Purpose: To present preliminary clinical results of the effects of a
new treatment with percutaneous electrolysis directed to peripheral tendon and
therapeutic resistance exercise, with or without the presence of degenerative
zone. Methods: 3 patients with patellar tendinopathy aged 37–45 years
with diagnostic of patellar tendinopathy with pain since 5–8 weeks were treated
with a novel, less invasive electrolysis technique. Pain severity was measured by
Numerical Pain Rating Scale (NPRS). Lower limb functionality was measured by a
Victorian Institute of Sport Assessment questionnaire (VISA-P). A clinical
interview and ultrasonography assesment were performed before study protocol were
carried out. Each participants received 4 to 7 sessions of percutaneous
electrolysis (350
Patellar tendinopathy is one of the most common injuries among runners [1]. However, there is a great difference in prevalence between different sports (between 14% and 22%) and its frequency is 2 times higher in men than in women. The highest prevalence is found in sports where there is a ballistic load with a high demand on the knee extensor apparatus, suggesting that there is a correlation between an excessive mechanical load on the tendon and the appearance of symptoms [2].
Considering the available scientific evidence [3], it is not yet clear whether structural alterations in tendon tissue may have a strong correlation with pain, but does indicate that those alterations are related to a loss of tendon function, increasing the probability of developing a symptomatic picture when the alterations are present [4].
In recent years, ultrasound has been widely used in the static and dynamic evaluation of abdominal and lumbar soft tissues [5]. In addition, the high reliability and precision that it has shown for objective measurements of oral motor function by measuring masticatory muscle thickness in healthy patients [6], Rotator Cuff tendon disease [7], or Achilles or patellar tendinopathies [8, 9] has made it possible that it has progressively replaced the traditional magnetic resonance imaging (MRI) or computed tomography (CT).
Moreover, there is literature that also strongly recommends its use not only as an evaluation tool due to its low cost, safety and portability, but also because of the possibility of obtaining quantitative measurements of tissue texture (thickness, cross-sectional areas or perimeters) and as a guide for invasive interventions such as dry needling or percutaneous electrolysis in anatomical regions of high risk due to space conflict [10, 11, 12].
The therapeutic exercise is one of the most effective conservative treatments in the management of patellar tendinopathy symptomatology [13]. Isometric contractions have shown to be an effective tool to control short-term tendon pain and increase muscle activation in the early stages of treatment [14]. In order to achieve collagen remodeling and improve long-term tendon function, a more demanding exercise involving higher mechanical stress on the tendon, such as eccentric contraction and Heavy Slow Resistance (HSR), is needed [13]. It seems that the active role of the patient triggers neurological adaptations that increase neuromuscular functionality [13]. Therefore, increasing strength and motor control are established as primary objectives to achieve results in treatments.
One of the most innovative techniques today for the treatment of tendinopathies is percutaneous electrolysis due to its powerful effect in reducing pain and increasing functionality, especially in load tendons in the lower limb [15]. There is only a modest scientific evidence demonstrating the effects of electrolysis in reducing pain and increasing function in both lower and upper extremity tendon structures, which is drawing the attention of physicians and physical therapists in both clinical and scientific arenas [16, 17, 18].
Percutaneous electrolysis is produced when the flow of a galvanic current goes through a sterile needle made of surgical steel (negative electrode). This generates a dissociation of water (H+OH) which leads to an oxidation reaction with the mineral salts of the tissue, producing mostly sodium hydroxide (NaOH) [15, 16, 17, 18, 19].
The negative electrode is attributed with the capability of liquefaction, membrane permeabilization, and an increase of metabolism and local vascularization. From a molecular point of view, increased concentrations of vascular endothelial growth factor (VEGFR) and activated peroxisome proliferator receptor (PPAR), instigators and modulators of regeneration have been found [19].
One of the possible effects that has been attributed to the technique is the change in pH of the injured area [20]. However, recent publications have emerged rejecting the existence of this mentioned action mechanism, observing that the effect is not statistically significant [21].
One of the hypotheses currently handled in publications [22] investigating evident clinical effects of electrolysis, is the clearing of algogenic and inflammatory substances that have been found in degeneration tendon areas and its periphery at initial stages of tendon degeneration. In a recent systematic review, Gomez-Chiguano et al. [23] presented results of moderate scientific evidence of electrolysis for the treatment of pain and musculoskeletal dysfunction. For this reason, the aim of our study was to determine the effects of a new treatment with percutaneous electrolysis directed to peripheral tendon, with or without the presence of degenerative zone.
From January to August 2020, at different times, 3 patients were referred for rehabilitation to the ONELIFE pain clinic in Madrid, Spain, after being diagnosed by a physician from traumatology service of the Quirón Sur Hospital, (Madrid, Spain) with local pain at the anterior region of the knee, located at the proximal insertion of the patellar tendon, with at least 5 weeks of evolution.
In all cases prior to the onset of symptoms, participants made a more or less sudden increase in the frequency or intensity of physical activity involving the lower limb, resulting in an increased mechanical stress on the patellar tendon. All patients had previously undergone a treatment program based on dry needling technique and therapeutic exercise (1–2 sessions during 4 weeks from the onset of symptoms), but the evolution of the symptoms worsened in all cases, therefore it was decided to begin a combined treatment of tendon electrolysis and a strength exercise program for the lower limb.
On the first day of rehabilitation program (RP) patients provided informed consent to use their information for publication. The study protocol was approved by the ethical committee of European University of Madrid (reference number CIPI/20/156). All patients received a physiotherapeutic evaluation based on the patient’s history, symptoms, palpatory examination and orthopedic tests for patellar tendon dysfunction.
Due to the situation in Madrid caused by the SARS-COV-2 virus, the protocol used in the clinic was the following:
(1) Before entering to the center, shoes were disinfected by stepping on a mat soaked in hydrogen peroxide.
(2) Then, the patient was given a temperature measurement with a digital thermometer and hydroalcoholic gel was applied to the hands.
(3) Mandatory use of surgical mask by the patient and FFP2 mask and face shield by the physiotherapist, in addition to a disposable gown.
(4) Verbal recognition of not having symptoms or having been in contact with people suspected of being with the current disease.
(5) During treatment, disinfection of stretcher, chair, ultrasound probe, and gloves.
(6) Safety distance was always maintained except during treatment.
The 3 patients in this case series were evaluated at the beginning of the treatment and at 3 and 8 weeks after the treatments were performed. Information related to each patient’s age, relevant history, and main symptoms are shown in Table 1.
| Patient | Gender | Age (years) | Sport | Duration of symptoms before treatment (weeks) | Symptom presentation | Relevant signs | Load modifications |
| 1 | M | 37 | Running | 5 | Up and down the stairs | Ecographic signs | Yes |
| Running | Local pain when palpated | ||||||
| Get up | |||||||
| 2 | M | 45 | Running | 8 | Up and down the stairs | Ecographic signs | Yes |
| Local pain when palpated | |||||||
| 3 | M | 38 | Running Padel | 5 | Down the stairs | Local pain when palpated | Yes |
| Abbreviation: M, male. | |||||||
All the patients indicated that they had undergone medical treatment with NSAIDs at the beginning of the appearance of the symptoms and only 2 of them reported treatment with injection, one with corticosteroids and the other with type III collagen. A case series study was performed, based on a CARE case report guidelines developed by Equator Network [24].
Three of the patients presented ultrasound images compatible with degenerative tendinopathy at the deep tendon interface in the area closest to the proximal insertion, as shown in Fig. 1A–C.
 Fig. 1.
Fig. 1.Ultrasound images compatible with degenerative tendinopathy. (A) In the image we can observe an evident thickening of the patellar tendon in a longitudinal cut. (B) In the image we can appreciate a series of hypo-echoic images and tissue heterogeneity in the inner pole of the patella in a longitudinal cut. (C) In the image we can observe the presence of intratendinous neovascularization in a longitudinal cut. (D) Regional anatomy details.
During the physical examination, patients presented pain when palpating the lower pole of the patella, placed on supine with the knee at 20º of flexion. Two provocative tests were performed, the single leg squat and the lateral step down.
Single leg squat: Subject standing, barefoot, starting at 0º of knee flexion, a 45º knee flexion is required. The patient’s heel must remain on the floor during the whole exercise. During the test, the patient should report pain using the Numerical Pain Rating Scale (NPRS).
Lateral step down: Subject in standing position with the lower limb to be tested on a step. The opposite leg in suspension. We ask you to perform a knee flexion greater than 60º. During the execution the patient will not modify the position of the pelvis or the lumbar spine. During the execution of the test the patient should reproduce pain on the NPRS scale.
Pain score values were taken according to the NPRS [25], and the Victorian Institute of Sport Assessment questionnaire (VISA-P) of lower limb functionality translated into Spanish [24]. The VISA-P scale is used to measure painful symptoms as well as the ability and functionality to perform activities of daily living. This scale is a reliable and validated measurement that has specific scales, such as the VISA-P for specific knee affections, and that presents psychometric properties of validity and reliability of 76.1% and 70%, respectively [26]. The maximum possible score which corresponds to an asymptomatic patient is 100 points. Thickness—tendinopathy can cause an increase in tendon thickness, due to a change in the number and type of cells of the tendon tissue, and this event provokes a swelling increase, and consequently an augmented tendon dimensión [27]. Tendon thickness is indeed moderately correlated with pain, for some authors [9, 28, 29, 30], so it is considered as an indirect measure of treatment outcome [31, 32]. The participants were assessed at the beginning of the treatment, at 3 weeks and at 8 weeks from the beginning of the treatment with electrolysis and strengthening exercise.
In an 8 weeks period of time, from 4 to 7 sessions of percutaneous electrolysis were performed [9], leaving at least one week between sessions (Table 2).
| Patients | VISA-P | NPRS | Thickness (mm) | Sessions (Accumulated) | ||||||||
| n | Pre | Post-3 | Post-8 | Pre | Post-3 | Post-8 | Pre | Post-3 | Post-8 | Pre | Post-3 | Post-8 |
| 1 | 31 | 50 | 74 | 6 | 3 | 0 | 4.4 | 4.4 | 4.3 | 0 | 3 | 6 |
| 2 | 26 | 32 | 60 | 7 | 6 | 2 | 6.7 | 6.5 | 6.5 | 0 | 4 | 7 |
| 3 | 20 | 35 | 65 | 7 | 5 | 1 | 7.2 | 7.3 | 7.1 | 0 | 3 | 4 |
| Abbreviations: VISA-P, Victorian Institute of Sport Assessment (0–100, the maximum possible score which corresponds to an asymptomatic patient is 100 points); NPRS, Numerical Pain Rating Scale; Pre, pre-treatment; Post-3 and Post-8, post-treatment at 3–8 weeks. | ||||||||||||
In all cases the tendon approaches were superficial, on it superficial interface
and on it deep interface due to the relationship with the Hoffa fat. The needle
insertion area was sterilized with chlorhexidine. Sterile ultrasound gel was then
applied to obtain the reference ultrasound image. Ultrasound examination was then
performed until a clear cross-sectional image of the destructured area or the
area of local pain of the patellar tendon was obtained. Once the image was
obtained, using the scale to the left of the ultrasound image, the distance of
the superficial and deep interface of the patellar tendon from the probe was
calculated. With these reference values, the needle was introduced completely
parallel to the probe (in in-plane approach), from the lateral margin of the
tendon at the necessary distance to access the superficial or deep interface of
the tendon (Figs. 1,2). We use the electromedical device EPTE System, to supply
in each approach a galvanic current with 350
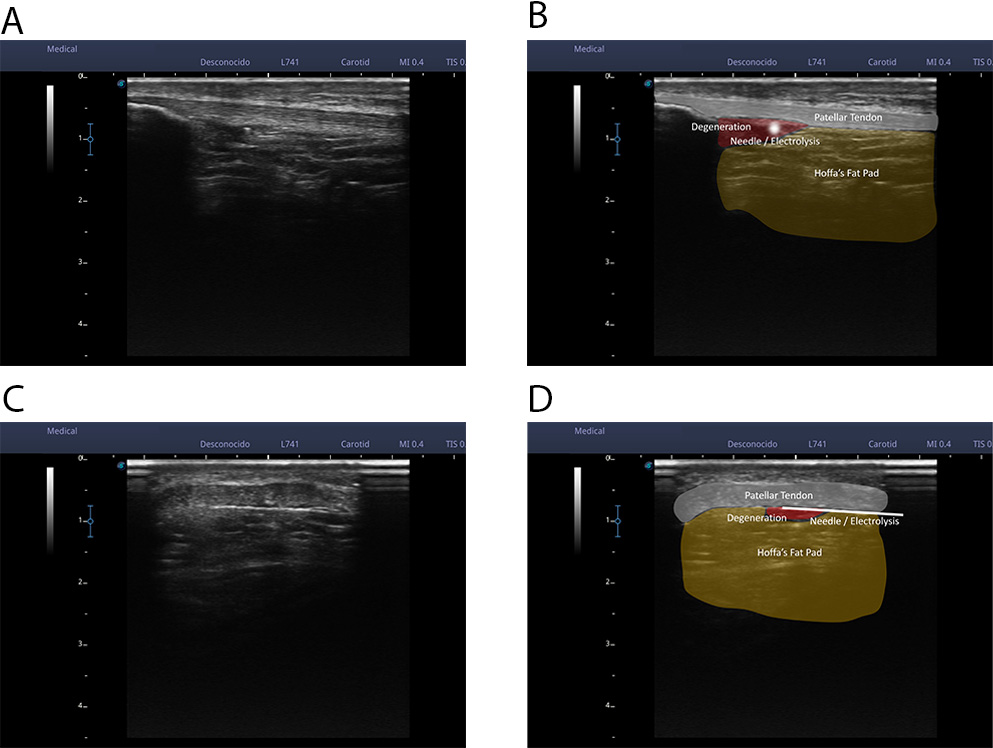 Fig. 2.
Fig. 2.An ultrasound examination was performed until a clear cross-sectional image of the unstructured area or the area of local pain of the patellar tendon was obtained. Once the image was obtained, using the scale to the left of the ultrasound image, the distance of the superficial interface and the deep interface of the patellar tendon from the probe was calculated. With these reference values, the needle was introduced completely parallel to the probe (an in-plane approach), from the lateral margin of the tendon at the necessary distance to access the superficial interface or the deep interface of the tendon (A,B). In these 2 images we can see a real image of an ultrasound cross-section of the patellar tendon (A) and a representation of the tissues that can be observed (B); patellar tendon, Hoffa fat, hypo-echoic area, and needle. (C,D) In these 2 images we can see a real image of a longitudinal ultrasound cut of the patellar tendon (C) and a representation of the observable tissues (D); patellar tendon, Hoffa fat, hypoechoic area, and needle.
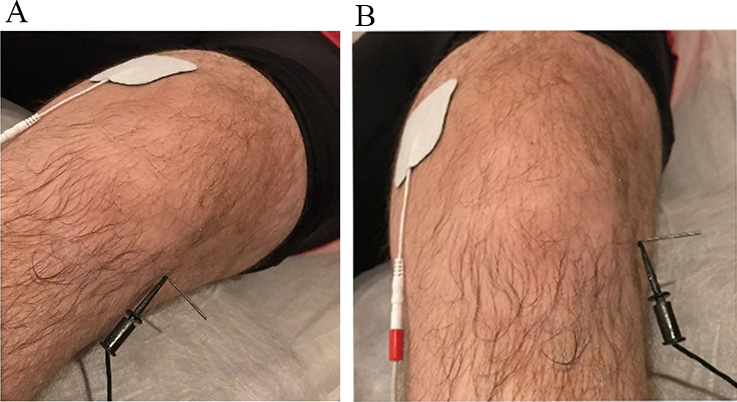 Fig. 3.
Fig. 3.Placement of the electrodes for the application of electrolysis on the patient’s knee. (A) Sagittal. (B) Frontal. We can see a real image of the treatment with electrolysis and how the positive electrode (anode) is located about 10 cm from the negative electrode (cathode). The positive electrode is connected to a self-adhesive patch. The negative electrode is connected to a sterile surgical steel needle 40 mm long, positioned at the deep interface of the patellar tendon.
All patients came to the center to learn the exercises and set the doses of the exercises [33, 34, 35, 36, 37, 38]. They all performed 3 sets of 15 repetitions with a 90 seconds rest between sets of each exercise in those involving a concentric or eccentric phase [13, 15, 18] of hip clams, single limb bridge on stable surface, pelvic drop, sidelying hip abduction, decline squat exercise, step squat, deadlift and short foot (Fig. 4A–H).
 Fig. 4.
Fig. 4.A to H: Exercise protocol performed by patients. The exercises are designed to improve the functional unit of the lower limb. We focused on the hip and foot stabilizers, and specifically the patellar tendon. The selection of the resistance exercise set was based on the available scientific evidence [13, 39, 40], and the patient must maintain the neutral position during its execution. In addition, the good execution of the exercises was supervised, and the quality of the technique was maintained. The hip clams (A), single limb bridge on stable surface (B), pelvic drop (C) and sidelying hip abduction (D) exercises focus specifically on the gluteal muscles. Decline squat exercise (E) focus on the stability of the foot and step squat exercise (F) as a global exercise works the eccentric control of the knee, the muscular activity of the gluteus and the stability of the foot. Finally, deadlift (G) and short foot (H) exercises we worked on the eccentric activity of the patellar tendon. All patients performed the same exercise protocol and showed pain less than 6/10.
Physical therapists (n = 2) with a range of clinical experience (10–20 years) performed examinations and provided treatment to all 3 patients. All two of the therapists are board certified in orthopedics and had fellowship training in electrolysis. All two therapists were previously trained and certified in therapeutic exercise.
After 3 weeks, patients were treated with percutaneous electrolysis between 2–4 sessions. After 8 weeks, patients had been treated for a total of 4 to 7 electrolysis sessions (Table 2).
Organized care physical therapy information as chronology is presented in Fig. 5.
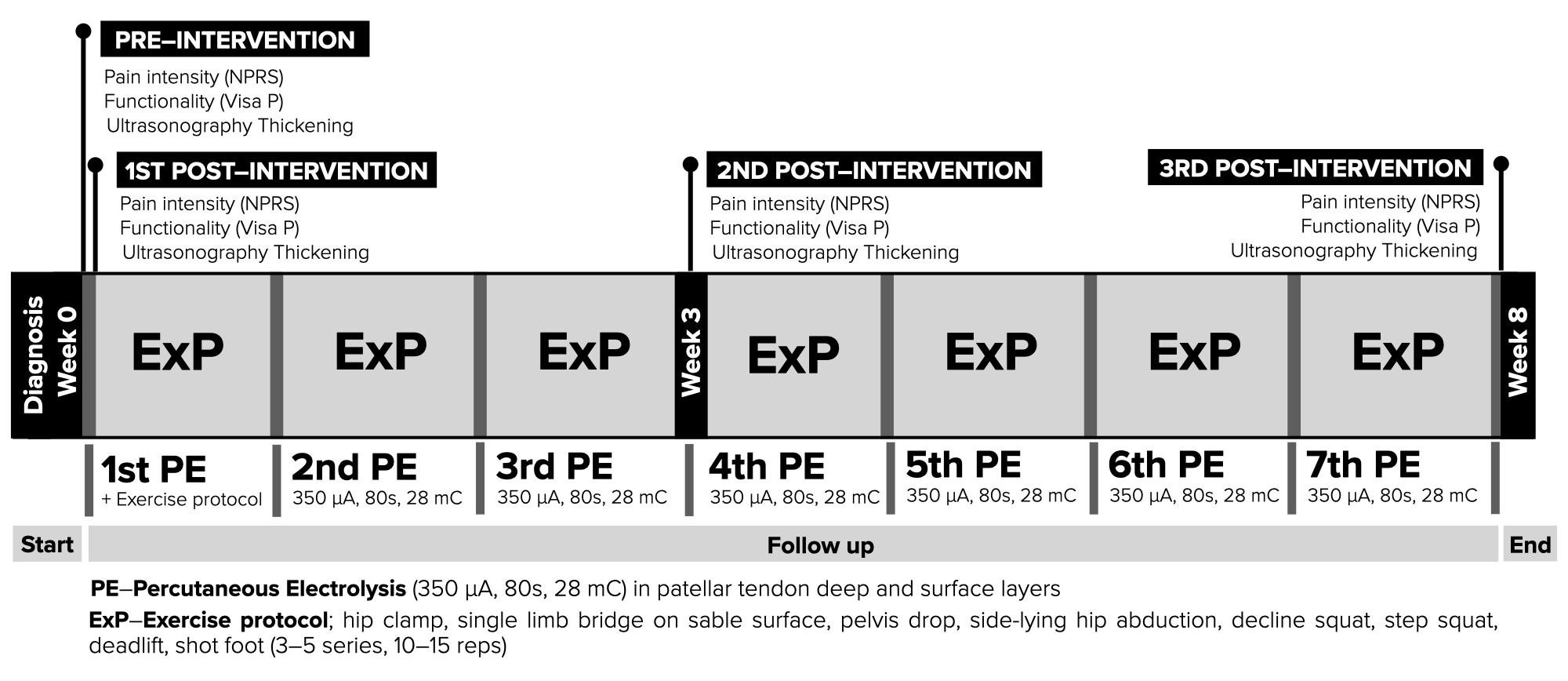 Fig. 5.
Fig. 5.Diagram of the interventions from the intervention, observation and follow up.
Data were analyzed using SPSS package version 25.0 (SPSS Inc, Chicago, IL, USA). Descriptive statistics, including frequency counts for categorical variables and measures of central tendency and dispersion for continuous variables were calculated to summarize the data. Paired samples T-test was used to compare the measurements pre, post-3 and post-8 the NPRS, VISA-P and thickness. For all the data of the study, p values lower than 0.05 were considered significant.
The 3 patients (3 males) in this case series ranged in age from 37 to 45 (mean
age 40 years
| Outcome | Mean ( |
p-value |
| VISA-P (Pre) | 25.6 |
- |
| NPRS (Pre) | 6.6 |
- |
| Thickness (Pre) | 6.1 |
- |
| VISA-P (Post-3) | 38.74 |
0.003* |
| NPRS (Post-3) | 4.6 |
0.011* |
| Thickness (Post-3) | 6.07 |
0.007* |
| VISA-P (Post-8) | 66.6 |
0.001* |
| NPRS (Post-8) | 1 |
0.015* |
| Thickness (Post-8) | 5.96 |
0.007* |
| Abbreviations: VISA-P, Victorian Institute of Sport Assessment; NPRS, Numerical
Pain Rating Scale; Pre, pre-treatment; Post-3 and Post-8, post-treatment at 3–8
weeks; SD, standard deviation. *Indicates statistical significance p | ||
The present work provides novel evidence that percutaneous electrolysis with a less invasive approach targeting the peripheral tendon in combination with therapeutic resistance exercise diminished pain, improved functionality and showed a tendency to decreases thickness in our cases with patellar tendinopathy.
Also, we found that, by means of the ultrasound evaluation it was observed that 3 of the patients presented structural alterations in the proximal insertion of the patellar tendon, in the deepest aspect at the inferior pole of the kneecap. Despite this clinical heterogeneity, there were no significant differences in the type of symptoms presented by the patients.
Our results agree with those of the clinical trial developed by Abat et al. [15], in which two groups of 30 subjects affected by patellar tendinopathy and treated with an identical eccentric exercise program were compared, but one group was also treated with electrolysis and another with electrotherapy, which showed better results in the VISA-P scale the group treated with electrolysis.
The changes in terms of decreased pain as well as increased functionality become evident long before the improvement in tissue disorganization [17, 22, 35]. These findings align with the controversial evidence widely described in the literature of whether or not the presence of structural alterations are responsible for the appearance of tendon pain [3, 30].
Therefore, the effectiveness of electrolysis in terms of pain and functionality is due not so much to the structural recovery of the tissue but to an analgesic effect due to the stimulation of the nervous tissue. We know that the periphery of the tendon is a very relevant area in terms of accumulation of algogenic substances and proliferation of both nerve endings and vascular endings [30]. It is possible that the application of galvanic current through the needle may have a modulating effect on pain and vascularization.
The type of approach chosen for treating the patellar tendon by means of electrolysis has been repeatedly used in previous studies with positive results [15, 18]. In our current case, we have sensibly modified the approach used by Abat in which intratendinous and peritendinous approaches were made. For this reason, approaches were made exclusively on the superficial interface and on the deep interface of the tendon. The absence of structural alterations in some of the tendons diagnosed with patellar tendinopathy that come to our attention does not justify, from our point of view, the intra-tendinous approach in which a certain homeostatic alteration is generated [19]. Even in patients with structural damage to the tendon, in most cases it is found in the deepest aspect of the tendon, coinciding with the deep interface of the tendon [41, 42]. There are a number of limitations to this case series. We recognize that the sample size was small (although sufficient to determine significance), in addition to differences in age and composition of men only, and the lack of a control group. The immediate therapeutic response to treatment leads us to believe that it is due to the electrolysis treatment, since the effects of exercise take at least 3 to 6 weeks to be present. The information extracted from the patients of this study comes from the clinical field, therefore it cannot be found in the same way as the information obtained from an experimental study in terms of extracting efficacy conclusions. An additional limitation to this case series is that there was no long-term follow-up of patients after their discharge from physical therapy.
The results of this retrospective case series suggest the combination of percutaneous electrolysis and therapeutic exercise may have the potential to improve pain and disability in individuals with patelar tendinopathy. Conservative management of individuals with patelar tendinopathy pain is often challenging and and the novel treatment exposed in the present work is the least invasive known in invasive physiotherapy to date, being able to prevent patients from having to undergo surgery. The clinical outcomes observed with the first description of a less invasive approach of electrolysis than those presented to date in this case series suggest that further controlled trials of this novel treatment approach are warranted.
Conceptualization—ACMF and EASR; methodology—JHV and EASR; software—ACMF; validation—all authors; formal analysis—JHV; investigation—all authors; resources—JLAP; data curation—EASR; writing—original draft preparation—JHV; writing—review and editing—EASR, CBC, RDM, DGJ and JHV; visualization—SMP; supervision—all authors; project administration—EASR; funding acquisition—EASR. All authors have read and agreed to the published version of the manuscript.
The study protocol was approved by the Ethical Committee of European University of Madrid (reference number CIPI/20/156). Informed consent was obtained from all subjects involved in the study. Written informed consent for publication must be obtained from participating patients who can be identified (including by the patients themselves). Written informed consent has been obtained from the patient(s) to publish this paper.
The authors would like to thank the 3 patients of the study for their collaboration. The authors would like to thank Samuel Fernández Carnero for his guidance.
The publication of this work has been financed by the European University of Canary Islands, C/Inocencio García 38300 La Orotava, 38300 Tenerife, Canary Islands, Spain.
The authors declare no conflict of interest.
NPRS, Numerical Pain Rating Scale; VISA-P, Lower limb functionality was measured by a Victorian Institute of Sport Assessment questionnaire; RP, rehabilitation program.
