Alzheimer’s, a progressive neurodegenerative disease affects brain and neurons through enormous reduction in nerve cell regenerative capacity. Dementia and impairment of cognitive functions are more prevalent in Alzheimer’s disease (AD) patients in both industrialized and non-industrialized countries. Various factors play significant role in molecular cascades that leads to neuronal inflammation, dementia and thereby AD progression. Current medications are symptomatic that alleviates pain while lack in absolute cure, urging researchers to explore targets and therapeutics. Interestingly, nanomedicines developed due to the onset of nanotechnology, are being extensively investigated for the treatment of AD. This review presents the advancement in nanotherapeutic strategies, involving the emergence of nanomaterials that offers advantage to pass through the blood-brain barrier and acts as a therapeutic modality against AD.
The neurodegenerative disorder, AD is the sixth leading cause of death that affects 5.8 million Americans and is estimated to reach 14 million by 2050. According to the Alzheimer’s association, AD or associated dementia victimize 1 in 3 senior patient which is higher than cancer disease (breast and prostate cancer) [1]. The economic burden levied by this disease and other form dementia is expected to rise to 1.1 trillion dollars by 2050 from 290 billion dollars in 2019 [1]. AD adversely progresses with age as the major risk factor, the disease doubles exponentially every five years after the age of 65 [2, 3, 4]. The persistent lacuna remains in the diagnosis of AD that fails to establish a full-proof diagnosis except for post-mortem identification of AD characteristics such as NFTs and SPs [5, 6]. Premortem reports of the neurological, cognitive and neurophysiological tests as well as in vivo brain imaging in addition to patient’s clinical history, have proven to provide maximal accuracy of 85% [5]. The trajectory of AD begins with healthy aging, preclinical AD that progresses to MCI and ultimately leads to dementia. These stages in trajectory are distinguished by associated symptoms represented in Fig. 1A. Though, the disease remains difficult to be diagnosed and distinguished between healthy aging, AD patients are characterized with specific hallmark features of brain.
 Fig. 1.
Fig. 1.Alzheimer disease and its pathogenesis. (A) Characteristic symptoms at various stages of AD. (B) The hallmarks and causatives of Alzheimer disease.
The cascade of AD resulted in progressive loss of cognitive function of the
brain. This impairment further leads to dysfunction of nerve synapsis. The
principal cause of AD on genetic evaluation found to be amyloid
Next, the blood vessels play a vital role in delivering oxygen rich blood and nutrients to all the tissues and organs of the body. The central nervous system (CNS) is also vascularized by the blood vessels that hold a unique property of allowing movement of ions, molecules, and cells in a regulated manner between the blood and the brain. This tight regulation having the unique property is termed as blood-brain barrier (BBB). The BBB maintains the homeostasis of the CNS and thus helps in proper functioning of neurons and protecting the tissues making up the neurons from toxins and pathogenic attack. It also helps in preventing the progression of various neurological diseases [17]. The BBB is known to have a major impact in the AD pathogenesis. It is a highly selectively semipermeable membrane which acts as a structural and chemical barrier to prevent the entry of any foreign substance that aims to invade the brain tissues. Dysfunction of BBB is known to induce the hindrance or failure in transporting beta-amyloid protein from brain to the peripheral circulation through the BBB [18].
Nanomaterials have been extensively used in the field of medicine and healthcare
over the past two decades because of their tiny size and their extraordinary
characteristics. Such nano-sized materials have been fabricated into various
nanoparticles (NPs) that can cross easily BBB. These NPs have the ability to act
on molecular structures and cellular components. These structures may be nucleic
acids, cellular membranous tissues, proteins, and peptides causing unexpected
changes in the functioning of biological processes in cells and tissues. The
therapeutic approach based on NPs is gaining attention continuously [19]. In AD,
the amyloid-
In this review, we comprehensively discussed different hallmarks of brain that are affected during AD progression, the role of various factors in the pathological development of AD, therapeutic modalities in the treatment of AD, and different types of nanomaterials used to deliver drugs via crossing BBB to the brain of AD patients.
Fig. 1B shows the schematic representation of the various causes and hallmarks
associated with AD. Two cellular features are hallmarks of an AD patients’ brain:
formation of Amyloid Plaques and formation of Tau Protein tangles. Amyloid
precursor protein (APP) is a transmembrane protein which is cleaved by two
enzymes
Cerebral lipids are one of the major biological components of brain constitutes
of about
The significance of lipids in AD came to light following the identification of apolipoprotein E (ApoE), variant E4, one of the prominent genetic risk factors for AD [24]. It has been shown to play a key role in the transport of lipids and metabolic pathways associated with it. Genome-wide association studies revealed that the list of other genes involved in lipid metabolism, which are connected with AD pathology. To name a few, there are APOC1, CLU, APOC2, APOC4, ABCA7, ABCA1 and many others [25]. Alterations in fatty acids at the level of lipid rafts and cerebral lipid peroxidation were found at the early stage of AD [26].
Dysregulated lipid metabolism is the most common symptom for Late-onset AD. This was concluded based on studies with fibroblast and peripheral blood mononuclear cells of peoples affected by AD [27]. More importantly, the amyloid precursor protein has been shown to regulate the pathways that are central to lipid synthesis, mainly cholesterol [27]. Among the omega-3 fatty acids, the levels of docosahexaenoic acid (DHA) in hippocampus region of brain were found to be reduced in AD patients [28]. Besides, the levels of numerous fatty acids found to change with onset of AD [23].
Proteins are known to bind to essential metal cofactors and its binding to
protein is very competitive. For maintaining neuronal functions, it is important
to regulate the homeostasis of metal ions. At the same time, heavy metals are
known to induce epigenetic changes and the AD associated pathological conditions
[29]. Dysregulated metal homeostasis and exposure to toxic metals such as
mercury, lead, aluminium, and cadmium aggravate the pathogenesis of AD [30].
During the progression of AD, there exists a good connecting link between the
imbalance in the biologically significant metals such as magnesium, zinc, copper,
calcium, manganese, iron and the abnormal expression of genes that code for
endogenous proteins to carry out the metal transport [30]. It is also known that
metal ions augment the reactive oxygen species production in the brain, which
hinder the functions of neurons. Alternatively, fluctuations in the metal ion
concentration have been shown to affect the A
De Toma and coworkers gave an elaborative review describing the interactions of
metal with amyloid peptides and islet of amyloid polypeptide (IAPP) that affect
the structural, catalytic and signaling function of the body. Various essential
metal ions as copper and zinc helps in neural synapsis and the balance of these
metals should be maintained for the proper functioning of the brain. Higher
concentration of metal ions reported in amyloid plaques found in AD about 15
Macromolecular crowding is an important aspect shown to influence the A
As an attempt to combat the AD, novel therapeutic approaches mainly the
development of (i) small molecule inhibitors which blocks
oligomerization step and (ii) catalytic antibodies for the hydrolysis of
A
The lack of no full-proof treatment and diagnosis against this dreadful disease urges the researchers to explore various therapeutic approaches that focus to ease the diagnosis and therapy for AD [48]. The current pharmacological and non-pharmacological therapies adopted by physicians aim at alleviating the symptoms and improving the quality of life in patients [49]. The clinical trials underway mainly targets towards symptomatic therapy, by attaining minimum production and reduction of pathology within brain [50]. The ideal hallmark target that effectively halts or slows down the progression of neurological disease is still under investigation [51].
Nanomaterials have gained considerable attention because of their relevant characteristics such as biocompatibility, and low toxic nature. Besides, these nanomaterials can be tailored by facile chemical modification to impart unique and desirable properties suitable for biomedical applications [52, 53, 54]. Nanotechnology promisingly revolutionizes drug manufacturing, drug delivery, medical diagnostics and treatments. Targeting of the drug and enhanced safety profile is the prime advantage of using NP approaches [55]. Further, in the next section, we describe the various nanomaterials including magnetic NPs, dendrimers, liposomes, carbon nanotubes, nanopores, and fullerene to combat AD progression.
With the advent of nanotechnology, the wide use of NPs as front-line tool in biomedical sciences is vastly recognized. In the last decade, a wide spectrum of organic and inorganic nanomaterials based nanocarriers viz., fullerenes, carbon nanotubes, quantum dots (QDs), dendrimers, liposomes (LIPs), magnetic NPs have been investigated, as potential means for targeted drug delivery, diagnostics, tissue regeneration, cell culture, biosensors, etc., in the field of biomedicine [56]. These nanocarriers can easily cross the blood brain barrier (BBB) or bypass the BBB and reach to the target region in the brain of AD patients. Due to which, these nanosized vehicles may display their improved clinical outcomes (Fig. 2). The importance of these nanomaterials in the design and development of therapeutic agent against the progression of the AD is discussed in the next section and summarized in Table 1 (Ref. [19, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75,]).
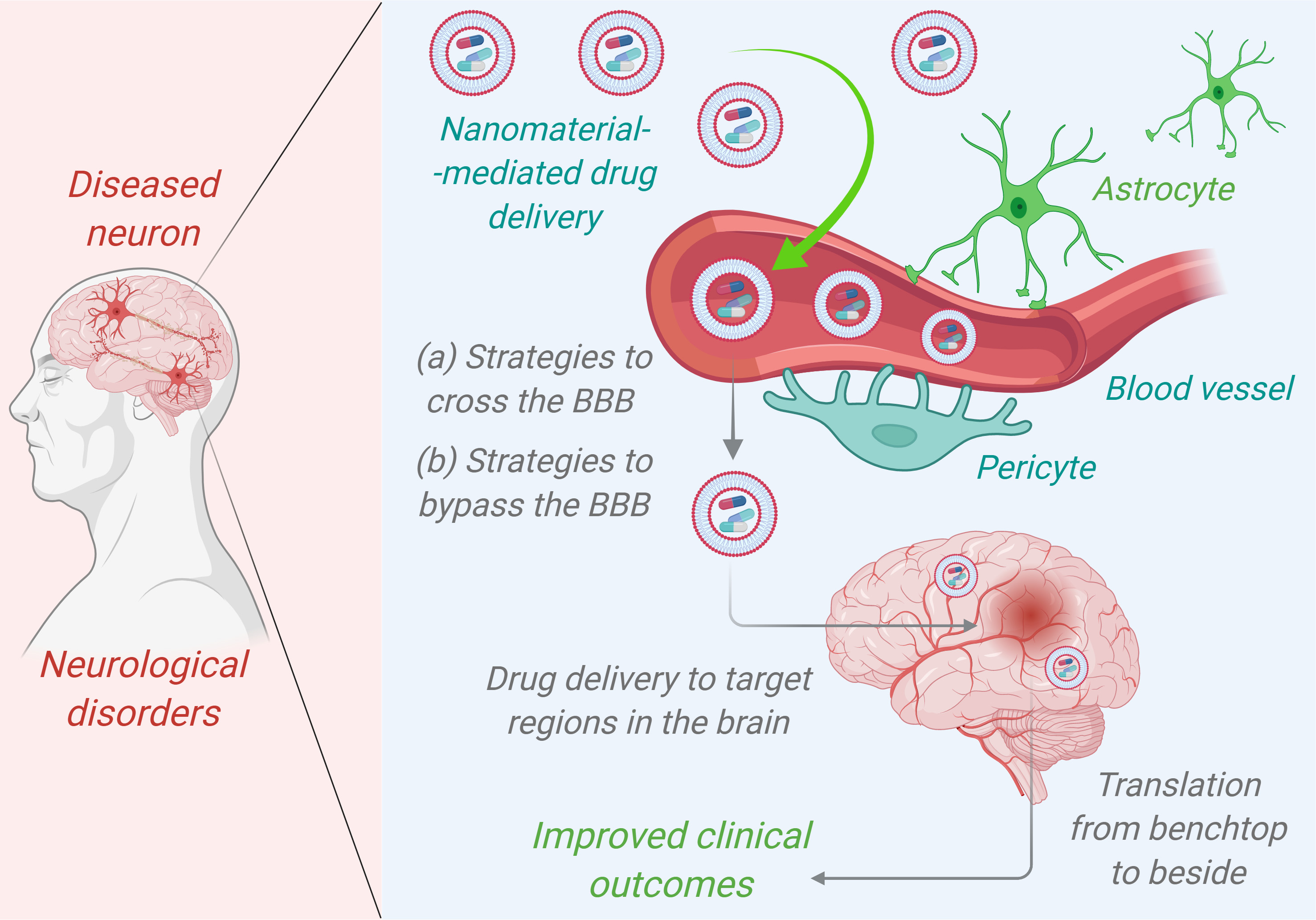 Fig. 2.
Fig. 2.Nanomaterials mediated drug delivery of therapeutic agents in targeting Alzheimer disease patients’ brain to improve clinical outcomes.
| Nanodrug carrier | Anti-AD drug | Preclinical or clinical | References |
| Polymeric Nanoparticles | |||
| PLGA-b-PEG | Galantamine | In vitro & In vivo | [57] |
| PLGA | Donepezil | In vitro | [58] |
| PLGA | Withaferin | In vitro | [59] |
| PEG–PLGA | Memantine | In vitro & In vivo | [60] |
| PAAM-Cardiolipin-PLGA | Rosmarinic acid & Curcumin | In vitro | [61] |
| Solid-lipid Nanoparticles | |||
| SLN-DSPE-ApoE | Resveratrol | In vitro | [62] |
| SLN-Palmitate-ApoE | |||
| S80-, PS-, PA-SNP | Nicotinamide | In vitro & In vivo | [63] |
| S80-SNP | Piperine | In vitro & In vivo | [64] |
| Liposomes | |||
| mApoE-PA-LIP | Modified ApoE-derived peptide | In vitro & In vivo | [65] |
| GSH-PEG-EYPC-LIP | VHH-pa2H | In vitro & In vivo | [66] |
| CPP-LIP | Rivastigmine | In vitro & In vivo | [67] |
| Carbon Nanotubes | |||
| MWCNTs | Berberine | In vitro & In vivo | [68] |
| Dendrimers | |||
| PAMAM-Lf | Memantine | In vitro & In vivo | [19] |
| PAMAM-DG4.0, DG4.5 | Tacrine | In vitro & In vivo | [69] |
| Pyridylphenylene | —— | In vitro | [70] |
| Magnetic Nanoparticles | |||
| MNPs-PEG-PLA | Curcumin | In vitro & In vivo | [71] |
| Au NPs | Anthocyanin | In vitro & In vivo | [72] |
| Nanodiscs | |||
| 4F Nanodiscs | —— | In vitro | [73] |
| rHDL-rApoJ Nanodiscs | rApoJ | In vitro & In vivo | [74] |
| Carbon Dots | |||
| CUR-Fe |
Curcumin | In vitro | [75] |
| CDs, Carbon Dots; CPP, Cell penetrating peptide; CUR, Curcumin; DG, Dendrimer generation; DSPE, 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine; EYPC, Egg yolk phosphatidylcholine; GSH, Glutathione; Lf, Lactoferrin; LIP, Liposomes; mApoE, Modified ApoE protein; MNPs, Magnetic Nanoparticles; MWCNTs, Multi-walled carbon nanotubes; PA, Phosphatidic acid; PAAM, Polyacrylamide; PAMAM, Poly(amidoamine); PEG, Poly(ethylene glycol); PLA, Poly(lactic acid); PLGA, Poly(lactic-co-glycolic) acid; PS, Phosphatidylserine; rApoJ, Recombinant apolipoprotein J; rHDL, Recombinant high-density lipoprotein; S80, Polysorbate 80; SLN, Solid lipid Nanoparticles; VHH-pa2H, Amyloid beta binding llama single domain antibody fragments. | |||
Buckyballs or Buckminster fullerenes is a carbon allotrope with diameter of
about 7Å constituting 60 carbon atoms in a geometry called truncated
icosahedrons [76]. Depending on application, fullerenes are classified into three
types: endohedral metallofullerenes, exohedral fullerenes and heterofullerenes.
Endohedral metallofullerenes is composed of radioactive metal within the
Buckyball and used for diagnostic purposes such as magnetic resonance imaging
(MRI) and other imaging procedures employing radiocontrast media. Being less
toxic and safer, these can be utilized as radioactive tracers for imaging organs
[77]. Exohedral fullerenes are produced by chemical reaction between fullerenes
and other chemical entities. These are derived from certain modifications of
fullerene, also known as functionalized fullerenes. These are used as
photosensitizers in photodynamic therapy where it produces harmful reactive
oxygen species on stimulation by light, induced the programmed cell death
[78, 79, 80]. Heterofullerenes contain other atoms like boron, nitrogen and few others
in the place of one or more carbon present in fullerene compounds. A large number
of conjugated double bonds present in the core of the fullerenes which scavenge
free radicals and protect mitochondria from the attack by free radical species
[81]. Oxidative stress induced as a result of free radicals generates
demyelination of neurons, mitochondrial dysfunction, damage to microtubules, and
apoptosis [82]. Ehrich et al. [83] investigated nanomaterials made of
fullerene derivatives and showed the potential to counteract the toxic effect
produced by organophosphatase-induced AChE inhibition, suggesting the antioxidant
property. Furthermore, fullerenes are believed to activate the host immune
response and generate antibodies specific to fullerenes [84]. REMD simulation
studies by Xie et al. [85] proved that C-60 fullerene NPs (where molar
ratio of fullerene: peptide was greater than 1:8) has immense ability to halt
Nanotubes are tube-like structures on which graphite is rolled and buckyballs
present either at one or both the ends. These are of two types: SWCNT with an
internal diameter of 1–2 nm and MWCNT having 2–25 nm diameter and 0.36 nm
spacing between the two layers. Its length is of few micrometers [87]. Nanotubes
enter the cell membrane through endocytosis or direct insertion or diffusion
phenomena. To make it accessible within the cell, facile carboxylic or ammonium
group can be introduced over this tube-like nanostructure. Thus, it serves as a
means to deliver peptides, nucleic acids and other therapeutic molecules into the
cell. Nanotubes have been explored in gene silencing therapy by conjugation of
siRNA to the nanotube. It is advantageous over other means of transfer since it
is non-immunogenic. Another way of targeting specific diseased cell is by
conjugating antibodies along with radiolabeled or fluorescent tagged isotope
[88, 89, 90]. SWNTs which were actually F-CNTs have been used by Yang et al.
[91] to target the brain cells. It was orally administered to mice for continuous
10 days. When observed under electron microscopy, SWNTs were present in traces in
absorptive cells, macrophages and neurons as well as in other organs such as
heart, liver and brain. Improvement in learning and memory and other cognitive
functions were observed as a result of acetylcholine transport with the help of
SWNTs in a mouse model with induced AD. MWCNTs loaded with berberine (BRB) and
coated with polysorbate and phospholipid formed complex of 186 nm, showed
exceptional reclamation of memory up to 201th day. This complex maintained the
biomolecules level in brain and thereby reduces A
QDs (2–10 nm) are composed of core-shell made of inorganic substances while
aqueous organic substance serve as coating to which biomolecule can be attached
that has the ability to target several biomarkers. Upon activation by light, it
emits fluorescence light and size of this nanocrystal determines the color of
fluorescence [92]. Functionalization of QDs increases the particle size that
restricts it to cross and filter through renal capillaries, and thus failed to
get eliminated in order to overcome the toxicity of accumulation of QDs within
body. In this regard, in vivo studies related to the metabolism and
excretion of QDs are scarce [92]. Quan et al. [93] designed quantum dot
nano-vehicle that have the ability to target surface cells and plays an important
role in the detection of AD. Nanoformulated probe comprise of fluorescent QDs
producing red light from the core which is enclosed in a shell made of
polyethylene glycol (PEG)-conjugated with benzotriazole (BTA). This QD-PEG-BTA
probe has shown 4 times more sensitivity for the detection of AD when compared to
the conventional thioflavin derivatives. The success rate is high due to the
fusion of high impact red fluorescence, presence of multivalent binding, and
reduced background signal and non-specific binding. As a result, QDs impart
increased sensitivity for the detection of amyloid-
Delivery of SPMNs under the influence of magnetic field have shown profound
applications in non-invasive MRI. Certain magnetic nanoparticles (MNPs) have
shown the potential to cross few biological and physical barriers, like BBB
studied using molecular dynamics (MD) approach and deduced the association
between BBB and NPs [96]. In a finding by Pansieri et al. [97] MNPs were
investigated as a tool to efficiently diagnose the amyloidosis through imaging
the amyloidogenic plaque or fibril depositions. This technique was reported to be
safe and non-toxic when used under optimized conditions. However, the assessment
of free or functionalized MNPs for biocompatibility with medical relevance
remains to be investigated [97]. Nasr et al. [98] worked to achieve AD
diagnosis in vivo and designed magnetic nanoparticle which cross BBB and
detect the presence of A
Dendrimers are tree-shaped nanosized structure comprises of three parts viz., central core, branches and functional groups which are present at the outer surface of macromolecule. These functional groups determine the efficacy of macromolecular complex which is composed of nucleic acid or entrapped drug. Dendrimers are multivalent molecules with a definite size and known structure with flexible features to modify the surface functional moieties to meet the requirements [100, 101]. It has lower viscosity as compared to the linear polymer equivalents and also exhibited good water solubility due to the presence of hydrophilic functional moieties on the surface. Further, engineering dendrimers with diverse chemical modification such as organic and inorganic groups at the branched site are also known [101, 102, 103]. As a replacement to conventional viral vectors, dendrimers are used for gene therapy. Dendrimers have shown promising results when tested in mammalian cell types and animal models. It enters the cells by endocytosis and transports DNA into nucleus for transcription of the desired gene and gene product [56]. The interesting advantage of dendrimer-based therapy is that it lacks the stimulation of immune reaction [56, 68].
Considered to be the original model of drug delivery vehicles, spherical in
shape, composed of lipid bilayer membrane, may be unilamellar or multilamellar
having aqueous interior environment, liposomes (LIP) hold a promising approach
against AD. LIP facilitate loading of hydrophilic drug in aqueous compartment and
lipophilic drug in LIP’s membrane for rapid delivery and efficacy (Fig. 3) [104].
To overcome the macrophage attack and opsonization in in vivo system,
LIPs are coated by a layer of biomaterial with stealth properties such as
polyoxyethylene, cholesterol, polyvinylpyrollidone polyacrylamide lipids,
distearoyl phosphatidylcholine to form stealth LIPs. These coatings enhance the
duration of drug action by prolonging its circulation time and protecting from
immune attack [105, 106, 107, 108, 109, 110]. Some of the modified LIPs include immuno-LIPs,
antibody-directed enzyme–prodrug therapy (ADEPT), and ligand bearing LIPs. In
principle, LIPs are conjugated with an antibody targeted against the desired site
and enzymes that activates prodrug and ligand specific for the target structure.
It offers advantages like reduction in undesired effects and harm to normal
cells, increases targeted drug delivery thereby boosts the drug’s efficacy and
safety level [111, 112, 113, 114]. Owing to the failure of conventional small drugs or
biological molecules to reach clinical trials, targeted nano-LIPs as drug
delivery vehicle are promising modalities for AD [115]. So far, it has not reached
clinical trials but it is found to be biocompatible, flexible with excellent
property of carrying various types of therapeutic agents to cross the BBB and
reach brain cells. LIPs can be designed for single therapeutic target or multiple
pathways/cascades as targets. Various transformations utilizing peptides that can
cross BBB, combined LIP-ligand complex involving phosphatidic acid, curcumin, and
a retro-inverted peptide have been designed to target and inhibit A
 Fig. 3.
Fig. 3.Liposomal nanodrug carrier in the rapid delivery of both hydrophilic and hydrophobic drugs targeting Alzheimer disease brain.
Kuo et al. [117] synthesized LIP containing cardiolipin and phosphatidic acid which provides target specificity against tau protein in hyperphosphorylated state. Trans-activator of transcription (TAT) peptide facilitated the ease of transport across BBB. LIP was loaded with NGF, rosmarinic acid (RA), curcumin (CURC), quercetin (QU), and phospholipid. The optimized TAT-NGF-RA-CURC-QU-CL/PA-LIP complex was found efficient in downregulating the expressions of pERK1/2 under phosphorylated state which is controlled by external signals such as c-Jun protein kinase present at N-terminal, p38, tau protein found at serine 202 and Caspase 3. This complex also enhanced the expression of p-ERK5 and p-cyclic adenosine monophosphate response element-binding protein [117]. Thus, LIPs are a promising delivery vehicle that pass-through BBB and protect nerve cell against the accumulated amyloid plaques.
Nanodiscs are a disc type structure having potential applications in proteomics
and biomedicine. It is around 7–50 nm in diameter and consists of two main
components: (i) phospholipids which are either of artificial origin or from the
cell membrane and (ii) stabilizing agent which is belt shaped and holds the
phospholipids together. Stabilizing agents can be protein or synthetic polymers
[118]. Nanodiscs aims to mimic the cellular phospholipids for structural and
functional studies of target molecules which are membrane proteins and peptides
including amyloids. Membrane proteins and membrane interacting peptides are
involved in numerous vital biological processes and are important targets for
drug development [119]. There is a great utility of nanodiscs in the study of
cellular signaling processes assembling on a membrane surface, by providing a
well-defined and structured bilayer surface. Klein developed nanodiscs that allow
unbiased high throughput screens that target binding sites for
Alzheimer’s-associated A
Carbon dots (CDs) are 0D carbon-based fluorescent nanomaterials less than 10 nm
in size and are generally classified into carbon quantum dots (CQDs), carbonized
polymer dots (CPDs), graphene quantum dots (GQDs) and carbon nitride dots (CNDs)
[122]. CDs were first discovered in 2004 during the purification of SWCNTs via
preparative electrophoresis [123]. Synthetic methodologies of CDs consist of
top-down and bottom-up approaches with optimized conditions and
precursors. CDs have demonstrated the abilities to penetrate the BBB due
to their special characteristics, such as low toxicity, high biocompatibility,
surface functional group modifications, excellent photoluminescence (PL), and
size distribution [124]. CDs have been surface functionalized with amine
and carboxyl groups to conjugate with various CNS drugs and also act as carriers
to deliver drugs into the CNS to treat AD [125]. Recently, Kuang et al.
[75] fabricated CUR-Fe
AD remain as the prime cause of dementia which has many uncommon risk factors and pathologies associated with it. The research progress directed towards unravelling the disease mechanism and developing therapeutics against AD has been remarkable. Integrative analysis of AD diagnostic pathways that vary between patients affected by different causatives is warranted for better understanding of the underlying mechanisms. Identification of AD biomarkers and other observable pathological mechanism such as aberrant inflammation, processing of beta-amyloid protein and tau proteins, neurotrophic functions, etc. enables development of advanced and new approaches that pave way for the early diagnosis and also to identify the most appropriate targets for therapy. The therapeutic efficacy of various inhibitors, antibodies, and other modalities have been limited due to BBB. To overcome this limitation, various nanoformulations have been designed and investigated their crossing across BBB and studied their therapeutic efficiency against AD (Fig. 4).
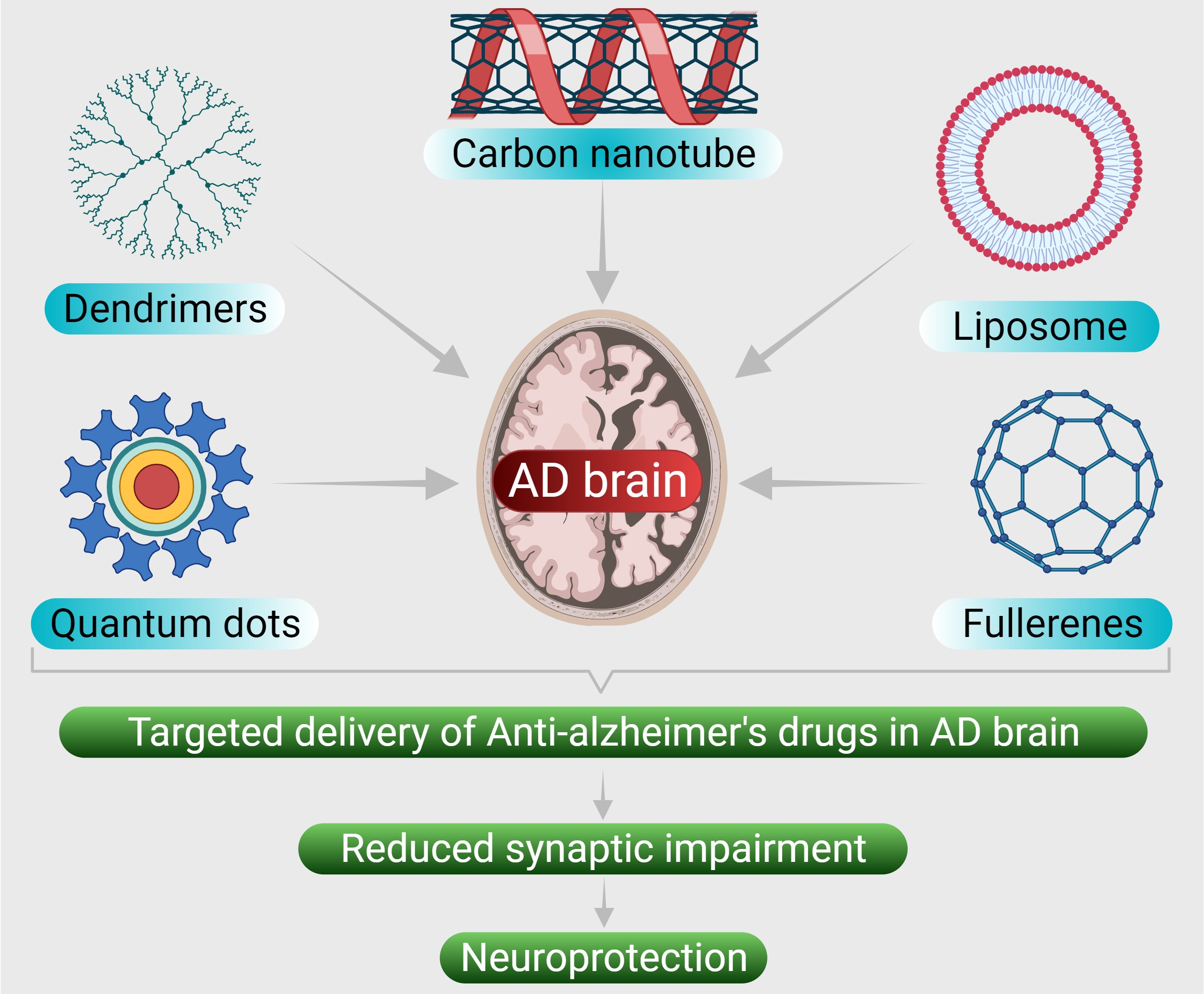 Fig. 4.
Fig. 4.Various nanoformulations that have been designed to deliver the anti-AD drugs in Alzheimer disease brain.
Further, the other major limiting factor in AD research is the lack of appropriate animal model that can be assigned as closely mimicking the human AD, which is imperative to evaluate the clinical performance of the designed nanoformulations targeted against AD. Due to this ongoing limitation, the translation of AD targeted nanoformulation-based drug delivery systems to clinics is delayed. Thus, successful translation of AD therapeutic modality prerequisite development of animal model that meticulously investigates the therapeutic potential as well as serve to apprehend the complex disease mechanism of AD. Systematic clinical studies involving animal models and humans conducted under the regulatory framework would be vital to collect information about the efficacy, toxicity and pharmacological aspects of these nanoparticle-based AD therapeutics.
MF and MAG contributed in the collection of the literature, writing and editing the manuscript drafts. SA, SK, NKJ, BF, DD, DKC, PN, and KD contributed in editing the draft and provided the critical inputs in the review discussion. PKG and KKK conceptualized, planned, edited, and finalized the manuscript.
Not applicable.
Piyush Kumar Gupta is thankful to the Department of Life Sciences, Sharda University, Greater Noida for providing the infrastructure and facility for research.
This research received no external funding.
The authors declare no conflict of interest.
AAT, Alpha-1 antitrypsin; AChE, Acetylcholinesterase; AD, Alzheimer’s Disease;
ADEPT, Antibody-Directed Enzyme Prodrug Therapy; APOE, Apolipoprotein E; APP,
Amyloid Precursor Protein; BACE1,
