Aim: This study aimed to determine the effect of mechanical
ventilation (MV) on the differentiation and proliferation of diaphragm satellite
cells. Methods: Diaphragm satellite cells were isolated from C57 mice
receiving 6 h of MV with optimized magnetic-activated cell sorting (MACS)
approach. The cells were stained with BrdU or antibody for differentiation marker
MYH3. The expression of MyoD and myogenin was detected by real-time PCR.
Results: Diaphragm satellite cells were successfully isolated from mice
by using MACS with a set of optimized parameters. About 1.5
Mechanical ventilation (MV) is known to cause damage to diaphragm muscle [1]. Especially, weaning from MV is challenging for the recovery after serious illness. Moreover, weaning failure is associated with life-threatening complications, leading to increased morbidity and mortality [2]. Accumulating evidence has indicated that prolonged MV longer than one week leads to respiratory muscle weakness because of contractile dysfunction and atrophy, especially in the diaphragm [3, 4, 5, 6]. Diaphragm dysfunction plays a key role in weaning failure from MV, and such a condition is named as ventilator-induced diaphragmatic dysfunction (VIDD) [7].
Diaphragm satellite cell prolifearion is a promising driving force to reverse VIDD. Satellite cells are usually mitotically quiescent but can be activated upon injury, leading to the production of myogenic precursor cells or myoblasts to promote muscle regeneration [8]. Stem cells are known to have great potential of differentiation and regeneration [9, 10]. Satellite cells are the primary myogenic cells that contribute to skeletal muscle regeneration after muscle injury [11, 12]. Myogenic cells in dystrophic muscle show accelerated differentiation [13]. However, it remains unknown whether such a phenomenon occurs in diaphragm muscle.
Previous study reported that pro-myogenic factors or the transplantation of muscle satellite cells (MuSCs) can enhance skeletal muscle regeneration [14]. Several protocols have been published to isolate MuSCs from skeletal muscle [15, 16]. However, the diaphragm is distinct from other skeletal muscles because it is activated constantly to drive ventilation and diaphragm satellite cells are myogenic cells with potential for proliferation and differentiation [17]. Therefore, it is difficult to isolate diaphragm satellite cells because of the challenge to preserve the quiescence state of diaphragm satellite cells during the isolaton. To overcome the problem, in this study we established an optimized method to purify diaphragm satellite cells, and determined the effects of MV on the differentiation and proliferation of satellite cells.
Male C57 mice (10–12 weeks of age) were housed under standard conditions, and divided randomly in two groups: control group and MV group (n = 6). Mice in control group were fasted for 6 h with free access to water. Mice in MV group underwent 6 h of controlled MV as described previously [18].
All the animals were sacrificed using CO
The cells were stained with 0.03 mg/mL BrdU (Cat # B5002, Sigma, St. Louis, MO, USA) for 24 h, and sequentially incubated with acetone and 1.5 M HCl. Next, the cells were fixed with 1:5000 Hoechest and covered using coveslips, the cells were observed under Zeiss M2 fluorescence microscope. The proliferation of satellite cells was determined according to the intensity of BrdU staining.
The cells were fixed in 4% PFA, washed and then incubated with primary antibody for MYH3 (Cat # sc-53091, Santa Cruz Biotech, Santa Cruz, CA, USA) and rabbit anti-mouse secondary antibody Alexa Fluor 488 (Cat #A11059, Invitrogen, Carlsbad, CA, USA). Next, cells were fixed with 1:5000 Hoechest and covered using coverslips. The cells were observed under Zeiss M2 fluorescence microscope.
Total RNA was isolated from satellite cells using TRIzol regent (Cat #15596018,
Invitrogen, Carlsbad, CA, USA) and RT-PCR was performed using iScript reverse
transcription supermix for RT-qPCR (Cat # 1708840, Bio-Rad, Hercules, CA, USA).
HPRT1 was selected as the housekeeping gene. The primer sequences were as
follows: MyoD 5
The data were shown as mean
Using MACS, 1.5
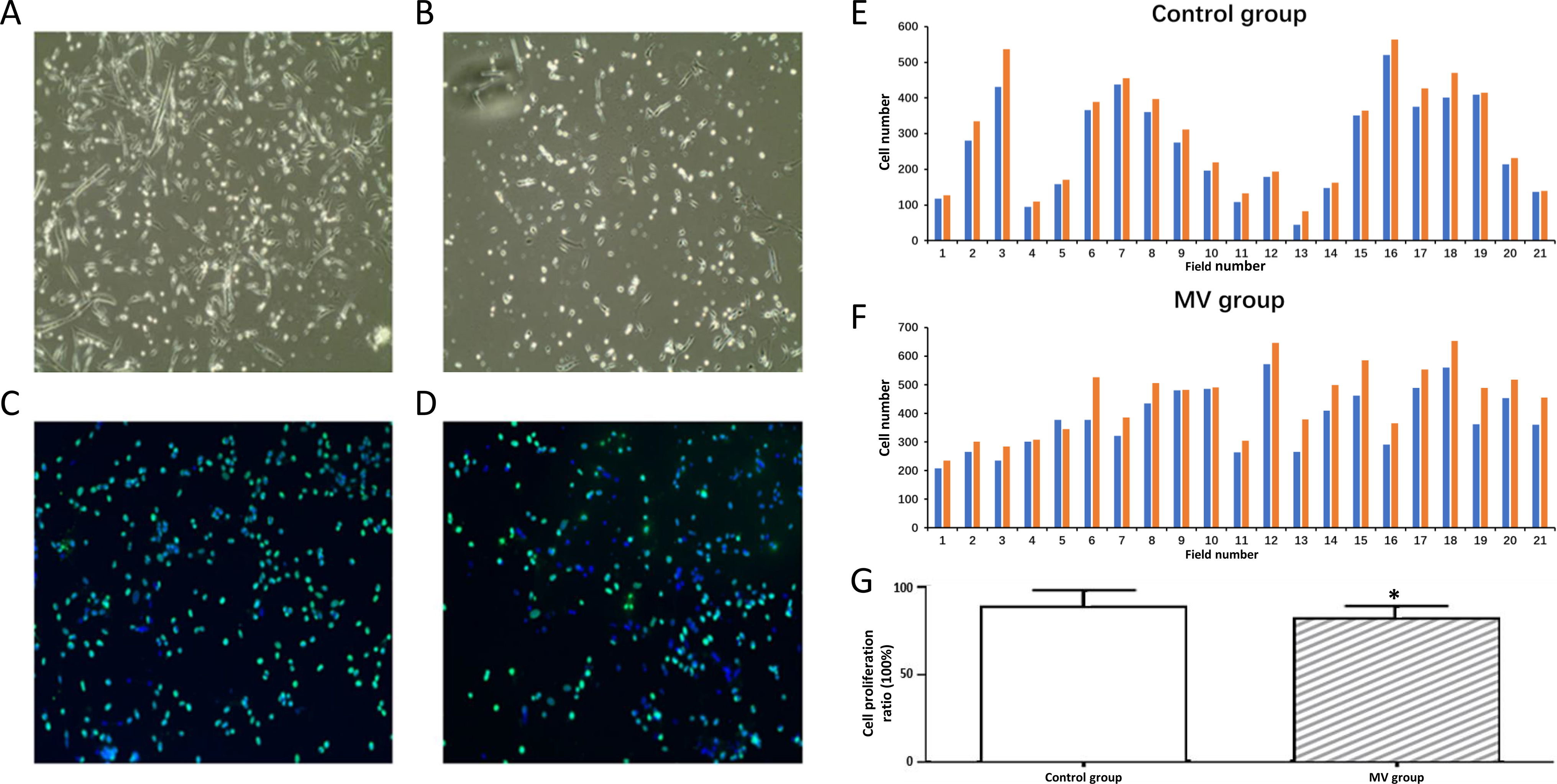 Fig. 1.
Fig. 1.Proliferation of diaphragm satellite cells. (A) Diaphragm
satellite cells in control group on day 4. (B) Diaphragm satellite cells in MV
group on day 4. (C) Diaphragm satellite cells in control group stained by BrdU
(green) after 5 days. (D) Diaphragm satellite cells in MV group stained by BrdU
(green) after 5 days. The nuclie were stained as blue by DAPI. Magnification 400.
(E) Number of cells in proliferation in control group from 21 randonly selected
areas in the slides. (F) Number of cells in proliferation in MV group from 21
randonly selected areas in the slides. Green indicated positive BrdU staining and
blue indicated positive DAPI staining. (G) Proliferation ratios of diaphragm
satellite cells. Proliferation ratio: number of BrdU positive cells/number of
DAPI positive cells
All satellite cells were detected with the antibody for differentiation marker
MYH3. We found that the diaphragm satellite cells in the control group exhibited
spindle-shaped morphology, and the nucleus was distributed orderly in fiber
bundles. However, the satellite cells of the MV group were not spindle-shaped,
and the nucleus was irregular and scattered, indicating that the cells of the MV
group tended to differentiate (Fig. 2A–D). Furthermore, we counted the numbers
of cells stained positive for differentiation in randomly selected areas in
control group and MV group (Fig. 2E,F). We found that the differentiation ratio
was increased from 17.94% to 27.58% by MV (p
 Fig. 2.
Fig. 2.Differentiation of diaphragm satellite cells. (A) Diaphragm
satellite cells in control group on day 4. (B) Diaphragm satellite cells in MV
group on day 4. (C) Diaphragm satellite cells in control group stained by
antibody for differentiation marker MYH3 (green) after 5 days. (D) Diaphragm
satellite cells in MV group stained by antibody for differentiation marker MYH3
(green) after 5 days. The nuclei were stained as blue by DAPI. Magnification 400.
(E) Number of cells in differentiation in control group from 12 randonly selected
areas in the slides. (F) Number of cells in differentiation in MV group from 9
randonly selected areas in the slides. Green indicated positive MYH3 staining and
blue indicated positive DAPI staining. (G) Differentiation ratios of diaphragm
satellite cells. Differentiation ratio: number of MYH3 positive cells/number of
DAPI positive cells
MyoD and myogenin are markers of muscle cell differentiation. Real-time PCR analysis showed that MyoD and myogenin mRNA levels were significantly higher in diaphragm satellite cells from MV group compared to control group (Fig. 3). These results supported that MV promoted the differentiation of diaphragm satellite cells.
 Fig. 3.
Fig. 3.MyoD and myogenin mRNA expression in diaphragm satellite cells.
Data were presented as mean
Currently, no reliable approach is available to isolate diaphragm satellite
cells, which has been a challenge for further investigation on diaphragm
satellite cells. In present study, we developed a method to isolate diaphragm
satellite cells with MACS. Based on parameter optimization, we successfully
established a stable method to obtain diaphragm satellite cells. The muscle
weight of a single diaphragm from C57 mouse was about 150 mg, and 1.5
MV is a life-saving supportive therapy for patients with respiratory failure, and has been associated with some complications such as infection, barotrauma, tracheal injury, and ventilator-induced lung injury [19]. A previous study indicated that diaphragmatic strength and endurance played critical role in successfully weaning patients from MV [20]. MV can cause sudden interruption of diaphragmatic contractile activity [21]. Taken together, the mechanisms underlying MV-induced diaphragm damage may involve oxidative stress, muscle atrophy and muscle fiber remodeling, but need further investigations.
In present study, we speculated that the differentiation and proliferation of diaphragm satellite cells played a critical role in weaning difficulties from MV. It is known that in adult muscle, satellite cells are myogenic progenitors [13]. It has been reported that the atrophy of the diaphragm was faster compared with other skeletal muscles under adverse stimulation [18]. Interestingly, myogenic cells from the dystrophic mouse showed accelerated differentiation [13]. In present study, we found that diaphragm satellite cells tended to differentiate after 6 h of MV. This result was consistent with the reduced diaphragmatic flexibility, disuse atrophy, and dysfunction after MV.
Myogenic regulatory factors (MRFs) are improant to regulate myocyte proliferation and differentiation, and mainly include Myf5, MyoD, myogenin, and MRF4 [13]. Among them, MyoD can regulate skeletal muscle differentiation vai the interaction with chromatin modifying complexes [22]. Myogenin is a target of MyoD and directs satellite cell regeneration to skeletal muscle [23]. In present study, we confirmed that MyoD and myogenin expression in diaphragm satellite cells significantly increased after 6 h of MV. These findings might help explain diaphragm cell differentiation tendency and diaphragm dysfunction after MV.
In conclusion, we developed a feasible and efficient approach to isolate diaphragm stem cells through optimized MACS and demonstrated that diaphragm stem cells tended to differentiate after MV, accompanied by increased expression of MyoD and myogenin.
JD performed experiments, conducted data analysis, and drew the manuscript. QL (Qian Li), FL and SB took part in the animal experiments and contributed to the tests. QL (Qingquan Liu) provided financial support.
All animal-related protocols were approved by the institutional animal care and use committee (IACUC) of Mcgill University Health Centre (Approval No. 20180911, date 2018-09-11), and efforts were made to minimize the suffering of animals according to the recommendations by European Commission (1997).
This work was performed at Mcgill University Health Centre. The authors are grateful to Basil Petrof for scientific advice.
This work was supported by the Beijing Natural Science Foundation [No. 7182071] and National Natural Science Foundation of China [No. 81503399].
The authors declare no conflict of interest.
All data are available from the corresponding author upon request.
