influence as a predisposing or triggering factor for ventricular arrhythmias, however, depending on the cardiac arrhythmogenic substrate, the effect might be stronger in different time periods.
Circadian variability also influences drug metabolism and treatment efficacy. Therefore, one way to optimize the effect of cardiovascular drugs is to carefully program the administration regimen [47]. On the other hand, some drugs can interfere with the normal circadian rhythm or with the chronobiology of cardiac arrhythmias [12]. Ivabradine reduces the average daily heart rate in sinus rhythm patients but also attenuates daily heart rate variability, suggesting a local effect at the local sinus node clock [5]. As previously mentioned, beta-blockers attenuate the circadian pattern and the morning peak of ventricular arrhythmias [38, 39]. Patients with implantable cardioverter defibrillator treated with beta-blockers have an almost even distribution of appropriate shocks [39]. The Cardiac Arrhythmia Suppression Trial (CAST) found an excess of deaths in patients treated with class IC antiarrhythmics, and these patients also showed the conventional circadian pattern with the morning peak [48]. Amiodarone reduces heart rate variability, suggesting a suppression of autonomic control on the heart [49]. Among the mechanisms by which antiarrhythmic drugs affect the circadian pattern of arrhythmias interactions with the autonomic nervous system are one of the potential explanations. Amiodarone has bradycardia and hypotensive effects resulting from increased vagal tone [50]. In addition, it has been observed that moricizine (an off-patent Class I anti-arrhythmic) regulates circadian and cardiac channel gene expression and is able to modulate the circadian clock, particularly by lengthening the circadian period [51].
Melatonin is a circadian hormone, which can be supplemented exogenously due to its pleiotropic cardioprotective [52, 53] and antiarrhythmic effects [52, 54]. These effects include up-regulation of connexin-43, a protein of intercellular channels at the gap junctions that ensures electrical signal propagation throughout the myocardium [52, 55, 56, 57]. Melatonin also lowers heterogeneity in the repolarization of myocardiocytes, therefore reducing the possibility of reentry circuits [52, 55]. Furthermore to electrical remodeling, melatonin has antifibrotic properties [57] and has a protective effect against ischemia/reperfusion injury [54, 58]. Interestingly, it is known that melatonin production decreases with age and this could be one of the main causes of the age-related increase in arrhythmias [53]. Future studies are needed to test the hypothesis that chronic melatonin supplementation may be helpful in preventing cardiac arrhythmias.
The maintenance of a normal sinus rhythm is linked to a state of cardiovascular health and its disruption can be the cause or the consequence of cardiovascular diseases [1, 59]. Circadian disruptors, such as shift-work or jet lag might cause misalignments in the regulation of circadian clocks [60, 61]. A reduced heart rate variability and a higher rate of premature ventricular beats have been described in shift workers [62, 63, 64] and in those with sleep deprivation states [65]. Shift-work or jet lag causing circadian rhythm dyssynchronization and autonomic stress [65] have been associated with an increased risk of cardiovascular events [3, 9]. Exposure to sunlight allows the natural re-synchronization of circadian rhythms but his effect is not observed with artificial light [66].
The regulation of transcription through methylation as the most common epigenetic mechanism has gained importance in explaining the circadian pattern of arrhythmias. There are cyclic changes in DNA methylation, which increases at night [67]. Importantly, environmental factors such as lifestyle, eating and sleeping habits exert epigenetic modifications in the expression of the genes responsible for circadian rhythms [68]. It has been observed that chronic stress in a murine model induces a methylation of adrenergic signaling of cardiomyocytes genes and that they lead to the development of arrhythmias [69].
A better understanding of the mechanisms and the impact of circadian rhythms on the cardiovascular system would allow the development of therapeutic targets or preventive measures aimed at correcting disruptions in the biological clock of patients with cardiovascular conditions [9, 70]. Chronotherapy could guide pharmacological treatments and determine the best time to administer specific drugs to achieve the highest efficacy and a better tolerability [9, 71]. In addition, circadian rhythm might guide patient monitoring, focusing in moments in which arrhythmic events are most likely to occur. Moreover, knowing the peak of presentation of malignant ventricular arrhythmias is of public health interest, for better resource planning [42].
In conclusion, circadian rhythms influence cardiac arrhythmias and sudden death. A better knowledge of the chronobiology of arrhythmias will allow the development of more specific and effective treatments, as well as preventive strategies to reduce the impact of the disruption of biological rhythms. More studies are needed on this promising topic. One of the goals of personalized medicine could include an individualized analysis of the disruptions of the circadian rhythm, with the intention of restoring a normal circadian pattern.
MMS conceived the manuscript. LV prepared the first draft and performed the first references search. Both authors worked in the next versions and approved the final manuscript.
Not applicable.
We would like to thank the reviewers for their comments and suggestions.
This research received no external funding.
The authors declare no conflict of interest.


