Parkinson’s disease (PD) is a progressive neurodegenerative disorder, pathologically characterized by abnormal alpha-synuclein aggregation and Lewy body formation, which leads to neurodegeneration and dopaminergic cell death. Currently there is no cure for PD. Thus, it is imperative to develop a new therapeutic approach. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a member of unconventional evolutionary conserved protein families. It has unique molecular structure and capable to detect and rescue apoptotic neurons. MANF protein could selectively enhance the survival and sprouting of nigral dopaminergic neurons in vitro. Studies have shown that MANF can protect and repair dopaminergic neurons in animal models of PD. MANF is localized in the endoplasmic reticulum (ER) lumen function in regulation of ER stress and unfolded protein responses. Its C terminal domain is complete homologous to SAP domain of Ku70, which functions in anti-apoptosis. In this review, we described molecular structure, tissue expression of MANF, and summarized preclinical studies using MANF for PD therapy. We also discussed the mechanisms of MANF for the treatment of PD.
Parkinson’s disease (PD) is a progressive neurodegenerative disorder, which typically occurs in individuals older than 60 years. The clinical symptoms of PD are mainly characterized by the impairment of motor function, including tremor, slowness of movement (bradykinesia), rigidity, and postural instability (1). The main pathology of PD is the abnormal aggregation of protein alpha-synuclein and the formation of Lewy bodies in the neurons. Currently there is no effective treatment for PD. The clinical therapies for PD include dopamine-replacement therapy (levodopa, dopamine agonists, MAO-B inhibitors), surgery, and physical treatment. However, the current therapies for PD can only improve the motor symptoms transiently, but not prevent or reverse the progressive neurodegenerative processes of the PD. Thus, it is imperative to develop new therapies.
A broad range of literatures describe that neurotrophic factors (NTFs) can improve the survival and functions of nigral dopaminergic neurons. NTFs are a family of small extracellular proteins. They can support the survival, growth, differentiation, and maturation of developing neurons (2). They can also promote survival and synaptic plasticity of mature neurons and protect neurons against injury (3-5). Currently, there are four families of NTFS: (I) neurotrophins family including nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophic-3 (NT-3) and neurotrophin-4 (NT-4); (II)glial cell-line derived neurotrophic factor family ligands (GFLs) including glial cell-line derived neurotrophic factor (GDNF), neurturin (NRTN), artemin (ARTN) and persephin (PSPN); (III) neuropoietic cytokines including ciliary neurotrophic factor (CNTF), neuropoietin, leukemia inhibitory factor (LIF), interleukin-6 (IL-6), interleukin-27 (IL-27), oncostatin M (OSM) (6, 7); (IV) a novel evolutionary conserved protein family including mesencephalic astrocyte-derived neurotrophic factor (MANF) and cerebral dopamine neurotrophic factor (CDNF). GDNF and NRTN are the two most used NTFs in rodent and non-human primate neurotoxin models of PD. Experimental studies have indicated that GDNF and NRTN can enhance the survival of dopamine cells in vitro and protect dopamine neurons in animal models of PD (8-10). However, GDNF exhibited only modest protection in the severe 6-OHDA models of PD, and failed to protect dopamine neurons in α-synuclein models of PD (9, 10). Both GDNF and NRTN have been tested in clinical trials on PD patients (Table 1). The data from phase I and II trials have evidenced that GDNF did not show beneficial effects for PD patients(10-13).The phase 2 trials indicated that NRTN showed a modest benefit only after 18 months of treatment or in an early stage of PD (14, 15). Thus, the unsatisfied effects of GDNF and NRTN in clinical trials have stimulated the further exploration for new neurotrophic factors for PD. In 2003, MANF was characterized and has a unique mechanism to rescue apoptotic neurons (16, 17). In the present review, we attempted to discuss the roles of MANF in PD treatment. We described the molecular structure, tissue expression of MANF, and summarized preclinical studies on therapeutic effects of MANF in PD treatment. We also discussed the potential protective mechanisms of MANF for PD treatments.
| Conditions | PD model | Effects | Mechanism | Refs |
|---|---|---|---|---|
| pAAV-hMANF | 6-OHDA; in vitro | Increased cell viability | Decreased ER stress | (45) |
| rhMANF protein | 6-OHDA; in vitro | Increased cell viability | Activated PI3K/Akt/mTOR pathway | (45) |
| MANF protein | alpha-synuclein; in vitro | Protection effect | inhibition of apoptosis | (44) |
| 6-OHDA; in vitro | Increased cell viability | Autophagic inhibition via activated AMPK/mTOR pathway | (46) | |
| 6-OHDA; in vitro | Increased cell viability | Ameliorated ROS via maintaining mitochondrial function | (46) | |
| 6-OHDA; in vitro | Increased cell viability | Up-regulation of ER stress relative genes | (52) | |
| 6-OHDA; in vitro | Protection effect | Activated PI3K/Akt/GSK3β pathway and Nrf2 nuclear translocation | (53)} |
MANF is a neurotrophic factor of CDNF/MANF family (7). It was found homologous to a human arginine-rich protein (ARP) of 234 amino acids (Figure 1.). MANF is initially discovered by Petrova et al from the culture medium of a rat mesencephalic type-1 astrocyte cell line (16). MANF is a secreted protein with a molecular weight of 18 kDa. The primary sequence of MANF contains a signal peptide with 21 amino acids at N-terminal amino acid domains. The signal peptide can direct primary sequence of MANF to ER and is cleaved off resulting in a mature MANF protein with 158 amino acids (16, 19, 20). MANF consists of N-terminal domain and C-terminal domain which are connected by flexible linker (17, 21). The crystal structure analysis of human MANF showed that N-terminal domain contains five α-helices (α1-α5) and a 310 helix in a closed globular saposin-like architecture, which are homologous to saposin-like proteins (SAPLIPs) (21). SAPLIPs are a family of small, cysteine-rich proteins which are able to interact with lipids and membranes. A solution structure determined by nuclear magnetic resonance (NMR) spectroscopy revealed that C-terminal domain of MANF consisted of α-helices (α6-α8), which are homologous to the SAP (SAF-A/B, Acinus, and PIAS) domain of Ku70 protein (17). It is well known that SAP domain of Ku70 could inhibit proapoptotic BAX (Bcl-2-associated X protein) and prevent mitochondrial cell death signaling (22-24). It has been reported that MANF protein or C-terminal domain MANF could protect neurons intracellularly, not extracellularly, against BAX-dependent apoptosis (17). Therefore, Hellman et al speculated that MANF prevented apoptosis via interaction with BAX. However, no evidence for a direct interaction between BAX and MANF has been described currently (25).
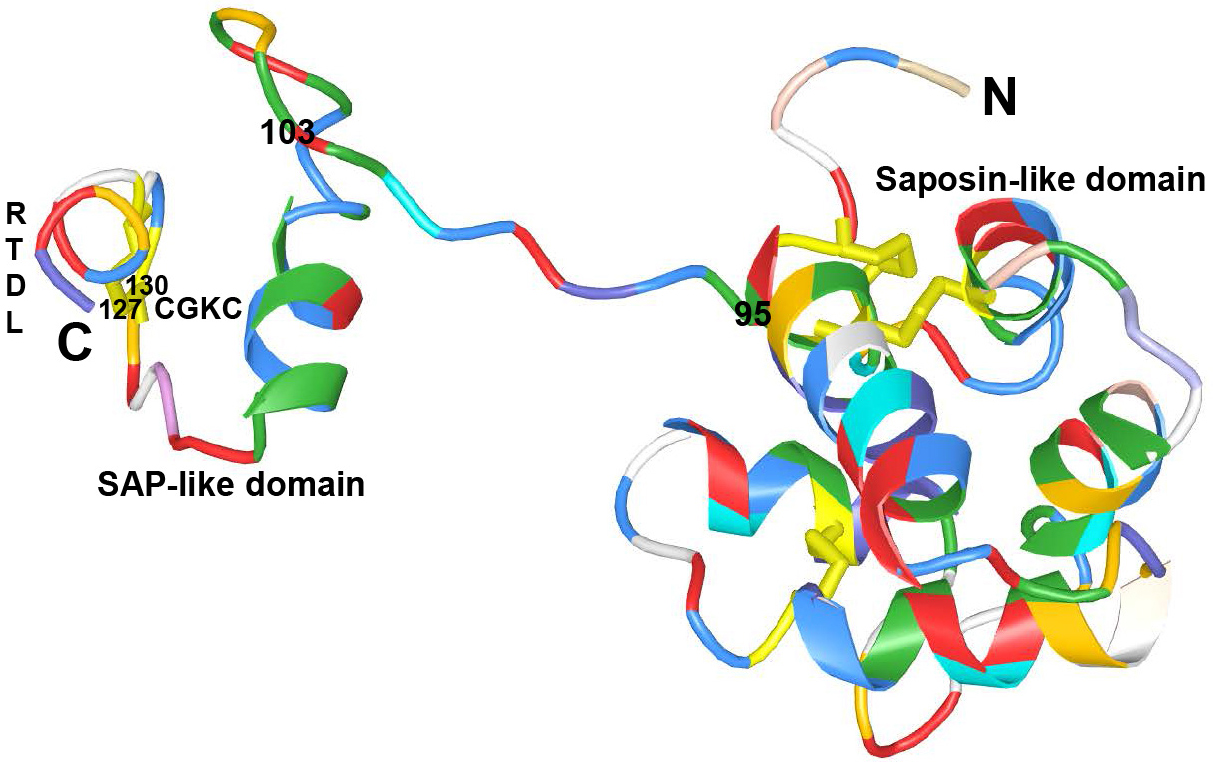 Figure 1
Figure 1Crystal structure of human mature MANF to show the Saposin-like domain (residues 1-95) at the N-terminal domain, SAP-like domain (residues 96-158) at the C-terminal domain and linker region (residues 96-103). 127CKGC130 and RTDL motifs in the C-terminal domain.
MANF has CXXC and RTDL motifs in the C-terminal domain. The CXXC motif forms a disulphide bridge with cysteine between the α7 and α8 helices. The electron density showed that the cysteine bridge is located at 127CKGC130 in mature human MANF (21). Mutation of CXXC motif of human MANF could completely abolish its protective effect intracellularly in cultured sympathetic or sensory neurons, and extracellularly in the rat model of cerebral ischemia (25). Thus it is evidenced that CXXC motif is crucial for MANF activity. It has also been evidenced that MANF does not have oxidoreductase activity (20, 25, 26). The RTDL motif at the very end of the C-terminal domain is homologous to the canonical Lys-Asp-Glu-Leu (KDEL) sequence for endoplasmic reticulum retention (27). It has been reported that deletion of RTDL motif inactivated MANF intracellularly and mutant MANF protein could relocalize from the ER to Golgi in vitro (25). It is concluded that intracellular MANF protects these primary neurons in vitro only when localized to the ER (25, 28-30).
MANF is widely expressed in the nervous system (31, 32). In the brain of postnatal and adult rats, MANF expression is mainly localized in neurons (31, 32). Relatively high levels of MANF mRNA and protein were distributed in the areas of brain including anterior olfactory nucleus and mitral cell layer of olfactory bulb, II-VI layers of cerebral cortex, piriform cortex, CA1-CA3 regions of hippocampus, dentate gyrus, paraventricular nucleus, bed nucleus of stria terminalis, hypothalamus, cerebellar Purkinje cells, and also in the spinal cord. In the striatum, low MANF expression was detected. Relatively intermediate levels of MANF expression was present in the pars reticulata of substantia nigra (SN). Of note, some of the tyrosine hydroxylase (TH) positive dopaminergic neurons in the pars compacta expressed MANF (31). MANF expression was high on postnatal day 3 and 5, and declined gradually during brain maturation (32). MANF expression is also widely distributed in the non-neuronal tissues. High MANF expression was detected in testis, seminiferous tubules, salivary gland and pancreas (31, 33).
Patterns of MANF mRNA and protein expression in mouse embryonic development were revealed by in situ hybridization and immunohistochemistry. MANF mRNA expression was widely present in the brain from E12.5. mouse embryos. High level of MANF expression were detected in the neopallial cortex, choroid plexus of the lateral ventricles. Relatively low levels MANF expression were detected in the embryonic midbrain, striatum, trigeminal and dorsal root ganglia. A wide MANF expression was also detected in the cartilage primordium of head and vertebra, liver, umbilical vessels, submandibular gland, and pancreas. A low level of MANF expression was detected in lung, metanephros and gut (31).
In human tissues, MANF expression has not been detected extensively. MANF mRNA expression was revealed by RT-PCR in several brain regions and in peripheral tissues (31). Recently, a robust MANF expression was detected by Immunofluorescence and Western blot in retinal ganglion cells of human retina (34). A recent study revealed that MANF expression was also present in human blood serum (35).
It has been evidenced that MANF is important for dopamine system maintenance and survival in Drosophila. In larval Drosophila brain, MANF expression was present in astrocyte-like glia surrounding dopaminergic cells (36). In Drosophila adult brain, MANF expression was detected both in glia and dopaminergic neurons (37). The knockout of MANF gene in Drosophila could lead to a loss of axons of dopaminergic neurons. In addition, the lack of MANF in Drosophila could decrease dopamine levels and increase the transcripts of the dopamine producing enzymes such as tyrosine hydroxylase and DOPA decarboxylase (38). The knockout of MANF gene in Drosophila resulted in several genes related to processes altered in PD including oxidative phosphorylation, protein ubiquitination, mitochondrial function and dopamine metabolism (38). Overexpression of MANF could rescue dopaminergic neurons against oxidative stress (38).
In zebra fish, a wide expression of MANF mRNA was detected by qPCR and in situ hybridization during embryonic development and in adult organs (39). Highest levels of MANF mRNA were expressed in whole embryo two hours after fertilization and declined gradually during brain maturation. In adult brain of zebra fish, MANF mRNA expression was most prominently detected in in neurons of several regions of brain including the thalamic, hypothalamic, forebrain, basal ganglia, cerebellum, and optic tectum. However, only few MANF positive cells were found to co-express tyrosine hydroxylase. Notably, the knockdown of MANF expression could decrease dopamine levels and tyrosine hydroxylase gene transcripts. In addition, these defects could be rescued by injection of exogenous MANF mRNA (39). This evidence suggests that MANF is involved in the regulation of the development of dopaminergic system in zebra fish.
It has been revealed that purified MANF protein could selectively enhance the survival and sprouting of nigral dopaminergic neurons in vitro (16). MANF protein has also been reported to mediate the presynaptic enhancement of GABAergic inhibition to protect dopamine neurons (40). Since MANF expression was present in the striatum and MANF is considered to involve in maintenance of dopaminergic neurons (31). There is an increasing interest to probe the function of MANF in PD treatments. MANF was able to protect nigrostriatal dopaminergic nerves from 6-OHDA-induced degeneration when it was intrastriatally administrated 6 h before neurotoxin (41). More importantly, MANF was also able to restore the function of the nigrostriatal dopaminergic system when microinjected 4 weeks after 6-OHDA administration in the striatum (41). In addition, 125I-labeled MANF was distributed throughout the striatum more readily than GDNF (41). These results suggest that MANF has significant therapeutic potential for the treatment of PD. In addition, the infusion of MANF and gadolinium-DTPA with convection-enhanced delivery (CED) catheter system was tested in porcine brain. The finding showed that distribution of gadolinium-DTPA on magnetic resonance imaging correlated well with the distribution of MANF with immunohistochemistry, suggesting that the MANF with the CED system may be a potential strategy in PD treatments (42).
A comparative study related to the effects of MANF, CDNF and GDNF, was carried out in a severe unilateral 6-OHDA model (43). It has been shown that CDNF with a dose of 40μg was able to inhibit 6-OHDA-induced loss of TH positive cells of the pars compacta of the substantia nigra and TH positive fibers in the striatum and attenuate amphetamine-induced turning behavior. The results suggest that CDNF could inhibit degeneration of nigrostriatal dopamine nerve tract after 6-OHDA injection. However, MANF at similar doses failed to protect nigrostriatal dopamine nerve tract against 6-OHDA induced severe degeneration (43). Although after chronic infusion, 125I MANF protein exhibited a larger distribution volume than CDNF in rat brain tissue and was retrogradely transported from the striatum to the substantia nigra and frontal cortex.
MANF protein has also been studied in a cellular model of PD. Purified MANF protein improved the viability of SH-SY5Y cells against 6-OHDA toxicity, and protected the cells from 6-OHDA or α-synuclein induced apoptosis (44). Recent studies have reported that extracellular application of MANF attenuated 6-OHDA-induced neurotoxicity in SH-SY5Y cells (45, 46).
Since intracranial injection of neurotrophic factors is difficult to develop into a long-term therapy, gene delivery using viral vectors or non-viral vectors mediated neurotrophic factors into rats brain would be an attractive therapeutic method for treatment of PD.
Cordero-Llana et al indicated that intrastriatal lentiviral vector-mediated overexpression of MANF did not decrease amphetamine-induced rotational behavior and did not increase TH positive fibers in the striatum and TH positive neurons in the substantia nigra in parkinsonian rats (47). In addition, intranigral lentiviral vector-mediated overexpression of MANF also had no beneficial effects on amphetamine-induced rotations or TH striatal fiber density but resulted in a significant preservation of TH positive cells (47). However, these results are inconsistent to the results in our recent study. Our study reported that intrastriatal injection of adeno-associated virus serotype 9 (AAV9) mediated human MANF could reduce rotational asymmetry and promote the regeneration of TH positive fibers in the striatum and survival of TH positive neurons in the substantia nigra in the 6-OHDA model of PD (45). We also reported that intrastrital injection of AAV9 – human MANF could increase the concentration of striatal dopamine (DA) and dihydroxyphenylacetic acid (DOPAC, indicator of DA metabolism) and homovanillic acid (HVA, indicator of DA metabolism) and reduce the ratio of DOPAC + HVA /DA (indicator of DA turnover) in 6-OHDA-lesioned rats (45). There are several factors for this inconsistency. Firstly, 6-OHDA injection in Cordero-Llana’s study resulted in a severe lesion (75-90% TH positive cells loss in the substantia nigra), whereas 6-OHDA injection in our study led to a 29-38% loss of TH positive cells in the substantia nigra. In the severe 6-OHDA-lesioned model, nigrostriatal dopaminergic pathway is prone to be damaged severely. Thus dopaminergic neurons in substantia nigra cannot be rescued because of impaired axonal transport and/or neuron death. Secondly, lentivirus application with a low titer (8× 108 TU/ml) only led to a local transduction, and the low-level expression of MANF failed to exert its neuroprotection in the severe 6-OHDA-lesioned model.
MANF plays a significant role in the regulation of ER stress, which triggers unfolded protein response to restore ER homeostasis. It has been observed that MANF remains inside the cells and localizes in the ER lumen (25, 28, 30, 45, 48). Endogenous MANF protein expression is up-regulated by inducers of ER stress in vitro (20, 28, 45, 48-50) and in ER stress response induced by cerebral ischemia, epileptic insult and myocardial ischemia in vivo (31, 49, 50).
We previously showed that endogenous MANF was up-regulated with a similar pattern as chaperone Bip in SH-SY5Y cells after 6-OHDA treatment (45). In addition ER stress chaperone Bip, downstream molecules including p-eIF2α/eIF2α/ATF4/CHOP, ATF6α and XBP1s were also induced by 6-OHDA. We also observed that overexpressed MANF localized surrounding nucleus and co-localized with protein disulfide isomerase (PDI, an ER-resident marker protein) (45). Notably, intracellularly overexpressed MANF can significantly increase cell viability against 6-OHDA induced insult, and inhibited the activation of protein kinaselike ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and ATF6α pathway (ER stress-associated three pathways) (45). Our results demonstrate that intracellular overexpressed MANF can at least partially alleviate ER stress to protect cells against 6-OHDA neurotoxicity (Table 2).
| Methods | Clinical trial | Type | Time period | Comments | Refs |
|---|---|---|---|---|---|
| r-metHuGDNF; Intraputamental pump | phase 1 safety trial | Open | One year | No serious clinical side effects | (11) |
| r-metHuGDNF; Intraputamental infusion | phase 1/2 | Randomized double blind | 6 months | No beneficial effects | (13) |
| AAV2-GDNF; intraputamental infusions | phase 1 safety and tolerability trial | Open | recruiting | Elucidating the necessary parameters for GDNF expression | (18) |
| AAV2-NRTN; bilateral injection into the putamen | phase 2 | Randomized double blind | 12 months | No significant improvement | (14) |
| AAV2-NRTN; bilateral injection into the putamen | phase 2 | Randomized double blind | 18 months | Modest but significant benefits improvement | (14) |
The C-terminal domain of human MANF is structurally similar to the SAP domain of Ku70 protein (17). Ku 70 binds to the proapoptotic protein BAX via its C-terminal SAP domain to exert its anti-apoptotic function (22-24). Due to the highest homologous to SAP domain of Ku70, Hellman et al. speculated that MANF may prevented apoptosis via interaction with BAX. Accordingly, cellular studies confirmed that overexpression of the full length or C-terminal domain MANF could protect neurons intracellularly against BAX-mediated apoptosis and C-terminal domain of MANF is responsible for the intracellular protection against the BAX-dependent apoptosis (17). However, various data for protein-protein interaction did not confirm any evidence for interaction between MANF and BAX, suggesting that MANF may mediate its anti-apoptotic function via upstream molecules of BAX (25).
In addition, caspase-3 is also involved in the protection effect of MANF. Caspases play essential roles in apoptotic pathway. Caspase-3 is a central member in the execution phase of cell apoptosis. MANF was found to inhibit the cleavage of caspase-3 apoptosis induced by focal cerebral ischemia (51). Two recent studies have also reported that MANF protein repressed cleaved caspase-3 expression induced by 6-OHDA treatment or overexpressed α-synuclein in vitro (44, 52). MANF also prevented 6-OHDA-induced apoptosis by activation of PI3K/Akt/GSK3ß signaling pathway, activation and nuclear translocation of Nrf2 (Nuclear factor erythoid-2-related factor, Nrf2) (53).
Autophagy and apoptosis are two common phases in programmed cell death to maintain cellular homeostasis. It has been indicated that autophagy plays an important role in dopaminergic cells in vitro (54, 55). Moreover, autophagy system was unregulated in both PD patients and animal models of PD (56, 57). Zhang et al recently described that MANF could inhibit autophagy induced by 6-OHDA via AMPK/mTOR pathway in vitro (46) (Table 2).
MANF is a member of CDNF/MANF family and has a neurotropic effect to rescue apoptotic neurons (7, 17). It was therefore hypothesized that MANF would have a neuroprotection in animal models of PD. Numerous in vitro and in vivo studies have confirmed this hypothesis (41, 44-47, 53). But the neuroprotective mechanisms of MANF have not been fully understood. Our recent study showed that extracellular MANF could protect SH-SY5Y cells against 6-OHDA mediated neurotoxicity. However, the neuroprotective mechanisms of extracellular MANF may be different from intracellular MANF (45). Our data demonstrated that extracellular MANF protein did not inhibit the levels of p-eIF2α/eIF2α/ATF4/CHOP, ATF6α and XBP1s, which were induced by 6-OHDA. We found that extracellular MANF protein could increase the levels of p-AKT/AKT and p-mTOR/mTOR, which were decreased after 6-OHDA treatment. Hellman et al. showed that extracellular administration of MANF cannot enter the cells (17). Therefore, we concluded that the neuroprotection of MANF on dopaminergic cells seem to be via both intracellular and extracellular modes of action. On one hand, intracellular overexpression of MANF protects dopaminergic cells by inhibiting the ER stress, and on the other hand extracellular MANF protein protects cells by activation of PI3K/Akt/mTOR pathway. It has also been reported that PKC and NFκB pathways are also involved in cytoprotective effects of MANF in inducible spinocerebellar ataxia 17 mice and in inflammation diseases (58, 59) (Table 2). Whether these pro-survival pathways are involved in MANF-mediated neuroprotective effects are still needed to be further determined.
MANF has been discovered more than ten years. However the details about its biology, therapeutic potential, and molecular mechanisms are still not fully understood. It is necessary to confirm the receptor of MANF. It is also important to understand the therapeutic potential of MANF in other animal models of PD. It is obvious that MANF has different models of action compared to other neurotropic factors, because of its unique location in the ER lumen. Therefore, the major challenge is to probe the underlying mechanism of MANF. It is also of interest to explore whether MANF could inhibit neuroinflammation in PD. Because it has been documented that MANF expression in the cortex could be unregulated in the activated microglia and astrocytes under focal cerebral ischemia (60). NF-κB functions as a central mediator at the onset of inflammation. MANF alleviated OGD-induced inflammation by negatively regulating the NF-κB pathway (61). MANF may be a novel regulator of neuroinflammation. Although the effect of MANF was examined in a limited series of PD animal models, the experimental results are still very encouraging. These hopeful studies will stimulate further exploration of MANF in clinical trials, and also in Alzheimer's disease, Huntington’s disease, stroke, spinal cord injury and other disorders.
This work was supported by grants from the National Key Basic Research Program of China (2011CB504100), National Natural Science Foundation of China (31171126, 81602532), the Beijing Natural Science Foundation (KZ201110025022) and the Capital Medical University Natural Science Foundation (2016ZR08).
PD
Parkinson's disease
neurotrophic factors
nerve growth factor
brain derived neurotrophic factor
neurotrophic-3
neurotrophin-4
glial cell-line derived neurotrophic factor family ligands
glial cell-line derived neurotrophic factor
neurturin
artemin
persephin
neuropoietic cytokines including ciliary neurotrophic factor
neuropoietin leukemia inhibitory factor
interleukin-6
interleukin-27
oncostatin M
mesencephalic astrocyte-derived neurotrophic factor
cerebral dopamine neurotrophic factor
endoplasmic reticulum
saposin-like proteins
nuclear magnetic resonance
Bcl-2-associated X protein
tyrosine hydroxylase
substantia nigra
convection-enhanced delivery
adeno-associated virus serotype 9
dopamine
dihydroxyphenylacetic acid
protein kinase like ER kinase
inositol-requiring enzyme 1
