Glycerol-lactate esters are energy supplements for exercise, but effects of trilactic glyceride (TLG) on intestinal function and hepatic metabolism are unknown. We found that dietary supplementation with 0.5% TLG to weanling piglets decreased plasma concentrations of low-density lipoprotein and gamma-glutamyl transferase but increased those of D-xylose and high-density lipoprotein. TLG supplementation enhanced mRNA levels for fatty acid synthase (FASN) and SLC27A2 in white adipose tissue; insulin receptor in duodenum; aquaporin-8 in ileum, jejunum and colon; aquaporin-10 in duodenum and ileum; nuclear factor like-2 in jejunum and colon; glutathione S-transferase and phosphoenolpyruvate carboxykinase-1 in intestines; and abundances of claudin-1 and occludin proteins. TLG supplementation decreased mRNA levels for: hepatic hormone-sensitive lipase E, lipoprotein lipase, FASN, insulin-like growth factor-binding protein-3, and SLC27A2; and intestinal lipoprotein lipase, FASN and NADPH oxidase. Furthermore, TLG supplementation enhanced abundances of genus Bifidobacterium, while reducing abundances of family Enterobacteriaceae in ileum, colon and cecum; jejunal caspase-3 protein and diarrhea rate. In conclusion, dietary supplementation with TLG modulated lipid metabolism and alleviated diarrhea by improving intestinal function and regulating intestinal microflora in piglets.
Much evidence shows that lactic acid has many beneficial effects on the growth and production of livestock and poultry especially young animals (1-4). Lactic acid is mainly used as an acidifier in animal feeds to activate digestive enzymes, improve the digestion and development of the intestinal epithelium, and inhibit the growth of microbes, especially E. coli and Salmonella (2-4). Several studies have demonstrated that supplementation with lactic acid to the diets of poultry and piglets can effectively inhibit the growth of pathogenic bacteria and increase body weight (BW) gain (5-7).
Trilactic glyceride (TLG) decomposes into lactic acid and glycerol in the gastrointestinal tract, thereby providing a good source of lactic acid for the hind gut. Several studies have reported positive effects of TLG on growth performance, anti-oxidative capacity, and energy metabolism in broilers (8-10). However, little is known about effects of TLG on growth performance, lipid metabolism, or intestinal function in piglets. To fill in this gap of knowledge, our group determined effects of dietary supplementation with TLG on lipid metabolism and intestinal function in weanling piglets.
The animal use protocol for this research was approved by the Institutional Animal Care and Use Committee at Wuhan Polytechnic University (WH2018-0604). Sixteen crossbred healthy piglets (Duroc × Landrace × Yorkshire) were weaned at 21 days of age. After weaning, piglets had free access to the basal diet for 3 days for adapting to solid foods. At 24 days of age, piglets (7.25 ± 1.13 kg BW; means ± SD, n = 8) were assigned randomly into one of the two treatment groups: the control group, where piglets were fed a corn- and soybean meal-based diet; and the TLG group, where piglets were fed the basal diet supplemented with 0.5 % TLG. There were 12 piglets per group. Each piglet was individually housed in a 1.20 × 1.10 m2 steel metabolic cage with eight replicate cages per treatment. All diets were isocaloric (11). On day 20 of the trial, 1 h after infusion of 10% D-xylose at 1 mL/kg BW (12), blood samples were collected from the anterior vena cava and centrifuged to obtain plasma. After all piglets were killed under anesthesia with an intravenous injection of pentobarbital sodium (50 mg/kg BW), liver, white adipose tissue (WAT) around the kidneys (leaf fat), and intestinal samples were collected and stored at -80 °C until analysis (13).
Blood biochemical parameters were assessed using a Hitachi automatic biochemistry analyzer 7100 with WAKO chemical reagents (Wako Pure Chemical Industries, Ltd., Osaka, Japan). D-xylose, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) in plasma, and liver lipids were analyzed using commercially available kits (Jiancheng Bioengineering Institute, Nanjing, China) (14). All assays were carried out in triplicate.
The mRNA levels for genes related to lipid and amino acid metabolism in the liver, WAT, and the intestine were quantitated by the method of real-time qPCR (15). The real-time qPCR was carried out with primers designed to span introns and intron-exon boundaries (Table 1) and was performed using the SYBR® Premix Ex TaqTM (Takara, Dalian, China) on the 7500 Fast Real-Time PCR System (Foster City, CA, U.S.A.). Data were analyzed by the 2-ΔΔCt method (16). Each biological sample was run in triplicate.
| Genes | Forward Sequences | Reverse Sequences |
|---|---|---|
| AQP8 | TGTGTCTGGAGCCTGCATGAAT | AGCAGGAATCCCACCATCTCA |
| AQP10 | TGTCTGCTTTCTGTGCCTCTG | GGATGCCATTGCTCAAGGATAGATAA |
| Nrf2 | GAAGTGATCCCCTGATGTTGC | ATGCCTTCTCTTTCCCCTATTTCT |
| NOX2 | TGTATCTGTGTGAGAGGCTGGTG | CGGGACGCTTGACGAAA |
| GSTO2 | GCCTTGAGATGTGGGAGAGAA | AAGATGGTGTTCTGATAGCCAAGA |
| INSR | GGGGCTAAAGAGGAACTATGAGG | AGAGGAAAGCGAAGACAGGAAA |
| PCK1 | CGGGATTTCGTGGAGA | CCTCTTGATGACACCCTCT |
| LIPE | CCAGCCCTGCCTTAATGTG | TCCCGAATACCCGCAAAG |
| GHR | TCCTCCTTGCGAAGAAGTTG | GTGTGATGGTTCGTCTGTCG |
| IGFBP-3 | AGAACAGATACCCAGAACTTCTTC | CGCCCTCCGACTGCTG |
| FASN | ACACCTTCGTGCTGGCCTAC | ATGTCGGTGAACTGCTGCAC |
| LPL | AGCCTGAGTTGGACCCATGT | CTCTGTTTTCCCTTCCTCTCTCC |
| SLC27A2 | TTTTCAGCCAGCCACTTTTG | CATTTGGTTTCTGGGGAGAGTT |
| RPL4 | GAGAAACCGTCGCCGAAT' | GCCCACCAGGAGCAAGTT |
| GADPH | CGTCCCTGAGAGACACGATGGT | GCCTTGACTGYGCCGTGGAAT |
| Total bacterium | CGGYCCAGACTCCTACGGG | TTACCGCGGCTGCTGGCAC |
| Enterobacteriaceae family | CATTGACGTTACCCGCAGAAGAAGC | CTCTACGAGACTCAAGCTTGC |
| Lactobacillus genus | AGCAGTAGGGAATCTTCCA | CACCGCTACACATGGAG |
| Bifidobacterium genus | GATTCTGGCTCAGGATGAACG | CGGGTGCTCCCACTTTCATG |
| Total eubacteria (16S rRNA) | CAGAAATGGGAATGGAAAGTTG | CCATTGGTCAGGTCATTCAATACA |
Bacterial DNA from intestinal digesta samples were extracted using the QIAamp Fast DNA Stool Kit (Qiagen, Hilden, Germany). The quantity of intestinal bacteria was analyzed by real-time qPCR as described previously (17). The primer pairs used for the present study are shown in Table 1. The 16S rRNA served as the reference gene.
Abundances of proteins related to lipid and amino acid metabolism were determined by Western blot analysis. The primary antibodies used in this study are as follows: caspase-3 (rabbit, 1:1000; Cell Signaling Technology, Inc., MA, USA), occludin (mouse, 1:1000; Sant Cruze Biotechnology, CA, USA), claudin-1 (mouse, 1:1000; Invitrogen, CA, USA), β-actin (mouse, 1:2000; Sigma–Aldrich Inc., St. Louis, USA). The secondary antibodies used in this study are as follows: anti-rabbit (mouse, 1:2000; Zhongshan Golden Bridge Biological Technology Co., Beijing, China) and anti-mouse (rabbit, 1:2000; Invitrogen, CA, U.S.A.). Blots were developed by utilizing a chemiluminescence kit (Amersham Biosciences, Uppsala, Sweden) and quantitated using an imaging system (Alpha Innotech, CA, USA) (17).
Data, expressed as means ± SD, were analyzed by one-way analysis of variance. All statistical analyses were performed by using the SPSS software (Version 23.0, SPSS Inc., Chicago, IL, USA). A p-value of < 0.05 was taken to indicate statistical significance.
Growth performance is a major criterion used to evaluate outcomes of animal production (11). There were no significant differences in average daily feed intake (ADFI), average daily weight gain (ADG), or the feed to gain ratio (F/G) between the control and TLG groups. However, dietary supplementation with 0.5% TLG substantially decreased (P < 0.001) diarrhea rate (DR) in weaned piglets during day 0 to 10 and day 0 to 21 post weaning (Table 2). This implies an improvement in intestinal function in TLG-supplemented piglets.
| Item | Control | TLG | P-value | Control | TLG | P-value | Control | TLG | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Day 0 to day 10 | Day 11 to day 21 | Day 0 to day 21 | |||||||
| ADG /g | 245 ± 52 | 236 ± 80 | 0.187 | 438 ± 70 | 412 ± 73 | 0.907 | 341 ± 56 | 324 ± 70 | 0.485 |
| ADFI /g | 308 ± 8.3 | 321 ± 66 | 0.059 | 662 ± 86 | 658 ± 43 | 0.317 | 485 ± 44 | 490 ± 52 | 0.669 |
| F/G | 1.3 ± 0.2 | 1.4 ± 0.2 | 0.623 | 1.5 ± 0.1 | 1.6 ± 0.1 | 0.514 | 1.4 ± 0.1 | 1.5 ± 0.1 | 0.701 |
| DR /% | 15.0 ± 4.9 | 8.8 ± 3.1 | < 0.001 | 2.5 ± 0.9 | 3.8 ± 1.4 | 0.413 | 8.3 ± 2.8 | 6.0 ± 2.0 | 0.045 |
Intervention trials have shown that hypercholesterolemia, especially increased concentrations of LDL and cholesterol, leads to the development of atherosclerosis (18-19). In contrast, other studies have demonstrated a negative correlation between plasma HDL cholesterol and cardiovascular disease (20). Hormone-sensitive lipase (lipase E, LIPE) is one kind of lipases, the main function of LIPE is to mobilize the stored fats (21). In adipose tissue and heart, LIPE primarily hydrolyzes triglycerides to free fatty acids, while in steroidogenic tissues, principally converts cholesteryl esters to free cholesterol for steroid hormone production (22). Lipoprotein lipase (LPL) is expressed in the heart, muscle, and adipose tissue, which functions as both triglyceride hydrolase and ligand/bridging factor of receptor-mediated lipoprotein uptake (23-24). The main function of fatty acid synthase (FASN) is to catalyze the synthesis of palmitate from acetyl-CoA and malonyl-CoA, in the presence of NADPH, into long-chain saturated fatty acids (25-27). Solute carrier family 27 member 2 (SLC27A2) is an isozyme of long-chain fatty-acid-coenzyme A ligase family, which converts free long-chain fatty acids into fatty acyl-CoA esters, and thus plays a key role in lipid biosynthesis and fat degradation (28).
In this study, the concentrations of plasma LDL (day 20) and cholesterol (day 10) in the TLG group were decreased, while HDL (day 20) and triglycerides (day 20) were increased relative to the control group (Table 3). Furthermore, TLG supplementation resulted in a significant increase in the content of leaf fat (Table 3). These results reflected that TLG could lower cholesterol and improve blood lipid profiles. Moreover, supplementation with TLG significantly increased the expression of FASN and SLC27A2 genes in WAT, decreased the expression of LIPE, LPL, FASN and SLC27A2 genes in the liver and the expression of LPL and FASN genes in the jejunum (Figure 1). These results indicated that TLG could promote fat synthesis in adipose tissue, modulate fat metabolism in the liver and small intestine. Thus, TLG plays a crucial role in the regulation of fat metabolism in swine.
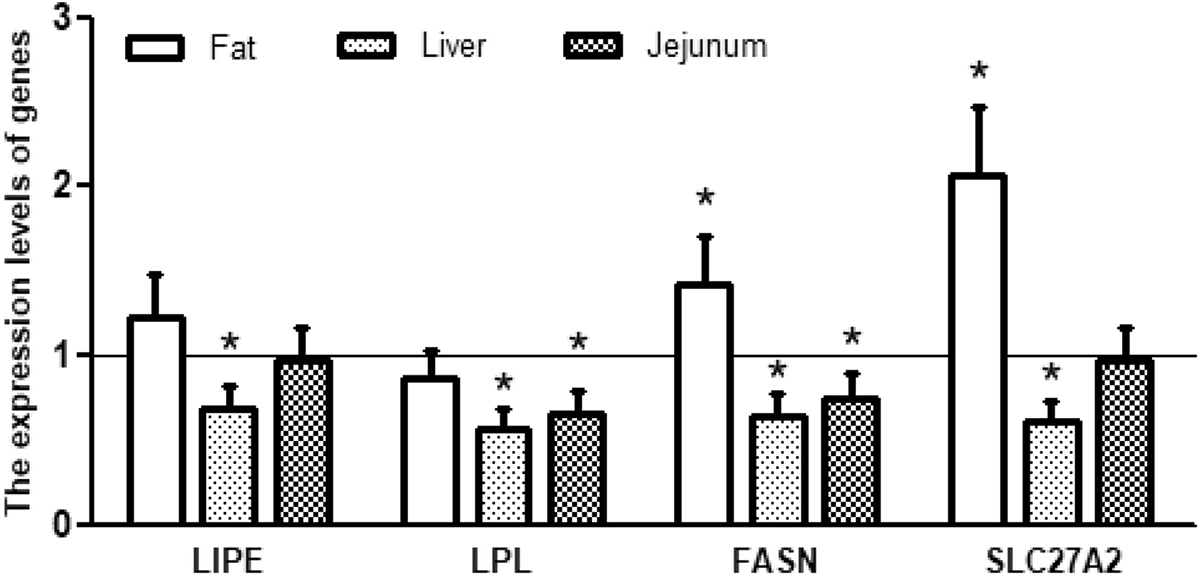 Figure 1
Figure 1Relative expression levels of genes associated with fat metabolism in piglets receiving dietary supplementation with or without 0.5% trilactic glyceride (TLG). Dietary supplementation with TLG up-regulated the expression of LIPE, FASN and SLC27A2, but down-regulated LPL expression in white adipose tissue (fat). Dietary supplementation with TLG down-regulated the expression of LIPE, FASN, LPL and SLC27A2 in the liver and the jejunum. mRNA levels in the control group were regard as 1. Values are means ± SD, n = 8. *p < 0.05.
| Item | Control | TLG | P-value | Control | TLG | P-value |
|---|---|---|---|---|---|---|
| Day 10 | Day 20 | |||||
| Blood urea nitrogen (mmol/L) | 3.1 ± 0.29 | 3.7 ± 0.20 | < 0.01 | 2.9 ± 0.29 | 3.5 ± 0.23 | < 0.05 |
| GGT (U/L) | 35.1 ± 5.2 | 29.1 ± 6.1 | < 0.05 | 30.4 ± 4.0 | 26.9 ± 2.5 | < 0.05 |
| Triglycerides (mmol/L) | 0.41 ± 0.11 | 0.43 ± 0.09 | 0.241 | 0.49 ± 0.11 | 0.63 ± 0.15 | < 0.01 |
| Cholesterol (mmol/L) | 1.8 ± 0.26 | 1.6 ± 0.1 | < 0.05 | 1.8 ± 0.1 | 2.0 ± 0.2 | 0.100 |
| Low-density lipoprotein (mmol/L) | - | - | - | 0.92 ± 0.12 | 0.81 ± 0.09 | < 0.05 |
| High-density lipoprotein (mmol/L) | - | - | - | 0.38 ± 0.10 | 0.61 ± 0.12 | < 0.01 |
| D-xylose (μmol/L) | - | - | - | 0.39 ± 0.13 | 0.62 ± 0.12 | < 0.01 |
| Liver fat content (%) | - | - | - | 1.00 ± 0.20 | 0.97 ± 0.25 | 0.294 |
| Leaf fat (g/kg body weight) | - | - | - | 2.44 ± 0.21 | 3.36 ± 0.9 | < 0.01 |
Intestinal biochemical indices, such as DNA concentrations, as well as RNA/DNA and protein/DNA ratios, can be used to assess intestinal development (28). DNA concentration reflects the rate of mitosis for producing new columnar epithelial cells, RNA/DNA ratios indicates cellular efficiency for transcription, and protein/DNA ratios implicate the efficiency of protein synthesis in cells (14). The growth hormone receptor (GHR) gene encodes a member of the type I cytokine receptor family, which is a transmembrane receptor for growth hormone (29). Binding of growth hormone to the receptor leads to receptor dimerization and the activation of an intra- and inter-cellular signal transduction pathway, leading to growth (30). Insulin-like growth factor binding protein (IGFBP-3) is mainly produced and secreted by the liver, and has been shown to regulate cell survival, proliferation and apoptosis (31). In the present study, TLG supplementation significantly decreased the ratio of RNA/DNA and total protein/DNA and the expression of IGFBP-3 in the liver, increased the ratio of total protein/DNA in the jejunum and trended to increase the ratio of RNA/DNA in the colon (Table 4). These results implied that TLG might affect hepatic metabolism, while promoting the growth and development of jejunal and colonic epithelial cells.
| Item | Control | TLG | P-value | Control | TLG | P-value | Control | TLG | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Liver | Jejunum | Colon | |||||||
| TP mg/g | 1.12 ± 0.14 | 1.09 ± 0.11 | 0.487 | 0.68 ± 0.03 | 0.70 ± 0.08 | 0.370 | 0.37 ± 0.07 | 0.41 ± 0.04 | 0.081 |
| RNA/DNA | 3.71 ± 0.66 | 2.49 ± 0.30 | < 0.05 | 9.19 ± 1.38 | 10.43 ± 1.58 | 0.760 | 2.13 ± 0.35 | 2.21 ± 0.87 | 0.068 |
| TP/DNA | 211.1 ± 34.8 | 165.0 ± 16.5 | < 0.05 | 336.1 ± 19.3 | 384.7 ± 62.0 | < 0.05 | 130.6 ± 17.1 | 194.1 ± 26.3 | 0.243 |
| GHR | 1.00 ± 0.08 | 0.97 ± 0.19 | 0.738 | - | - | - | - | - | - |
| IGFBP-3 | 1.00 ± 0.16 | 0.65 ± 0.12 | < 0.01 | - | - | - | - | - | - |
Absorption of D-xylose from the intestinal lumen into plasma is a useful marker of in vivo intestinal function in animals (32). Generally, one-hour blood D-xylose test is used to measure intestinal absorption capacity and mucosal integrity (32). In our study, the concentration of plasma D-xylose in the TLG group was greater than that in the control group (Table 3), suggesting that TLG could substantially improve the intestinal absorptive capacity in piglets.
The insulin receptor (INSR) is a transmembrane receptor which binds insulin, IGF-I, and IGF-II, and belongs to the large class of tyrosine kinase receptors (33). Binding of insulin or other ligands to this receptor activates the insulin signaling pathway, which regulates glucose uptake and release, as well as the synthesis and storage of carbohydrates, lipids and protein (34). Phosphoenolpyruvate carboxykinase 1 (PCK1) is a main control point for the regulation of gluconeogenesis and can be regulated by insulin, glucocorticoids, glucagon, cAMP, and diet (35). In this study, supplementation with TLG remarkably increased gene expression of INSR in the duodenum and PCK1 in the four intestinal segments (Figure 2). The result suggested that trilactic glyceride had positive effects on the regulation of glucose metabolism in weaned piglets.
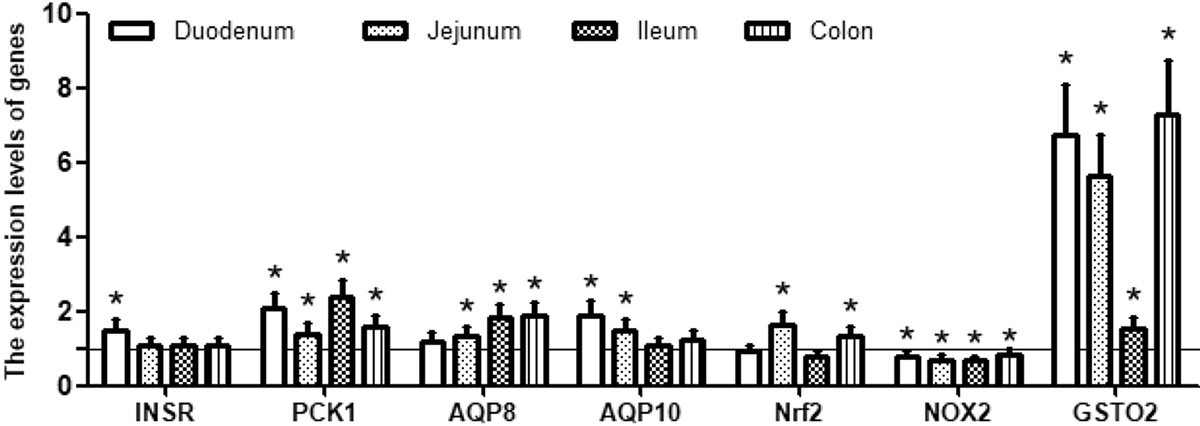 Figure 2
Figure 2Relative expression levels of genes associated with intestinal function in piglets receiving dietary supplementation with or without 0.5% trilactic glyceride (TLG). Dietary supplementation with TLG up-regulated the expression of INSR, PCK1, AQP8, AQP10 and GSTO2, but down-regulated the expression of NOX2 in the duodenum, jejunum, ileum and colon. Expression of Nrf1 was up-regulated in the jejunum and the colon, but down-regulated in the duodenum and the ileum. mRNA levels in the control group were regarded as 1. Values are means ± SD, n = 8. *p < 0.05.
Aquaporin-8 and 10 (AQP8 and AQP10) are two of the most important water channel proteins and mainly distributed in intestinal epithelial cells and facilitate the transport of water across epithelial cell (36). The results of our study showed that TLG supplementation resulted in a noticeable increase in the expression of AQP8 and AQP10 (Figure 2), indicating that TLG enhanced the intestinal capacity of water absorption.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a basic leucine zipper (bZIP) protein and regulates the expression of antioxidant proteins that protect cells against oxidative damage triggered by injury and inflammation (37). Several drugs that stimulate the Nrf2 pathway have been studied for the treatment of diseases caused by oxidative stress (38). NOX2 is one member of the NADPH oxidase family which generates superoxide by transferring electrons from NADPH inside the cell across the membrane and coupling these electrons to molecular oxygen to produce superoxide anion, a reactive free-radical (39). Glutathione S-transferase omega-2 (GSTO2) participates in detoxification of inorganic arsenic and catalyzes the reduction of monomethylarsonic acid to monomethylarsonous acid, the rate limiting step in detoxification of inorganic arsenic (40). In this study, supplementation with TLG significantly decreased the expression of NOX2 in four intestinal segments but increased the expression of Nrf2 in the jejunum and colon as well as GSTO2 in the intestines (Figure 2), suggesting that TLG improved intestinal antioxidant capacity.
Intestinal epithelial integrity is maintained by cohesive interactions between cells via the formation of tight junctions (41). Claudin-1 and occludin integrate such diverse processes as gene transcription, tumor suppression, and cell proliferation to modulate intestinal-mucosal structure and function (42). Caspase-3 is one of the key components of the apoptotic pathway in the small intestine. This protein is either partially or fully responsible for the proteolytic cleavage of many key proteins (43). The data of this study showed that TLG supplementation enhanced the abundances of claudin-1 and occludin proteins, while decreasing the abundance of the caspase-3 protein (Figure 3). These results supported the notion that TLG exerted beneficial effects on epithelial barrier as well as cell growth and survival.
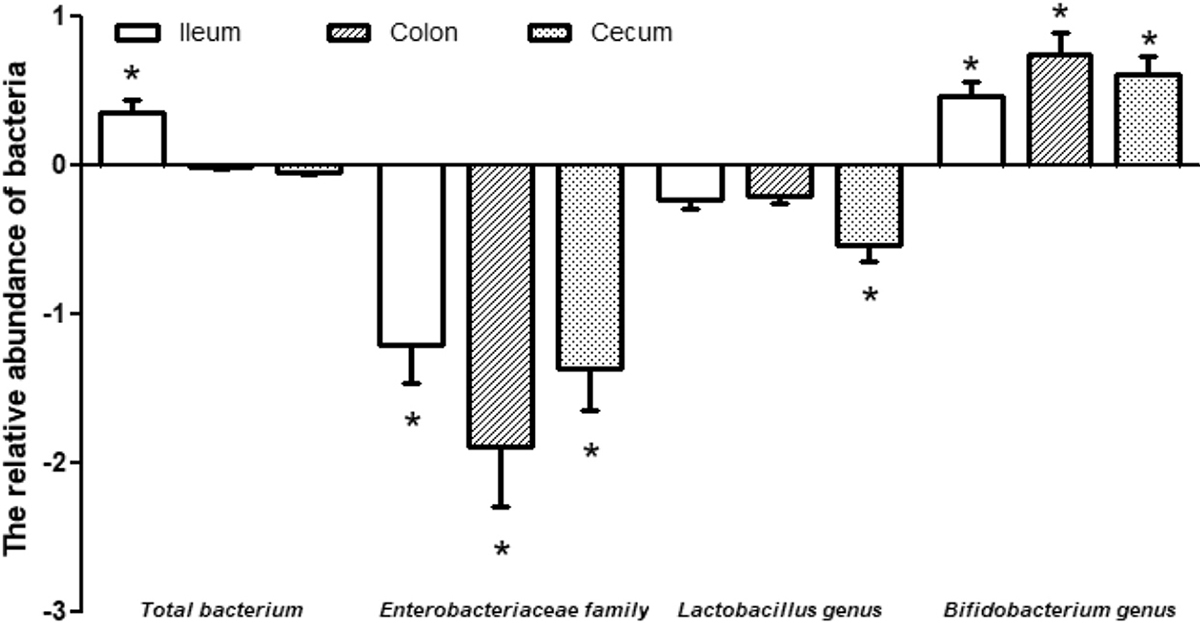 Figure 3
Figure 3Expression levels of jejunal proteins in piglets receiving dietary supplementation with or without 0.5% trilactic glyceride (TLG). Dietary supplementation with TLG increased the abundance of occludin and claudin-1 proteins, but decreased the abundance of the caspase-3 protein in the jejunum of weaned piglets. Values are means ± SD, n = 8. *p < 0.05.
In animals, the intestine is colonized by different bacteria that protect the host from the invasion of pathogenic bacteria, regulate intestinal growth, and produce metabolites for the host (44-45). The Enterobacteriaceae family are divided into five groups: Escherichia coli, Klebsiella, Proteus, Yersinia and Erwinia, which are almost pathogenic bacteria (46). Bifidobacterium is a gram-positive anaerobic bacterium with its number decreasing with age in the intestine, and is often used as a probiotic for the treatment of diarrhea (47). In this study, TLG supplementation significantly decreased the expression of the Enterobacteriaceae family and increased the expression of the Bifidobacterium genus in the ileum, colon and cecum (Figure 4), demonstrating that TLG supplementation had beneficial effects on the intestinal microbiota in weaned pigs. This has important implications for intestinal metabolism of amino acids (including glutamate, glutamine and arginine), as well as intestinal ATP production and health (48-52).
 Figure 4
Figure 4The relative abundances of bacteria in the ileum, colon and cecum of piglets receiving dietary supplementation with or without 0.5% trilactic glyceride (TLG). Dietary supplementation with TLG decreased the abundance of Enterobacteriaceae family and Lactobacillus genus, but increased the abundance of Bifidobacterium genus in the ileum, colon and cecum. The abundance of total bacterium was increased in the ileum but decreased in the colon and cecum. Values are means ± SD normalized by -log2 (TLG/control), n = 8. *p < 0.05.
Dietary supplementation with TLG to piglets reduces diarrhea incidence, improves blood lipid profiles (indicated by decreases in blood LDL and cholesterol and an increase in blood HDL), modulates lipid metabolism in the liver (indicated by downregulations of the genes for LIPE, LPL, FASN and SLC27A2), and promotes fat synthesis in adipose tissue (indicated by upregulations of gene expression of FASN and SLC27A2). In addition, TLG supplementation enhances intestinal absorptive function (indicated by increases in plasma D-xylose concentrations and the upregulation of intestinal expression of AQP8 and AQP10 genes), antioxidant capacity (indicated by the downregulation of NOX2 gene expression and the upregulation of expression of Nrf2 and GSTO2 genes), epithelial barrier, and cell growth and survival (indicated by increased abundances of the claudin-1 and occludin proteins and decreased abundances of the caspase-3 protein). TLG also modulates the intestinal microbiota (indicated by an increase in genus Bifidobacterium and a decrease of family Enterobacteriaceae) in piglets. Furthermore, TLG supplementation may regulate their hepatic growth (indicated by decreases in RNA/DNA, TP/DNA and IGFBP-3 levels in the liver). These findings have important implications for the nutrition and health of both swine and humans.
Drs Tao Wu, Kang Li and Yang Lyu contributed equally to this work and should be considered co-first authors. This work was jointly supported by the National Key R&D Program (grant number 2017YFD0500505, 2016YFD0501210), the Program of National Agricultural Research Outstanding Talents of China (2015), Hubei Provincial Key R&D Program (2019ABA083), the Open Project of Hubei Key Laboratory of Animal Nutrition and Feed Science (grant number 201805) and Texas A&M AgriLife Research (H-8200). We thank our students and technicians for their contributions to this research.
ADFI
average daily feed intake
average daily gain
albumin
aquaporin
basic leucine zipper
cholesterol
diarrhea rate
estrogen receptor-alpha
fatty acid synthase
ratio of feed intake to gain
gamma-glutamyl transferase
growth hormone receptor
glutathione S-transferase omega-2
high-density lipoprotein
insulin-like growth factor-binding protein 3
insulin receptor
low density lipoprotein
hormone sensitive lipase E
lipoprotein lipase
NADPH oxidase family-2
nuclear factor like 2
phosphoenolpyruvate carboxykinase 1
solute carrier family-27 member-2
trilactic glyceride
