Background: Corona Virus Disease 2019 (COVID-19) is an acute respiratory infectious disease caused by severe respiratory syndrome coronavirus 2 (SARS-CoV-2). The primary pathogenesis is over-activation of the immune system. SARS-CoV-2 continues to mutate and spread rapidly and no effective treatment options are yet available. Mesenchymal stem cells (MSCs) are known to induce anti-inflammatory macrophages, regulatory T cells and dendritic cells. There are a rapidly increasing number of clinical investigations of cell-based therapy approaches for COVID-19. Objective: To summarize the pathogenic mechanism of SARS-CoV-2, and systematically formulated the immunomodulation of COVID-19 by MSCs and their exosomes, as well as research progress. Method: Searching PubMed, clinicaltrials.gov and Chictr.cn for eligible studies to be published or registered by May 2021. Main keywords and search strategies were as follows: ((Mesenchymal stem cells) OR (MSCs)) AND (COVID-19). Results: MSCs regulate the immune system to prevent cytokine release syndrome (CRS) and to promote endogenous repair by releasing various paracrine factors and exosomes. Conclusions: MSC therapy is thus a promising candidate for COVID-19.
According to real-time WHO network data, the worldwide number of confirmed
COVID-19 cases to April 22, 2021 was 143,488,236 and 3,055,587 deaths, posing an
unprecedented threat to the global economy and to human health [1]. The
International Committee on the Taxonomy of Viruses named the COVID-19 pathogen as
SARS-CoV-2. This virus gains phagocytic entry into AT2 via interaction with
angiotensin-converting enzyme 2 (ACE2) (See Fig. 1). It increases Angiopoietin-2 (Ang-2) levels, leading to long-term and intense activation of pro-inflammatory
Ras-related pathways. A high concentration of Ang-2 in the lung interstitium
promotes cell apoptosis, releases pro-inflammatory cytokines and triggers the
inflammatory response, thereby causing immune-induced tissue damage and increased
vascular permeability [2, 3]. In patients with severe disease, the development of
CRS is an abnormal systemic inflammatory response that manifests clinically as a
rapid and sharp rise in the level of cytokines. These include C-X-C Motif
Chemokine Ligand 10 (CXCL10), monocyte chemo-attractant protein-1 (MCP-1/CCL2),
macrophage inflammatory protein-1 (MIP-1), platelet-derived growth factor (PDGF),
tumor necrosis factor-
 Fig. 1.
Fig. 1.The pathological processes of COVID-19 (blue arrows) and multiple therapeutic mechanisms of MSCs and their exosomes in COVID-19 (green arrows). (a) SARS-CoV-2 gains entry into AT2s via ACE2. (b) Immune cells recognize SARS-CoV-2 via TLRs. (c) T cells are polarized into pro-inflammatory phenotypes. (d) Excessive activation of M1 causes CRS. (e) Inflammation and oxidative damage cause lung fibrosis and remodeling. MSCs reduce fibrosis by various paracrine factors. (f) MSCs force T cells to polarize into Tregs. (g) MSCs and MSC-exosomes increase the number of anti-inflammatory M2 macrophages. (h) MSCs restore microvascular permeability. (i) MSCs activate the Na+/K+ pump to remove lung fluid and reduce pulmonary edema. Abbreviations: ECM, extracellular matrix; PGE 2, Prostaglandin E 2; COX, Cyclooxygenase; HGF, hepatocyte growth factor; KGF, keratinocyte growth factor.
Although the efficacy of vaccines in preventing COVID-19 ranges from 50% to 95%, an increasing number of COVID-19 cases still require effective treatment options [7, 8]. There is currently no standard drug therapy for COVID-19. Antiviral, anti-malarial and anti-inflammatory drugs are unable to repair or regenerate lung tissues, especially in patients with severe complications such as acute respiratory distress syndrome (ARDS) [9, 10, 11]. Since the outbreak of COVID-19, a number of studies have been carried out on MSCs and MSC-exosome therapy for this disease. MSCs have good safety for the treatment of COVID-19 and show particular clinical efficacy in shortening the course of disease, alleviating lung injury and reducing the level of inflammatory factors [6, 12, 13]. This is expected to provide a new approach for the treatment of severe and critical COVID-19. MSC-exosomes also show promise as a cell-free substitute for COVID-19 [14, 15, 16] (Table 1, Ref. [17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]). The potential mechanisms of action of MSCs and MSC-exosome therapy are shown in Figs. 1,2.
| Functions | Involved genes/factors/pathways | Diseases | References |
| (+) Immunomodulatory activities (+) repair (+) angiogenesis (–) fibrosis | miR-21, miR-24, miR-124, miR-145, miR-122, KGF, VEGF, HGF, VE-cadherin, Occludin-1, Claudin-1, PCNA, Cyclin D1, IDO, Wnt/ |
COVID-19 | [17, 18, 19, 20, 21, 22] |
| (–) EMT | miR-182-5p, miR-23a-3p (-) NF-κB, Hedgehog | LPS induced lung injury | [23] |
| (–) Inflammation, proliferation | miR-34a, miR-122, miR-124, miR-127 | IPAH | [24] |
| (–) Leukocyte infiltration | ICAM-1 | ARDS | [25] |
| Anti–viral | COX-2, PGE2 | Influenza | [26] |
| (–) Airway hyperresponsiveness | miR-146a-5p | Allergic airway inflammation | [27] |
| (–) Autophagy, apoptosis | miR-125b, Bcl-xL, Bcl2, and BIRC8 (-) Caspase1, Caspase 8, lymphotoxin |
Myocardial infarction, acute kidney injury. | [28, 29] |
| (–) Fibrosis | (-) Col-I, Col-III, TGF- |
Liver fibrosis | [30, 31] |
| (+) M2 macrophage polarization | miR-1260b (-) Wnt5a/RANKL | Periodontitis, Tendon healing | [32, 33] |
| (+) Treg cell induction, T–cell apoptosis | FASL/FAS | Acute colitis | [34] |
| (–) Dendritic cell maturation | a blockade in G0/G1 phase of the cell cycle | LPS intervention | [35] |
| (+) Autophagy | AMPK/ /mTOR, Akt/mTOR | Myocardial I/R | [36] |
| (+) indicates stimulation. (–) indicates inhibition. | |||
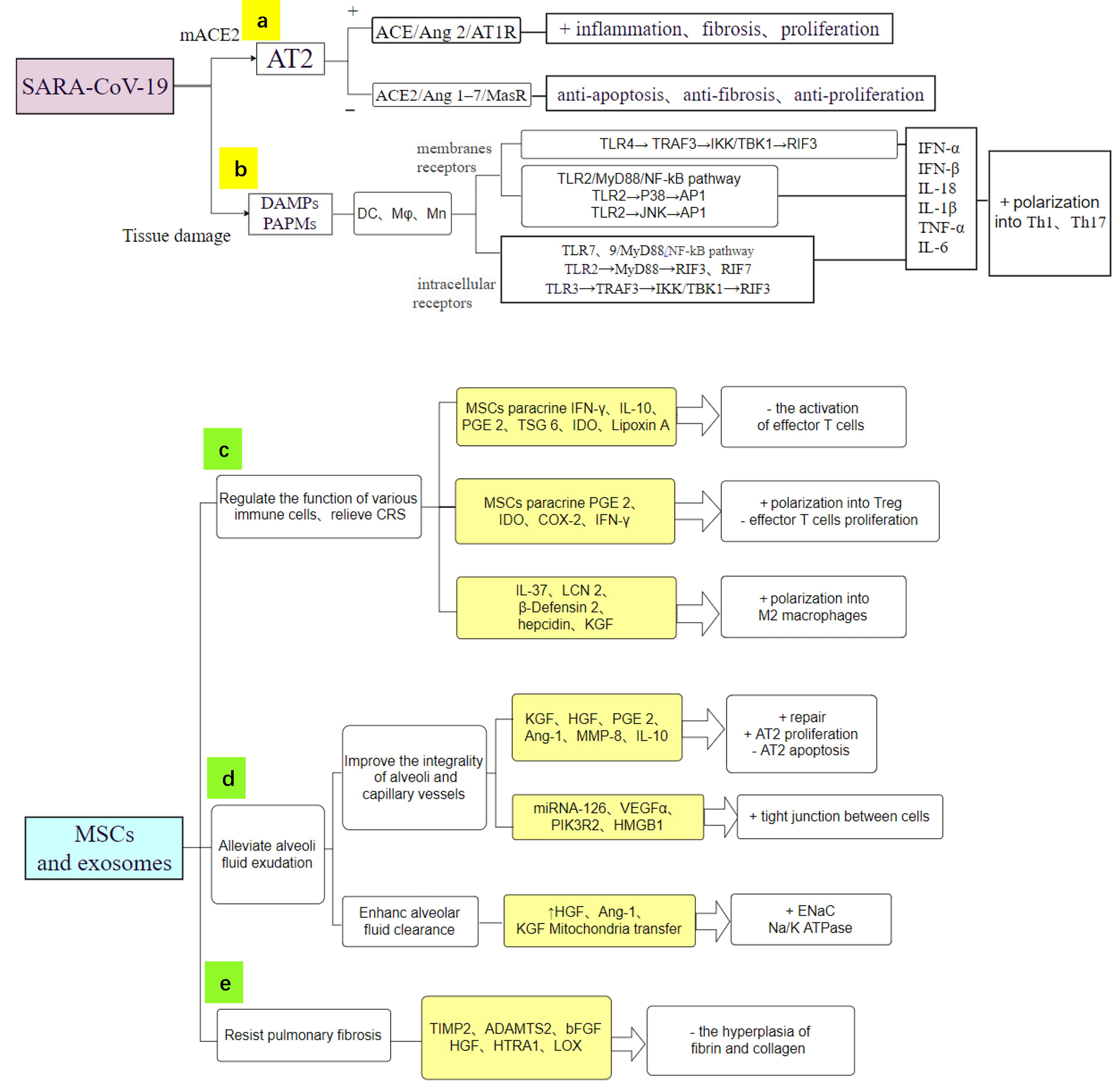 Fig. 2.
Fig. 2.MSCs act on COVID-19 through multiple mechanisms and specific secretory products (light yellow squares). (a) SARS-CoV-2 causes immune-induced tissue damage via pro-inflammatory Ras-related pathways. (b) TLRs of immune cells recognize SARS-CoV-2, leading to CRS. (c) MSCs have powerful anti-inflammatory and immunomodulatory functions. (d) MSCs repair microvascular permeability and alleviate pulmonary edema. (e) MSCs secrete substances to inhibit the hyperplasia of fibrin and collagen, thereby alleviating PF. + indicates stimulation. – indicates inhibition.
Once SARS-CoV-2 infects the human lung epithelium and is internalized (Fig. 1a),
SARS-CoV-2 RNA activates the intracellular receptors TLR3/7 and membrane
receptors TLR2/4. TLR3 in dendritic cells (DCs) specifically recognize dsRNA, an
intermediate product of viral replication, thereby activating nuclear factor
kappa-B (NF-
MSCs can interfere with the antigen-presenting function, differentiation and
maturation of DCs by paracrine IFN-
Pro-inflammatory macrophages were reportedly more abundant in the
bronchoalveolar lavage fluid from severe compared to mild COVID-19 cases [42].
Zhang et al. [43] proposed that CRS in severe COVID-19 is mainly a
virus-triggered macrophage activation syndrome. Viral RNA stimulates macrophages
to produce various soluble factors via the activation of TLRs. These factors
include IFN-
MSCs regulate macrophage polarization to limit inflammation and to promote
tissue healing following injury. Anti-inflammatory soluble factors and two main
inhibitory molecules secreted or expressed by MSCs trigger the immune system’s
inhibitory response. MSCs are stimulated to produce IL-10, thyrotropin-releasing
hormone-6 (TRH-6), human leukocyte antigen (HLA), human growth factor (HGF), heme
oxygenase-1 (HO-1) [45], superoxide dismutase (SOD), cyclooxygenase-2 (COX-2),
PGE2 and IDO [46]. Meanwhile, MSCs are induced by IFN-
Flow cytometry analysis has shown that the number of CD4+ and CD8+T cells in the
peripheral blood of COVID-19 patients was significantly reduced, with the degree
of reduction being related to the severity of COVID-19 [53]. This phenomenon may
be related to the recruitment of T cells from peripheral blood to lung tissue,
and to the apoptosis of T cells induced by the virus [54]. COVID-19 patients
present with lymphocyte deficiency and over-activation of T cells. These effector
T cells are stimulated by pro-inflammatory mediators produced by DCs, macrophages
and neutrophils [55, 56]. A significant rise in HLA-DR+CD38+ cell levels can
manifest in the over-activation of T cells. The proportion of highly
pro-inflammatory CCR4+CCR6+ Th17 cells amongst CD4+ T cells then increases [57].
High expression of IL-17A in Th17 induces the migration of inflammatory white
blood cells, leading to inflammatory infiltration and destruction of lung tissue.
Additionally, the major histocompatibility complex 1 (MHC-1) of infected cells
presents viral antigens, thus activating CTLs to produce high levels of cytotoxic
granules such as perforin and granzymes. This implies that over-activation of T
cells and the elevated cytotoxicity of CD8+T cells leads to an excessive immune
response. T cell-derived cytokines and chemokines such as TNF-
Leng et al. [40] reported that on day 4 after MSC transplantation, the
absolute lymphocyte count increased to 0.58
The mechanism of action of MSCs in reversing lymphocytopenia and reducing
inflammatory mediators in COVID-19 is mainly attributed to the more than 30
soluble paracrine factors such as PEG2, IDO and COX-2 [61]. These have been shown
to inhibit the proliferation of CD4+ Th1 and Th17 cells as well as CD8+T cells,
and to induce Foxp3+ Treg differentiation (Fig. 1f). They also indirectly inhibit
excessive T cell proliferation by interacting with APCs and other immune cells.
IL-10 is a critical negative regulator of T cell responses and directly inhibits
the ability of T cells to produce pro-inflammatory mediators. IL-10 also reduces
the antigen presenting capacities and co-stimulation of macrophages and DCs,
thereby decreasing T cell-derived IL-6 and TNF-
Through their expression of PD-L1 and FasL, MSCs can inhibit abnormally
activated Th1 cells, thus inhibiting IL-
At the same time, MSCs release TGF-
ACE2 is down-regulated following the entry of SARS-CoV-2 into alveolar epithelial cells, resulting in an imbalance of ACE/Ang II/AT1R and ACE2/Ang 1-7/MASR. Elevated Ang-2 levels then lead to cell apoptosis and trigger inflammatory responses, giving rise to immune-induced tissue damage and increased vascular permeability [69, 70, 71] (Fig. 2b).
In COVID-19 patients the average time from symptom onset to dyspnea is 5 days,
the average hospital stay is 7 days, and the average time for onset of ARDS is 8
to 9 days [72]. By day 8 to 14 of disease onset, the overexpression of cytokines
such as IL-2, IL-6, IL-7, IP10, MCP1, MIP1A and TNF
MSCs and MSC-exosomes can effectively alleviate COVID-19-induced ARDS in a
dose-dependent manner by increasing alveolar fluid clearance and by improving
airway and hemodynamic parameters [76]. MSC-exosomes have been used as
intravenous infusion therapy for ALI and pulmonary fibrosis (PF) [23, 77, 78]. An
earlier study showed the exosomes release keratinocyte growth factor (KGF) and
Lipoxin A4 which act to prevent long-term lung damage caused by COVID-19 and to
promote tissue repair by activating Na+/K+ pumps [79] (Fig. 2d). Importantly,
MSCs have been shown to restore epithelial protein permeability, stabilize
endothelial fluid leakage, and maintain alveolar-capillary barrier function by
secreting Ang-1 [80, 81, 82]. In addition, MSCs can inhibit cellular signaling
pathways mediated by TLRs or PRRs, as well as reducing local immune cell
recruitment (Fig. 2c). miRNA-126, VEGF-
PF is a refractory lung disease that develops due to persistent alveolar injury, repeated destruction, repair, reconstruction, and excessive deposition of extracellular matrix (ECM) [83]. Current studies have determined that only 1% of AT2 cells can regenerate following SARS-CoV-2-induced lung injury [84, 85].
High expression of IL-17A by Th17 in COVID-19 can induce the migration of
inflammatory leukocytes, leading to inflammatory infiltration and the destruction
of lung tissue [86]. High levels of TNF-
Human embryonic stem cells (hESCs) derived from immune and stromal regulatory cells (IMRCs) have been used to treat lung injury and fibrosis in vivo. IMRCs have superior efficacy to FDA-approved pirfenidone [14] and show excellent efficacy and safety in both mice and monkeys [90].
MSC-exosomes are able to transfer cargoes such as mRNA, miRNA, proteins, lipids and even mitochondria to target cells and tissues, resulting in changes to gene expression and in the behavior of target cells. Hence, MSC-exosomes could have a therapeutic role in COVID-19 [91, 92]. Preclinical studies have confirmed that MSC-exosomes are able to serve as acellular alternatives [78].
A growing number of studies have established that the healing, nutritional, immunoregulation, and anti-inflammatory effects of administered MSCs are due to the exosomes they release. These effects of MSCs have been observed in vitro after the addition of MSC-exosomes [37]. MSCs were cleared from the circulation within 24 hours, but MSC-exosomes were detected in lung parenchymal cells and macrophages just 1 hour after injection and remained there for up to 7 days [93]. The efficacy and safety of a single intravenous injection of MSC-exosomes were recently assessed in 24 COVID-19 patients who presented with moderate to severe ARDS. The clinical symptoms, oxygenation, serum markers of acute inflammation, neutrophil and lymphocyte counts all improved in patients who received MSC-exosomes, with no side effects reported [18].
The immunomodulatory effects of MSC-exosomes have also been attributed to their
anti-inflammatory cargo, such as IDO, HLA-G, PD-L1, galectin-1, IL-10,
TGF-
MSC-exosome-transferred miRNAs cause APCs to produce fewer Ag/MHC molecules on their surface, thus resulting in reduced activation of effector T cells. miRNAs carried by MSC-exosomes also mediate the function of macrophages, NK cells, T cells and B cells to inhibit infection [98].
In the context of COVID-19, MSCs are known to aggregate in the peripheral microvasculature and exacerbate vascular clots, causing central or peripheral vascular dysfunction. This is probably because MSCs express procoagulant tissue factor (TF/CD142) on their surface [99, 100].
The small size and low immunogenic effect of MSC-exosomes allows them to pass through small blood capillaries without triggering a blood clot [101]. Because of their strong ability for self-replication and differentiation, the carcinogenicity of MSCs is also another clinical challenge. MSC-exosomes cannot replicate and hence there is no risk of endogenous tumor formation [102].
The challenges surrounding the use of MSCs for COVID-19 that still need to be overcome include their immuno-compatibility, stability, heterogeneity, differentiation and migration. The low homing rate of MSCs is also the focus of current research. Although Xiao et al. raised the possibility that CD90 binding to the specific integrins b3 and b5 could to some extent promote MSC homing [103], MSC-exosomes have an important advantage in their homing ability. Due to their nanosized dimension, intravenously injected MSC-exosomes accumulate in COVID-19-damaged organs through blood circulation [104]. MSC-exosomes from allogenic sources can also be used immediately after thawing and without washing. In addition, MSC-exosomes are easier to use routinely in hospitals compared to MSCs. Finally, the cost of using MSC-exosomes is much lower than that of MSCs.
Designing miRNAs that specifically bind to the SARS-CoV-2 genome could allow disruption of SARS-CoV-2 without any side effects on human gene expression [105]. Thus, MSC-exosomes that carry miRNAs may be a promising new approach to COVID-19 therapy. MSC-exosomes can be loaded with miRNAs either by direct insertion of the nucleic acids, or by collecting the exosomes from genetically-modified MSCs [106]. For example, miR-32, the first miRNA found to target viral RNA, binds to retrovirus PFV-1 transcripts to reduce viral replication [107], while miR-146a has been shown to specifically inhibit COX-2 in lung epithelial cells. miR-375 inhibits the trans-differentiation of myofibroblasts and their synthesis of collagen by blocking P38 [108].
MSC-exosomes are thus a novel intervention tool for COVID-19 treatment that can successfully deliver exogenous miRNAs to exert antiviral function. When combined with antiviral drugs such as Remdesivir, MSC-exosomes can therefore serve as an effective drug delivery system [109].
Spike protein is one of the structural proteins of SARS-CoV-2 that facilitates viral entry into the host cells. Therefore, spike protein is a good target for the development of anti-SARS coronavirus vaccines. Seraphin et al. showed that MSC-exosome-based vaccines containing the SARS-CoV-2 spike protein could induce high levels of neutralizing antibodies [17, 110, 111, 112].
Current treatment trials for COVID-19 include corticosteroids, PD-1/PD-L1 checkpoint inhibitors, cytokine absorption devices, convalescent plasma [113] and anti-malarial and antiviral drugs [114]. Definite clinical benefits from these treatments have yet to be established and their safety and efficacy still need to be validated through Phase II and III clinical trials.
However, clinical trials have shown that MSC therapy and its derivatives are
promising candidates for COVID-19 with known safety and efficacy. The United
States FDA has approved MSCs for severe COVID-19 patients as compassionate use
and progress has been made in this field. A study from Spain involving 13
COVID-19 patients requiring mechanical ventilation reported that no
treatment-related adverse events (TRAEs) were observed [115]. After the first
intervention with MSCs, clinical improvements were observed in 9 patients (70%)
after a median follow-up of 16 days. Seven patients were extubated and
discharged, while 4 patients continued intubation (2 with improved ventilation
and radiological parameters, and 2 with stable conditions). The research team
compared the clinical progress and mortality rates of their study cohort with
similar cases in the intensive care unit (ICU). The mortality rate of patients
who received MSC therapy dropped from 70–85% to 15% (2/13). Only 2 patients
died during the study, one from massive gastrointestinal bleeding unrelated to
the MSC treatment, and the other from secondary fungal pneumonia caused by
Candida spp. We searched for “COVID-19”, AND “exosome” OR “extracellular
vesicles” OR “mesenchymal stem cells” up to April 22, 2021. Clinicaltrial.gov had
83 registered trials for the clinical use of MSCs, MSC-exosomes or MSC-exosome.
Of these, 38 are ongoing and are recruiting patients. Nine trials had been
completed to that date (Table 2). Using similar methodology, 16 registered
clinical trials of MSCs for the treatment of COVID-19 were found in the Chinese
Clinical Trial Register (Chictr).cn (Table 3). One clinical trial enrolled 101
patients with severe COVID-19 lung injury. Patients received human umbilical cord
MSCs (HU-MSCs) (4
| NCT Number | Phase | Interventions | Outcome measures | Enrollment | Allocation |
| NCT04713878 | NA | Biological: MSCs | Change of clinical symptoms as respiratory distress or need for oxygen support | 21 | Randomized |
| Change of cytokine storm parameters | Parallel Assignment | ||||
| Change of pulmonary functions | Open Labe | ||||
| Change of clinical symptoms | Primary Purpose: Treatment | ||||
| NCT04288102 | 2 | Biological: UC-MSCs | Change in lesion proportion (%) of full lung volume from baseline to day 28 | 100 | Randomized |
| Biological: Saline containing 1% Human serum albumin | Change in ground-glass lesion proportion (%) of full lung volume | Parallel Assignment | |||
| Time to clinical improvement in 28 days | Masking: Quadruple | ||||
| Oxygenation index | Primary Purpose: Treatment | ||||
| NCT04573270 | 1 | Biological: PrimePro | Survival Rates | 40 | Single Group Assignment |
| Other: Placebo | Contraction Rates | Masking: Triple | |||
| Primary Purpose: Treatment | |||||
| NCT04355728 | 1/2 | Biological: UC-MSCs + Heparin along with best supportive care. | Incidence of pre-specified infusion associated adverse events | 24 | Randomized |
| Other: Vehicle + Heparin along | Incidence of Severe Adverse Events | Parallel Assignment | |||
| with best supportive care | Survival rate after 90 days post first infusion | Masking: Triple | |||
| Ventilator-Free Days | Primary Purpose: Treatment | ||||
| Change in Oxygenation Index (OI) | |||||
| C-Reactive Protein levels | |||||
| NCT04522986 | 1 | Biological: MSCs | Safety: Adverse Event | 6 | Single Group Assignment |
| Open Labe Primary Purpose: Treatment | |||||
| NCT04535856 | 1 | Drug: allogeneic MSCs | Incidence of TEAE in Treatment group | 9 | Randomized |
| Other: Placebo | Survival rate | Parallel Assignment | |||
| Duration of hospitalization | Masking: Quadruple | ||||
| Clinical improvement Ordinal scale | Primary Purpose: Treatment | ||||
| Clinical improvement Oxygenation index | |||||
| Inflammation markers change | |||||
| NCT04276987 | 1 | Adverse reaction (AE) and severe adverse reaction (SAE) | 24 | Single Group Assignment | |
| Biological: MSCs-derived | Time to clinical improvement (TTIC) | Open Labe Primary Purpose: Treatment | |||
| exosomes | Number of patients weaning from mechanical ventilation | ||||
| Duration (days) of ICU monitoring | |||||
| Duration (days) of vasoactive agents usage | |||||
| Rate of mortality | |||||
| NCT04492501 | NA | Procedure: Therapeutic Plasma exchange | Survival | 600 | Non-Randomized |
| Biological: Convalescent Plasma | Duration of hospitalization | Factorial Assignment | |||
| Drug: Tocilizumab | Time to resolution of cytokine release storm | Masking: Open Label | |||
| Drug: Remdesivir | Time of viral clearance | Primary Purpose: Treatment | |||
| Biological: MSCs | Complications | ||||
| NCT04491240 | 1/2 | Drug: EXO 1 inhalation | Adverse Events | 30 | Randomized |
| Drug: EXO 2 inhalation | Time to Clinical Recovery (TTCR) | Parallel Assignment | |||
| Drug: Placebo inhalation | SpO2 Concentration | Masking: Double | |||
| LDH | Primary Purpose: Treatment | ||||
| NA, Not Applicable. | |||||
| ChiCTR number | Biological | Interventions | Phase | Enrollment | Registration date |
| ChiCTR2000031430 | umbilical cord | MSCs + Routine treatment | 2 | 200 | 2020/2/5 |
| Routine treatment | |||||
| ChiCTR2000030835 | umbilical cord | High dose group: routine treatment + MSCs (2 × 10 |
NA | 20 | 2020/3/15 |
| Low dose group: routine treatment + MSCs (1 × 10 |
|||||
| ChiCTR2000030866 | umbilical cord | MSCs based on conventional treatments | NA | 30 | 2020/3/16 |
| ChiCTR2000030261 | exosomes | Aerosol inhalation of exosomes | NA | 13 | 2020/2/26 |
| Blank | 13 | ||||
| ChiCTR2000030088 | Wharton’s Jelly | Iv injection of Wharton’s Jelly MSCs (1 × 10 |
NA | 40 | 2020/2/22 |
| saline | |||||
| ChiCTR2000030020 | MSCs | MSCs therapy | NA | 20 | 2020/2/20 |
| ChiCTR2000029580 | Ruxolitinib combined | Ruxolitinib combined with MSCs | NA | 70 | 2020/2/5 |
| with MSCs | Routine treatment | ||||
| ChiCTR2000029990 | MSCs | MSCs therapy | 1–2 | 60 | 2020/2/18 |
| saline | 60 | ||||
| ChiCTR2000030116 | MSCs | MSCs in dose 1 | NA | 8 | 2020/2/1 |
| MSCs in dose 2 | 8 | ||||
| ChiCTR2000030138 | UC-MSCs | UC-MSCs | NA | 30 | 2020/2/24 |
| Routine treatment + placebo | 30 | ||||
| ChiCTR2000030173 | UC-MSCs | UC-MSCs | NA | 30 | 2020/2/17 |
| Routine treatment | 30 | ||||
| ChiCTR2000030484 | HUMSCs | HUMSCs: intravenous infusion, 5 × 10 |
NA | 30 | 2020/2/2 |
| HUMSCs: intravenous infusion, 5 × 10 |
30 | ||||
| The same amount of placebo (stem cell solvent) | 30 | ||||
| ChiCTR2000030944 | NK cells and MSCs | NK cells and MSCs + Routine treatment | 1 | 10 | 2020/9/1 |
| Routine treatment + placebo | 10 | ||||
| ChiCTR2000031319 | Human dental pulp | Human dental pulp stem cells + Routine treatment | 1 | 10 | 2020/4/1 |
| stem cells | Routine treatment + placebo | 10 | |||
| ChiCTR2000031430 | MSCs | MSCs + Routine treatment | 2 | 100 | 2020/3/20 |
| Routine treatment | 100 | ||||
| ChiCTR2000031494 | MSCs | MSCs + Routine treatment | 1 | 18 | 2020/2/1 |
| Routine treatment | 18 | ||||
| NA, Not Applicable. | |||||
Compared with MSCs, MSC-exosomes have the ability to transmit and exchange
intracellular chemical information. However, MSC-exosomes have received less
attention than MSCs in COVID-19 research to date. At April 22, 2021, only two
clinical trials using MSC-exosomes to treat COVID-19 had been completed
(NCT04276987 and NCT04491240; see Table 2). Preliminary results of NCT04491240
released on 21 October 2020 showed that compared to placebo, the clinical
recovery time, C-reaction protein (CRP) and layered double hydroxide (LDH) levels
were lower for 10 consecutive days after inhalation of a solution containing
0.5–2
For these reasons, MSC-exosomes are a highly promising, cell-free therapy for COVID-19 [124, 125]. The U.S. Food and Drug Administration has in fact allowed the expanded use of MSC-exosome preparations for the treatment of COVID-19 [126]. These include aerosol inhalation of MSC-exosomes, targeted drug delivery based on MSC-exosomes, and the development of MSC-exosome-based vaccines [127, 128]. However, a phase 3 trial is needed to further evaluate the effects of MSC-exosomes on mortality and long-term lung dysfunction from COVID-19.
COVID-19 treatment is currently very challenging, especially because of its complications and sequelae. Intravenous MSC administration or inhalation of MSC-exosomes can improve the overall prognosis for COVID-19 by a variety of mechanisms: (1) through their immune regulation, (2) by promoting tissue repair and regeneration, (3) through their anti-fibrosis effect, and (4) by resuming normal vascular permeability. All these mechanisms can interact to strengthen lung repair and to protect the organs from damage caused by the excessive immune response. Despite the readily available sources, high proliferation rate, minimally invasive or noninvasive administration, and no ethical concerns, several challenges remain to be addressed with MSC and MSC-exosomes therapy. In particular, the dosing and timing of MSC and MSC-exosome therapy require careful consideration, since improper use may aggravate immunosuppression and lead to an unfavorable prognosis for COVID-19.
JM contributed to the conception of the study and led to the submission. XC performed the tables and wrote the manuscript; LL helped perform the figures with constructive discussions; MJ contributed significantly to manuscript preparation; All authors approved the final version.
Not applicable.
Thanks to all the peer reviewers for their opinions and suggestions.
This work was supported by The National Natural Science Foundation of China (NSFC) [grant numbers 82070070].
The authors declare no conflict of interest.
ACE2, angiotensin-converting enzyme 2; ADAMTS2, ADAM Metallopeptidase With
Thrombospondin Type 1 Motif 2; Ang-2, Angiopoietin-2; APCs, Antigen presenting
cells; ARDS, acute respiratory distress syndrome; AZD1222, Oxford-AstraZeneca
1,2’s Chadox1 nCoV-19; bFGF, basic fibroblast growth factor; COL15A1, Collagen
15A1; COL-I, collagen-1; COVID-19, Corona Virus Disease 2019; COX-2,
cyclooxygenase-2; CRS, cytokine release syndrome; CRP, C-reaction protein;
CXCL10, C-X-C Motif Chemokine Ligand 10; DC, Dendritic cells; ECM, extracelluar
matrix; FDA, Food and Drug Administration; G-CSF, granulocyte colony stimulating
factor; hESCs, Human embryonic stem cells; HGF, human growth factor; HMGB1, High
mobility group box chromosomal protein 1; HLA, human leukocyte antigen; HO-1,
heme oxygenase-1; HTRA1, high temperature requirement A1; HU-MSCs, human
umbilical cord mesenchymal stem cells; ICU, intensive care unit; IDO, indoleamine
2,3-dioxygenase; IFN, interferon; IgM, immunoglobin M; IL, interleukin; IMRCs,
immune and stromal regulatory cells; IP-10, interferon gamma induced protein-10;
KGF, keratinocyte growth factor; LOX, lipoxygenase; LDH, layered double
hydroxide; MCP-1/CCL2, Monocyte chemoattractant protein-1; MHC-1, major
histocompatibility complex 1; MIP-1, Macrophage inflammatory protein-1; MMPs,
matrix metalloproteinases; mRNA, messenger RNA; MSCs, Mesenchymal stem cells;
MSC-EVs, MSC-derived extracellular vesicles; NLRP3, NOD-like receptor protein 3;
PGE2, prostaglandin E2; PIK3R2, phosphoinositide-3-kinase regulatory subunit 2;
SARS-COV-2, severe respiratory syndrome coronavirus 2; SOD, superoxide dismutase;
TGF-
