Starvation induces tertiary hypothyroidism in adult rodents. Response of the hypothalamus-pituitary-thyroid (HPT) axis to starvation is stronger in adult males than in females. To improve the description of this sexual dimorphism, we analyzed the dynamics of HPT axis response to fasting at multiple levels. In adult rats of the same cohort, 24 and 48 h of starvation inhibited paraventricular nucleus Trh expression and serum concentrations of TSH and T4 earlier in males than in females, with lower intensity in females than in males. In adult females fasted for 36-72 h, serum TSH concentration decreased after 36 h, when the activity of thyrotropin-releasing hormone (TRH)-degrading ectoenzyme was increased in the median eminence. The kinetics of these events were distinct from those previously observed in male rats. We suggest that the sex difference in TSH secretion kinetics is driven not only at the level of paraventricular nucleus TRH neurons, but also by differences in post-secretory catabolism of TRH, with enhancement of TRH-degrading activity more sustained in male than female animals.
The sexual dimorphism of energy metabolism is evident at many levels; women differ to men in fat distribution, storage and energy expenditure. Females resist fat loss at the expense of decreased energy expenditure better than males in situations of food restriction and have a higher ratio of lipid/protein loss than males during starvation; this may be an evolutionary advantage for survival (1).
Thyroid hormones are crucial for energy homeostasis, being responsible for >30% of basal metabolic rate, and in concert with the sympathetic system modulate thermogenesis, lipid and carbohydrate metabolism, as well as fuel mobilization in energy demanding situations (2). Thyroid hormone levels are controlled by the hypothalamus-pituitary-thyroid (HPT) axis whose activity is coordinated centrally by the hypophysiotropic neurons of the paraventricular nucleus of the hypothalamus that synthesize thyrotropin-releasing hormone (TRH) and integrate metabolic information. Once released into portal veins at the median eminence (ME), TRH reaches the pituitary where it controls thyrotropin (TSH) synthesis and release (3-4). The thyroid gland responds to TSH by increasing synthesis and release of thyroxine (T4), and 3,3',5-triiodo-L-thyronine (T3). Conversion of T4 to T3 is performed by deiodinases 1 and 2. These enzymes are expressed and regulated in a tissue specific manner; and together with deiodinase 3, they set the levels of bioactive T3 in target tissues (5). Among these targets, tanycytes are glial cells surrounding the ventro-lateral portion of the third ventricle; β2-tanycytes have prolongations intermingled with TRH terminal endings and portal vessels in the lateral layer of the median eminence; from this site, tanycyte deiodinase 2 may provide T3 to TRHergic neurons where it exerts negative feedback regulation (3). Furthermore, β2-tanycyte synthesizes TRH-degrading ecto-enzyme (TRH-DE). Thyroid hormones up-regulate TRH-DE and the activity of this enzyme may control TRH half-life in the extracellular space in median eminence before reaching the thyrotrophs (6-7). A circulating isoform of TRH-DE, secreted by the liver and termed thyroliberinase (8), may contribute to extracellular TRH turnover and is also up-regulated by thyroid hormone (9).
The activity of the HPT axis is inhibited in situations of energy deficit as food restriction, fasting and pathological situations as cachexia (10-11). Male rats starved for various days have decreased synthesis and release of TRH, TSH and T3; thyroid hormone metabolism is also disturbed; deiodinase activity or expression in liver, as well as that of thyroid hormone receptors, transporters and UDP-glucuronidase 1A1 are diminished (12). The tertiary hypothyroidism produced, despite decreased serum concentration of thyroid hormone, is triggered by enhanced synthesis and activity of deiodinase 2 in tanycytes and levels of T3 available to TRHergic hypophysiotropic neurons (3, 13). Furthermore, median eminence TRH-DE and thyroliberinase activities increase during prolonged fasting, which may contribute to suppress the activity of HPT axis in adult male rats (14). Much less information has been obtained in female animals. In two-months old female rats fasted for 3 days, HPT activity was reduced as evidenced by decreased mRNA levels of Trh in PVN and Tshb in anterior pituitary, TRH concentration in portal blood, as well as reduced T4 and T3 serum concentrations; however, serum TSH concentration is barely changed (15).
Since contradictory results about fasting effect on concentrations of circulating hormones have been reported, that could be due to variations in the duration of starvation, metabolic status (obese or lean) or animal species (3, 11, 16-17), any conclusion about a sexual difference should be independently confirmed. Nevertheless, few reports have evaluated within the same experimental settings, HPT response of males and females to starvation. In rats 30-42-days old, circulating levels of TSH, T3 and T4 decrease more in females than in males after 2-4 days of fasting (17). In contrast, adult male rats (63-97 days of age) have a higher percent of decrease than females (48-94 days) in circulating TSH and T4 concentrations after 2 or 5 days of food deprivation (18-19). Consistent with a higher decrease of serum TSH concentration in adult males starved for 48 h than in females, expression of Trhde in the mediobasal hypothalamus increases more in males than in females although, the expression of Trh in PVN tends to decrease more in female than in male rats (19). These results suggest stronger effects of starvation on peripheral hormones of the thyroid axis in adult males than females.
The HPT axis is inhibited by stressors; in rodents, corticosterone is one of the mediators of this effect, acting both on PVN Trh mRNA levels and TSH secretion (10). In the report from Rondeel et al. (17), rats were studied in their adolescent period, when brain circuits between limbic areas are forming (20), and there is a strong sexual dimorphism in stress response (21). Females are more susceptible than males in this period whereas as adult, females might be more resilient specially to stress and metabolic threats (22). Thus, the developmental changes in sexual dimorphism of the susceptibilities to stress associate with inversion of sex-specific responsiveness of the HPT axis to fasting. Furthermore, the fasting-induced tertiary hypothyroidism is triggered in part by increased corticosterone serum concentration in male rats (3, 13), but in females is dependent on increased release of corticosterone (15). These data suggest that the intensity of HPA responses to stress contributes to sexual differences in HPT axis reactivity to fasting.
In this study, we compared the responses of the HPA and HPT axes to 24 and 48 h fast in adult female and male Wistar rats from the same cohort, to correlate both axes activities. These time points were chosen because they reveal the earliest adaptations to fasting in the central arm of the adult male HPT axis (3). Because sexual differences in HPT reactivity were confirmed, the second aim of this work was to evaluate the dynamics (36 to 72 h fasting) of TRH-DE activities and serum concentrations of TSH, thyroid hormones and corticosterone in adult female rats in conditions identical to those chosen for adult male rats in a previous study from our laboratory (14).
Female or male Wistar rats were raised and kept under standard conditions in a pathogen-free vivarium at the Institute of Biotechnology (UNAM). Adult rats were housed according to sex in separate temperature-controlled rooms (21 ± 1°C) on a 12 h light/12 h dark cycle (lights on at 7:00 a.m.). Animals had ad libitum access to standard laboratory chow (Teklad 2018SX, Envigo), except where indicated, and water was always freely available. Animals were group housed (2-3 rats/cage) since weaning, until beginning of experiment (body weight: male: 300-340 g, female: 175-250 g). Experiments were approved by the Committee of Bioethics of the Institute of Biotechnology, UNAM (project # 276) and, performed according to the Guide for the Care and Use of Laboratory Animals (8th edition), National Research Council of the National Academies of USA, and the Official Mexican Norm for production, care and use of laboratory animals (NOM-062-ZOO-1999).
Paradigm 1. Effect of 24 or 48 h fasting in male and female rats. Three different experiments were performed with males and females from the same cohort but choosing one female and one male from different mothers to avoid littermates of the same sex within each study group. After an initial body weight measurement, two-month old male or female rats were housed in individual cages and randomly assigned to the control group (feeding ad libitum), or to the 24 or 48 h fasting group (food deprived). Body weight was registered before sacrifice and control and fasting animals were killed on the same day.
Paradigm 2. Effect of 36 to 72 h fasting in female rats. Three independent experiments were performed. Animals were single housed at the start of the experiment. For experiments 1 and 2, animals were deprived of food for 36, 48, 60 or 72 h; and for experiment 3, for either 36 or 60 h. Control animals were allowed free access to food. Body weight was registered before and after 36, 48, 60 or 72 h of fasting.
Each experimental group included 5 animals. Animals were killed in an independent room by decapitation between 9:00 and 12:00 a.m.; control and fasting animals were killed alternatively to reduce time of day or stress differences. The brain and the anterior pituitary were carefully removed and immediately frozen on dry ice; trunk blood was collected for hormone concentration and thyroliberinase activity quantifications. To keep track of the stereotaxic coordinates, the frozen brain was mounted on a cryomicrotome and sections stained with hematoxylin-eosin. Two coronal slices were obtained to dissect the PVN (Bregma -0.84 to -2.4 mm) or the median eminence (Bregma -2.4 to -3.6 mm) (23), using a sample corer of 1 or 0.5 mm internal diameter for the PVN or median eminence respectively. White abdominal and brown adipose tissues were dissected and weighted.
Total RNA was extracted from each of the punched-out PVN samples using the thiocyanate-guanidine method (24); purity and quality was verified by spectrometry and electrophoresis, respectively. One microgram of RNA of each sample was reverse transcribed using the M-MLV Reverse Transcriptase; cDNA samples were stored at -20 °C until PCR amplification. Relative mRNA levels of Trh, thyrotropin-releasing hormone receptor 1 (Trhr), corticotropin-releasing hormone (Crh), corticotropin-releasing hormone receptor 1 (Crhr1), corticotropin-releasing hormone receptor 2 (Crhr2) and nuclear receptor subfamily 3 group C member 1 (Nr3c1; the gene that encodes the glucocorticoid receptor) in PVN were evaluated by PCR as previously described (25-28). Expression levels were normalized against peptidylproline isomerase A (Ppia) mRNA, except for Crhr1 which was normalized against glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (27).
TRH-DE and thyroliberinase activities were determined as described (14). Membranes were collected by centrifugation. Protein concentration was determined by the Bradford assay. TRH-DE and thyroliberinase activities were determined using thyrotropin-releasing hormone-β-naphtylamide as substrate in a coupled assay with excess dipeptidyl-aminopeptidase IV (EC 3.4.1.4.5) and inhibitors of pyroglutamyl peptidase I (EC 3.4.1.9.3), and of prolyl endopeptidase (EC 3.4.2.1.26). Enzymatic assays were performed at 37 °C under initial velocity conditions. Since expression and activity drastically diminish from the third ventricle wall into the brain parenchyma (6), the activity of TRH-DE in median eminence was not expressed per mg protein, but reported by structure, to minimize variation due to dissection error.
TRH was extracted from frozen median eminence and quantified using a specific polyclonal antibody by radioimmunoassay (RIA), as described (29). Samples were assayed in triplicate, taking their mean as one determination. Limit of sensitivity was 10 pg/tube; intra- and inter-assay variation coefficients were <10 %.
Serum TSH levels were analyzed by RIA using rNIDDK reagents (Bethesda, MD). Serum corticosterone levels were analyzed by RIA with reagents from Merck-Millipore, Perkin Elmer and Sigma. Total serum T3 and T4 (Diagnostica International, México) and 17-β estradiol (Arbor Assays) concentrations were quantified using ELISA kits. Serum of hypothyroid rats (25 µl) was added to the standard curves of thyroid hormone assays (30). Serum leptin concentrations were measured with an ELISA kit (Crystal Chem, Inc). Detection limits: TSH: 25 ng/ml; T4: 13 nMol/L; T3: 0.25 ng/mL; 17-β estradiol: 5 pg/mL; corticosterone: 25 ng/mL; leptin: 0.2 ng/mL. The intra-assay precision was <5 %, < 10 %, and < 4.3 %, and the inter-assay precision was <9 %, < 10 %, and < 4.5 % for TSH, corticosterone, and leptin respectively. The intra assay precision was < 4.3 % for T4, < 9.6 % for T3 and < 24 % for 17-β estradiol. Samples were analyzed in duplicate.
Except were indicated, data from independent experiments of paradigm 1 or 2 were pooled after transformation in percentage of mean control values (paradigm 1) or mean control value at 36 h (paradigm 2) taken as 100 %. Data were analyzed using GraphPad Prism 8.3. A two-way ANOVA was performed to evaluate main effects of sex or feeding status in paradigm 1, or to determine main effects of feeding status or time in paradigm 2. ANOVA data are indicated in Table 1. When ANOVA was significant, Tukey’s post hoc analysis was performed for multiple comparisons; data of post hoc analyses are shown in Table 2 or Figures. Pearson correlation coefficients were calculated and a two-tailed t-test performed. Level of significance was set at p< 0.05. The results are presented as mean ± S.E.M.
| Paradigm 1. Effects of 24 or 48 h starvation in adult male or female rats | |||
|---|---|---|---|
| Parameter | Sex | Feeding | Interaction |
| Body weight | F(1, 69)=0.001038, p=0.9744 | F(3, 69)=351.4, p<0.0001 | F(3, 69)=20.30, p<0.0001 |
| Percentage of initial body weight | F(1, 114)=2.832, p=0.0951 | F(2, 114)=112.0, p<0.0001 | F(2, 114)=0.1932, p=0.8246 |
| Weight of abdominal white fat pads | F(1, 25)=10.68, p=0.0031 | F(2, 25)=6.902, p=0.0045 | F(2, 25)=3.396, p=0.0496 |
| PVN Crhr1 expression | F(1, 45)=0.3142, p=0.57 | F(2, 45)=1.926; p=0.15 | F(2, 45)=2.05, p=0.14 |
| PVN Crhr2 expression | F(1, 41)=0.8938, p=0.3500 | F(2, 41)=3.123, p=0.0547 | F(2, 41)=4.874, p=0.0126 |
| PVN Nr3c1 expression | F(1, 56)=4.962, p=0.0299 | F(2, 56)=9.166; p=0.0004 | F(2, 56)=1.314, p=0.2769 |
| PVN crh expression | F(1, 43)=0.8765, p=0.354 | F(2, 43)=0.1065; p=0.8992 | F(2, 43)=2.75, p=0.07 |
| Serum corticosterone | F(1, 79)=8.268, p=0.0052 | F(2, 79)=21.59, p<0.0001 | F(2, 79)=2.321, p=0.1048 |
| PVN Trhr expression | F(1, 64)=38.01, p<0.0001 | F(2, 64)=0.7109, p=0.4950 | F(2, 64)=13.00, p<0.0001 |
| PVN trh expression | F(1,46)=0.6103, p=0.4387 | F(2, 46)=10.15, p=0.0002 | F(2, 46)=3.320, p=0.045 |
| Median eminence TRH | F(1, 25)=1.105, p=0.3032 | F(2, 25)=6.425, p=0.0056 | F(2, 25)=0.3701, p=0.6944 |
| Serum TSH | F(1, 59)=15.88, p=0.0002 | F(2, 59)=39.17, p<0.0001 | F(2, 59)=5.076, p=0.0092 |
| Serum T4 | F(1, 67)=0.02410, p=0.8771 | F(2, 67)=8.506, p=0.0005 | F(2, 67)=0.2532, p=0.7770 |
| Serum T3 | F(1, 48)=1.185, p=0.2818 | F(2, 48)=7.166, p=0.0019 | F(2, 48)=0.3356, p=0.7165 |
| Paradigm 2. Effects of 36-72 h starvation in adult female rats | |||
| Parameter | Time | Feeding | Interaction |
| Body weight | F(3, 32)=6.745, p=0.0012 | F(1, 32)=632.6, p<0.0001 | F(3, 32)=5.573, p=0.0034 |
| Serum 17-β Estradiol | F(3, 35)=0.667, p=0.577 | F(1, 35)=5.861, p=0.02 | F(3, 35)=1.6; p=0.206 |
| Serum Leptin | F(3, 32)=0.7411, p=0.5354 | F(1, 32)=105.6, p<0.0001 | F(3, 32)=0.1966; p=0.8979 |
| Serum corticosterone | F(3, 70)=2.166, p=0.0997 | F(1, 70)=34.20, p<0.0001 | F(3, 70)=0.8943, p=0.4486 |
| Serum TSH | F(3, 78)=2.505, p=0.06 | F(1, 78)=10.74, p=0.0016 | F(3, 78)=0.538, p=0.657 |
| Serum T4 | F (3, 91)=2.753, p=0.047 | F (1, 91)=14.33, p=0.0003 | F(3, 91)=0.654, p=0.582 |
| Serum T3 | F(3, 90)=3.327, p=0.023 | F(1, 90)=1.168, p=0.28 | F(3, 90)=1.429, p=0.239 |
| Median eminence TRH-DE activity | F(3, 91)=4.026, p=0.0097 | F(1, 91)=5.304, p=0.023 | F(3, 91)=2.534, p=0.0618 |
| Anterior pituitary TRH-DE activity | F(3, 91)=6.297, p=0.0006 | F(1, 91)=1.006, p=0.3185 | F(3, 91)=1.477, p=0.2260 |
| Thyroliberinase activity | F(3, 92)=14.51, p<0.0001 | F(1, 92)=9.638, p=0.0025 | F(3, 92)=2.26, p=0.0867 |
| Hormone | Feeding status | Duration of treatment (hour) | |||
|---|---|---|---|---|---|
| 36 | 48 | 60 | 72 | ||
| 17β-Estradiol (ng/mL) | Fed | 14.1 ± 1.8 | 18.3 ± 4.1 | 15.2 ± 0.8 | 21.8 ± 2.7 |
| Fasted | 11.9 ± 2.9 | 10.9 ± 2.7 | 14.7 ± 0.2 | 11.8 ± 2.09* | |
| Leptin (ng/mL) | Fed | 3.84 ± 0.2 | 4.77 ± 0.9 | 4.24 ± 0.6 | 3.92 ± 0.5 |
| Fasted | 1.21 ± 0.6*** | 0.74 ± 0.2*** | 0.81 ± 0.3*** | 0.31 ± 0.08*** | |
| TSH (ng/mL) | Fed | 1.21 ± 0.12 | 1.18 ± 0.09 | 1.1 ± 0.13 | 0.8 ± 0.05 |
| Fasted | 0.75 ± 0.12* | 1.15 ± 0.22 | 1.09 ± 0.38 | 0.65 ± 0.07 | |
| T4 (nmol/L) | Fed | 90.7 ± 8.2 | 83 ± 8.7 | 90.6 ± 10.8 | 93.5 ± 14.2 |
| Fasted | 58.1 ± 6.4 | 60.5 ± 3.6 | 61.8 ± 6 | 52.5 ± 3.7* | |
| T3 (nmol/L) | Fed | 7.23 ± 0.9 | 7.44 ± 0.6 | 5.38 ± 0.3 | 7.33 ± 0.9 |
| Fasted | 5.47 ± 0.2 | 4.67 ± 0.3 | 5.73 ± 0.8 | 5.9 ± 0.5 | |
Ponderal parameters. Fasting induced body weight loss; male rats lost more weight than females after 48 h of fasting (Figure 1A). However, as males are heavier than females, weight changes were calculated as % of initial weight; female and male rats lost the same weight, proportionally to their original weight (Figure 1B). Weight of abdominal white fat pads decreased only in females (Figure 1C,D). Brown adipose tissue weight was not significantly changed (not shown).
 Figure 1
Figure 1Effect of fasting on ponderal variables in male and female adult rats. Animals were either maintained with food or fasted during 24 or 48 h (paradigm 1). In each experiment, each group consisted of 5 male or female animals coming from the same cohort. At time 0, male and female body weights were: 332 ± 9 g and 223 ± 6 g, respectively. At sacrifice, abdominal adipose tissue weight were: male: 9.5 ± 0.9 g, female: 5.2 ± 0.5 g. Data (mean ± SEM) were pooled. Tukey’s post hoc analysis: *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001 compared to same sex fed animals; a: p<0.01 compared to other sex; #: p<0.0001 compared to 24 h.
HPA axis. Crhr1, Crhr2 and Crh gene expression was quantified in the PVN, to determine the level of stress attained by the animals after 24 or 48 h fasting. Both Crhr1, Crhr2 are coexpressed with Crh in the PVN (31) but are also expressed in other cell types (32). PVN CRH-R1 expression is enhanced by psychological stress (33); there were no significant changes in Crhr1 expression due to either sex or fasting (Figure 2A,B). Expression of Crhr2, which codes for a receptor that mediates the anorexic effects of CRH and urocortins, and is modulated by feeding conditions in the PVN (28, 34, 35), was not significantly changed by fasting, although there was a statistically significant interaction of sex with fasting; Crhr2 expression was significantly reduced between 24 and 48 h in males (Figure 2A,B). Expression of Nr3c1, which codes for the glucocorticoid receptor, decreased in males after 24 h (Figure 2A,B). Crh mRNA levels tended to decrease only in males after 48 h fasting (Figure 2A,B) (t-test: p=0.0516). Basal serum concentrations of corticosterone were higher in females than in males, and the relative increase was faster and higher in females than in males (Figure 2A,B).
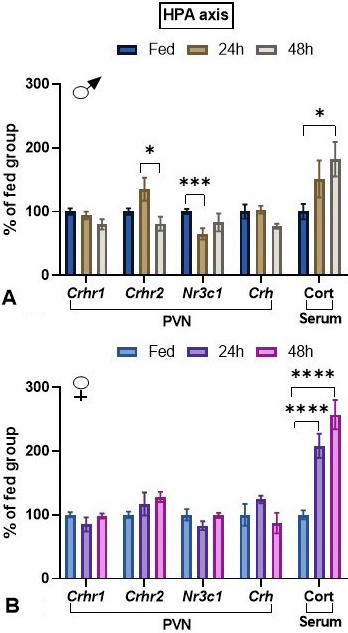 Figure 2
Figure 2Effect of fasting on HPA axis parameters in male and female adult rats. Animals were either maintained with food or fasted during 24 or 48 h (paradigm 1). In each experiment, each group consisted of 5 male or female animals coming from the same cohort. Data (mean ± SEM) were pooled. Tukey’s post hoc analysis: *: p<0.05, ***: p<0.001, ****: p<0.0001 compared to same sex fed animals. Abbreviation: Cort: corticosterone.
HPT axis. In the PVN, TRH nerve endings are in apposition with TRH (36, 37) and CRH (38) neurons, although the origin of these TRH afferents is unknown. After 1 or 2 days of starvation, Trhr mRNA levels decreased in males while increased in females (Figure 3A,B). Fasting decreased HPT axis activity. In male rats Trh expression in PVN was reduced, albeit the decrease at 48 h was significant only by t-test (p=0.04); in females, Trh expression decreased only after 48 h (Figure 3A,B). Although in male rats fasting did not increase significantly TRH content in median eminence, it did at 24 and 48 h in female rats, supporting decreased release (Figure 3A,B). Fasting decreased serum concentration of TSH at both time-points in male animals, but only at 48 h in females (Figure 3C,D). Fasting reduced serum T3 concentration significantly at 24 and 48 h in male rats, but only at 48 h in female rats (Figure 3C,D).
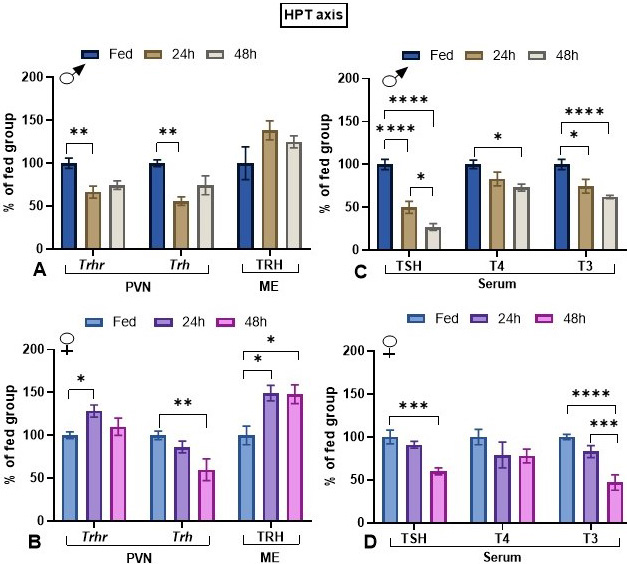 Figure 3
Figure 3Effect of fasting on HPT axis parameters in male and female adult rats. Animals were either maintained with food or fasted during 24 or 48 h (paradigm 1). In each experiment, each group consisted of 5 male or female animals coming from the same cohort. In fed animals, serum concentrations were: TSH, male: 2.69 ± 0.19 ng/mL, female: 2.34 ± 0.65 ng/mL; T4, male: 5.6 ± 0.3 ug/dl, female: 4.4 ± 0.4 ug/dl; T3, male 72 ± 3.7 ng/dl, female 85 ± 3 ng/dl. Fed animal median eminence (ME) TRH concentrations were: male: 143 ± 16 pg/mg, female: 134 ± 30 pg/mg. Data (mean ± SEM) were pooled. Tukey’s post hoc analysis: *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001 compared to same sex fed animals.
Correlations. Significant correlations were observed only in males. Corticosterone serum concentration correlated with PVN Trh mRNA levels, positively in controls (r=0.629, p=0.0095), while negatively in fasted rats although not significantly (r=-0.685, p=0.06), negatively with PVN Crh mRNA levels in controls (r=-0.96, p=0.00086) and positively with PVN Nr3c1 mRNA levels in fasted rats (r=-0.61, p=0.05). PVN Crh mRNA levels had a negative correlation with PVN Nr3c1 mRNA levels in fasted rats (r=-0.91, p=0.05).
This set of experiments aimed at studying the effects of starvation on the dynamics TRH-DE activity in 3 critical compartments in adult female rats, as well as of serum TSH, thyroid hormone and corticosterone concentrations. Experiments were performed exactly as described for adult male rats (14). Female rats lost body weight rapidly in the first 36 h and then progressively as fasting time augmented (Figure 4A).
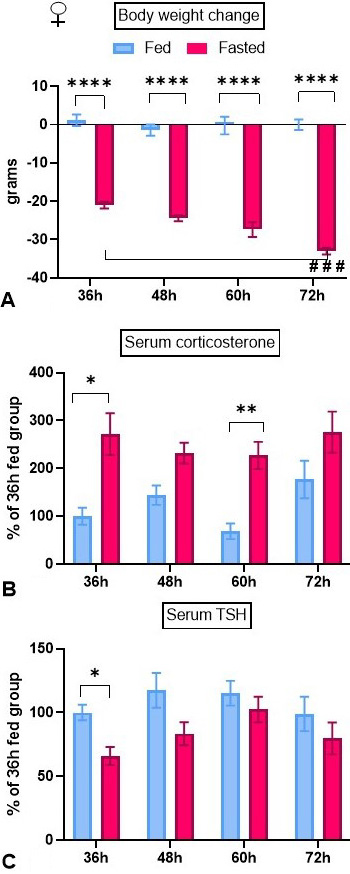 Figure 4
Figure 4Effect of fasting on body weight, and serum TSH and corticosterone concentration dynamics in female adult rats. Animals were isolated and either maintained with food or fasted during 36 to 72 h (paradigm 2). In each experiment, each group consisted of 5 female animals. At time 0, body weights were: Exp 1: 220 ± 1.9 g, Exp 2: 184 ± 1 g, Exp 3: 304 ± 7 g. In fed animals, serum concentrations at 36 h were: corticosterone: 299 ± 99 ng/ml, TSH: 0.68 ± 0.05 ng/ml. Compared to control fed male rats submitted to the same experimental protocol (14), in females serum corticosterone concentration was higher and TSH concentration lower. Data (mean ± SEM) were pooled. Tukey’s post hoc analysis: *: p<0.05, **: p<0.01, ****: p<0.0001 compared to fed animals; ###: p<0.001 compared to 36 h.
Endocrine parameters. Fasting decreased 17-β estradiol serum concentration at 72 h (Table 2). Serum leptin concentration was strongly decreased in fasted rats at all time-points (Table 2). Serum corticosterone concentration tended to be lower in control animals in the 36 and 60 h groups, that corresponded to animals that initiated the experiment in the evening. Fasting significantly increased serum corticosterone concentration at 36 and 60 h; increases were not significant at 48 and 72 h (Figure 4B). Serum TSH levels diminished after 36 h of fasting but not afterwards (Figure 4C). Serum concentration of T4 was reduced at 72 h fasting (Table 2). Serum concentration of T3 did not change (Table 2).
Activities of the TRH-degrading ectoenzyme. Median eminence TRH-DE activity was enhanced after 36 h of fasting but not later; activity in the fasting group at 72 h was lower than at 36 or 60 h (Figure 5A). In contrast, fasting did not change anterior pituitary TRH-DE activity, but we detected an increase in control activity in the 60 and 72 h groups compared to the 36 h group (Figure 5B). Thyroliberinase is a serum isoform of TRH-DE; fasting increased transiently its activity at 48 h (Figure 5C).
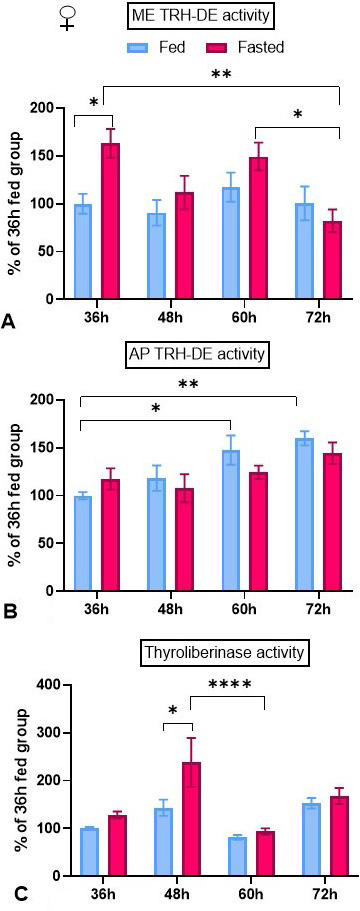 Figure 5
Figure 5Effect of fasting on TRH-DE activity dynamics in female adult rats. Animals were isolated and either maintained with food or fasted during 36 to 72 h (paradigm 2). In each experiment, each group consisted of 5 female animals. In fed animals, TRH-DE activities at 36 h were: in median eminence (ME): 0.97 ± 0.06 pmoles β-naphtylamide/min/ median eminence; in anterior pituitary (AP): 5.01 ± 0.5 pmoles β-naphtylamide/min/mg; in serum (thyroliberinase): 0.28 ± 0.01 pmoles β-naphtylamide/min/µl. These values were similar to values in fed male rats at 36 h (14). Data (mean ± SEM) from were pooled. Tukey’s post hoc analysis: *: p<0.05, **: p<0.01, ****: p<0.0001.
Correlations. Median eminence TRH-DE activity correlated positively with serum corticosterone concentration at the 60-h time-point (Figure 6A), but not at other or with all time-points (not shown), and negatively with serum TSH concentration at the 36 and 60 h time-points (Figure 6B), but not at other or with all time-points (not shown).
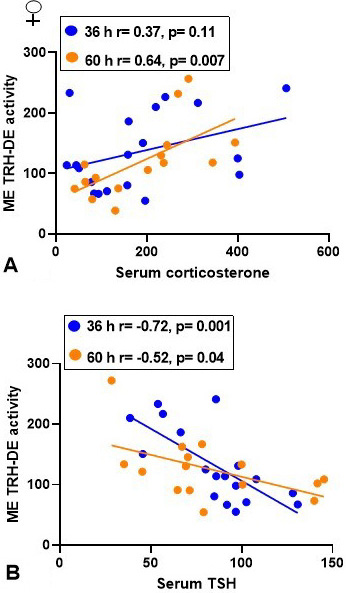 Figure 6
Figure 6Correlation between median eminence (ME) TRH-DE activity, serum corticosterone and TSH concentrations in adult female rats either fed or fasting for 32 or 60 h in individual cages (paradigm 2). Individual data are in percentage of mean control value at 36 h taken as 100 %. Pearson correlation coefficients were calculated and a two-tailed t-test performed.
Many aspects of metabolism are sexually dimorphic. Greater energy expenditure in female than in male rats fed ad libitum may involve higher metabolic rate and thermogenic activation (40), which could be partially due to higher serum concentration of T3 in females than in males, and to the effects of estrogen on energy expenditure (40-41). Furthermore, basal concentrations of corticosterone are higher in females (42). In response to starvation and energy deficits, females show a stronger decrease in facultative thermogenesis than males (40). In the present study, we observed that at 24-48 h of starvation adult females had a higher relative loss of abdominal adipose tissue weight than males, who lost a similar percentage of body weight than females, probably due to lean tissue loss (43-44). The inhibition of HPT axis activity is considered an adaptation to reduce energy utilization, decreasing basal metabolic rate and energy expenditure; it occurs in both males and females, but few studies have directly compared responses in adults of both sexes (18-19), and none the temporal changes of the responses on multiple HPT axis parameters.
In this study, we evaluated parameters of HPA axis activity in response to starvation to identify relationships between HPA and HPT responses. A gender difference in the response of CRH neurons from the PVN is observed in a variety of stressful stimuli (42, 45); part of this difference is due to the effects of hormones since estrogen stimulate the activity of HPA axis while testosterone inhibits it (46); however, metabolic cues play an important sexually dimorphic role too. In agreement with previously published data (47-49), PVN Crh mRNA levels tended to decrease only in male animals at 48 h food deprivation. In addition, we measured Crhr2 expression, which is expressed in the PVN CRH neurons, since we have previously shown that PVN Crhr2 expression is slightly stimulated after one week of strong food restriction in males but not in females, whereas it is inhibited in rats subjected to the dehydration model of anorexia (28). Although the effect of fasting on Crhr2 expression was almost significant, statistical interaction between sex and fasting was significant. Because CRH-R2 can mediate stress-induced activation of the HPA axis (50), our observation suggests that differential activation of the receptor may contribute to sexual differences of Crh response. In contrast to males in which Nr3c1 expression decreased in the first day of starvation, there were no changes in females. Finally, the relative corticosterone response did not show consistent differences between sexes, since it was higher in females than in males in paradigm 1 (Figure 2) while the reverse occurred in paradigm 2 (compare data in Table 2 with (14). Data like those in paradigm 1 have been explained by reduced hormone clearance in female compared to male fasted rats (51), but our results suggest that increased sensitivity to corticosterone feedback in male animals may also contribute to diminish CRH release and serum corticosterone responses. Discrepancy between both paradigms is likely due to experimental protocols differences. To avoid effects of circadian cycle at time of sacrifice, in paradigm 2 rats that were submitted to fasting for 36 or 60 h were left without food at the beginning of the active phase; inevitably hunger will generate a stronger initial stress than in the 24 and 48 h fasted groups.
Data from our study give further support to the existence of a sexual dimorphism in the response of various central parameters of HPT axis to fasting. Thus, at 24 h fasting only males showed diminished PVN Trh expression while at 48 h, Trh expression diminished more in females, in agreement with previous data at 48 h (19). In male rodents, the mechanism of starvation-induced HPT axis inhibition involves decreased serum leptin concentration and increases in T3 concentration in the median eminence (3, 52). Leptin concentration is higher in adult females than males, and the rate of its reduction by fasting differs between sexes. For example, 12 h food restriction induces a greater leptin decrease in females than males (53). Leptin action can be direct on TRH neurons, but is predominantly relayed through arcuate nucleus (ARC) neurons (3). ARC neurons respond differentially to fasting in male and female rats (47). Furthermore, the structure of these neurons is sexually dimorphic; for example, male mice have a decreased density of pro-opiomelanocortin (POMC) fibers in the arcuate nucleus, and decreased Pomc gene and protein expression, which is associated with an increased energy intake (54). Since ARC POMC and Agouti Related Protein neurons regulate PVN TRH neurons (3), it is likely that they strongly induce sex-specific responses.
The hypothesis that part of the sexual difference in PVN Trh expression response to fasting is due to differential corticosterone action is compatible with ours, and with published data. Adrenalectomy and corticosterone substitution blunt the fasting induced inhibition of Trh mRNA levels in the PVN of Zucker obese rats (55). A negative relationship between serum corticosterone concentration and PVN Trh expression was observed only in fasting males. It is thus apparent that the effects of elevated corticosterone concentration cannot solely account for the differential response in PVN Trh expression observed between males and females.
The sexual dimorphism of PVN Trh expression was associated with that of Trhr expression in the PVN. At least part of PVN Trhr expression likely maps to PVN TRH neurons, since TRH endings make synaptic contact on TRH neurons of the PVN (36, 56). TRH injections into the PVN of male rats rapidly promotes the activity of the HPT axis (57). Since TRH-R1 receptors are subject to ligand-induced down regulation (58), and PVN Trhr mRNA levels were decreased after 24 h of starvation in males while increased in females, this suggests increased TRH signaling in males and the opposite in females during fasting. This may contribute to the sex difference of Trh expression kinetics during fasting. We have previously shown that Trhr expression is downregulated in PVN of females that have been strongly food-restricted for 7 days (59), suggesting that PVN Trhr expression is indeed sensitive to energy balance. Finally, since CRH endings make synaptic contacts on TRH neurons of the PVN (56), and the long-term injection of an antagonist of Crhr2 into the PVN can reduce HPT axis activity in dehydration-induced anorexic male rats, including PVN Trh mRNA levels (28), and sex interacted with fasting to regulate PVN Crhr2 expression, further studies on Crhr2 role in sexual differences of HPT axis function are warranted.
TRH content in the median eminence can reveal changes in synthesis/transport and release of TRH from PVN hypophysiotropic neurons (10). Fasting increased TRH content in median eminence of females, as reported (18), but not in males. Thus, despite a strong reduction of PVN Trh mRNA levels, median eminence TRH levels were maintained or enhanced. If we assume that there were no changes in TRH precursor processing, the data are consistent with reduced TRH release during fasting; however, they do not correlate with the sexually dimorphic response in serum TSH concentration, pointing to additional factors which shape TSH response.
Once released into the median eminence extracellular space, and before reaching the thyrotrophs, TRH can be hydrolyzed by TRH-DE expressed on tanycyte cell surface and maybe by thyroliberinase in the portal blood. In male rats, we had previously observed a late (at 72 h) increase of median eminence TRH-DE activity and an earlier increase in thyroliberinase activity, which may have contributed to maintain TRH output low when fasting is prolonged (14). The impact of fasting on Dio2 expression in tanycytes has been attributed to the combined effect of decreased leptin and increased corticosterone serum concentrations (3, 60). Since median eminence TRH-DE activity of fed animals was not affected by adrenalectomy and corticosterone replacement (14), and thyroid hormones can rapidly enhance Trhde expression in tanycytes, we proposed that during fasting enhanced deiodinase 2 activity in tanycytes (6, 61) promoted T3 production and Trhde expression (14). In the same experimental paradigm, female rats had transient increase of median eminence TRH-DE and of thyroliberinase activities at 36 and 48 h respectively. In this case, we observed a positive correlation between TRH-DE activity and serum corticosterone concentration, which suggests this hormone could positively regulate TRH-DE activity in tanycytes of female rats. Elucidating the role of corticosterone, if any, in this sex difference will require functional experiments. On the other hand, the sexually dimorphic regulation of median eminence TRH-DE activity is consistent with a previous study in which we observed that median eminence Trhde expression was increased by 48 h fasting in adult male rats, and much less in female rats (19). As for adult male animals (14), we did not find changes at anterior pituitary level, where the activity may control TRH action on prolactin secretion, but not on thyrotropin release (62). Failure to detect an effect on TRH-DE anterior pituitary activity is consistent with a previous study showing that 7 days of food restriction in adult female rats does not change Trhde expression (59). The most striking difference between both sexes was the increase of median eminence TRH-DE activity at the earliest time-point (36 h) in females, which suggests that hydrolysis of TRH in the median eminence extracellular space increased early and may contribute to limit TRH-induced TSH secretion; correlation data were consistent with this hypothesis in the 36-60 h time span.
In response to fasting, circulating concentrations of TSH decreased earlier (24 h) in males than in females and were maintained low for up to 72 h in male (14), but not in female whose values rapidly reversed to control values. Although there are probably other determinants of the shape of TSH secretion, it appears that PVN Trh and Trhr expression, and median eminence TRH-DE and thyroliberinase activities demonstrate a sex difference that may contribute heavily to differentiate the intensity and rate of change of TSH secretion during fasting. Thus, we suggest that the early drop in males is likely driven by a strong reduction of TRH neurons activity, while the later plateau is maintained in part by increased thyroliberinase and median eminence TRH-DE activity. In contrast, in female rats the delayed reduction of TRH neurons activity coincided with a transient increase of TRH-DE activity and led to a lag in reduction of serum TSH concentration, which was transitory. In addition, there is a negative association between serum TSH and corticosterone concentrations during starvation (15), which is partially prevented by adrenalectomy and corticosterone substitution (15, 63), and that may be explained, not only by hypothalamic effects, but also through pituitary control (10).
In spite of the sex difference in the kinetics of serum TSH concentration, serum T4 and T3 concentrations showed no differences related to sex. The serum concentration of both hormones diminished slowly and was relatively low at later time-points. This is consistent with data that show that serum T3 and T4 concentrations depend not only on TSH concentration but also on many tissue specific bio-transformations that impact on serum values (64-65).
In conclusion, the central arm of the HPT axis showed a response to fasting that was more rapid and intense in male than in female adult rats, in agreement with previous studies that directly compared both sexes in adult rats (18-19). We suggest that the sexual dimorphism in TSH secretion patterns is driven in part at the level of PVN TRH neurons, as well as by additional differences in median eminence TRH-DE and thyroliberinase activities, with enhancement of TRH inactivation more sustained in male than female animals (Figure 7). The proximal causes for these sex differences likely lie in the structure of the hypothalamic circuits controlling the TRH neurons and in the dynamic of peripheral signals; among these, corticosterone dynamics may be relevant. Although the sexual dimorphism of the central arm of the HPT axis in response to fasting does not impact much on serum thyroid hormone concentrations, it is likely that it has target tissue specific consequences.
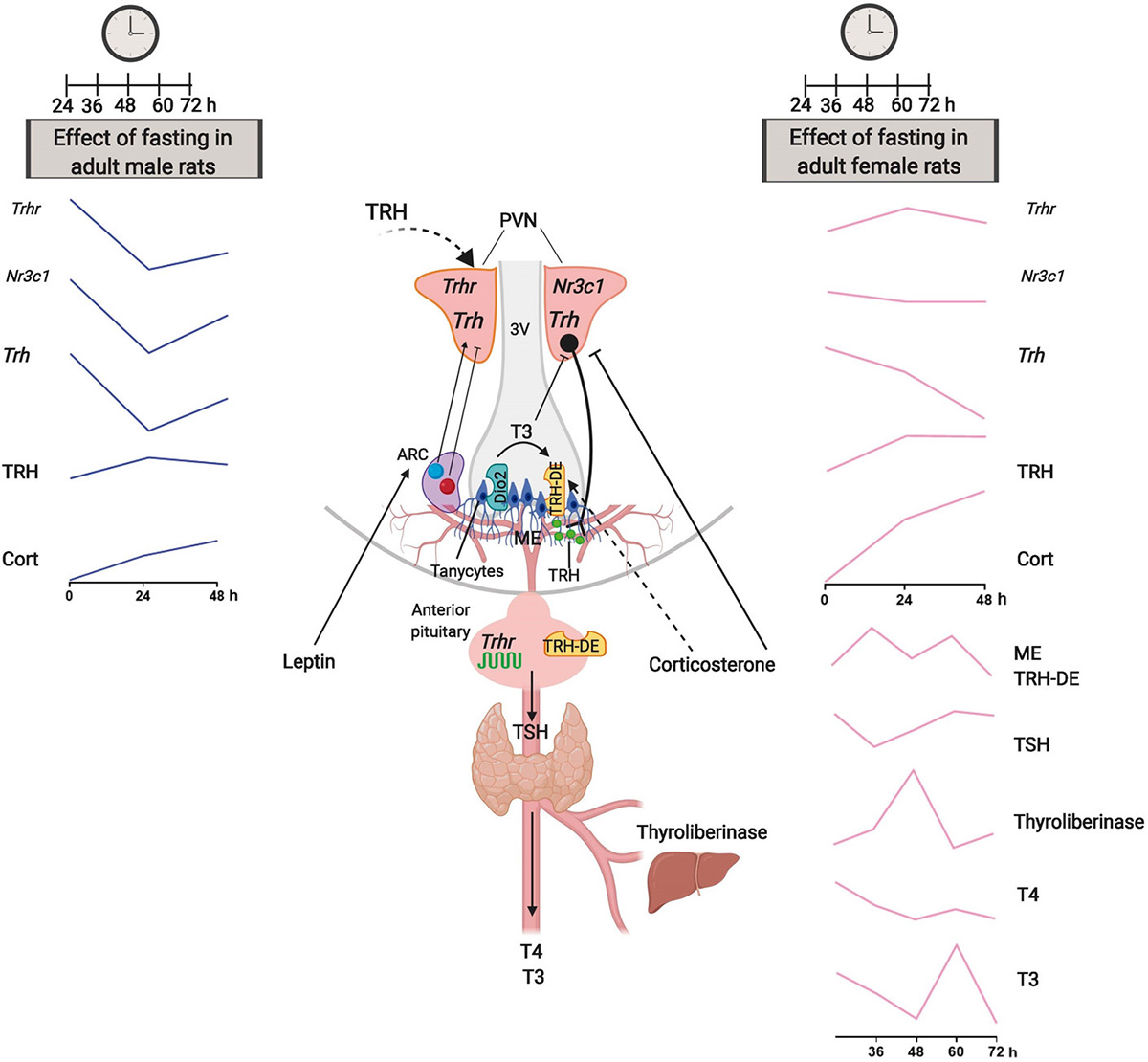 Figure 7
Figure 7Sexual dimorphic kinetics of HPT axis response to fasting in adult rats. Central part: schematics of the HPT axis, with emphasis on major structures and signals. The left and right panels describe the kinetics of the multiple parameters that determine the activity of the HPT axis, in male and female rats, respectively. Notable differences between male vs female rats include enhanced response at PVN level (Trhr, Nr3c1 and Trh), delayed enhancement of catabolism of TRH (TRH-DE and thyroliberinase activities), and sustained decreases of serum TSH and thyroid hormones concentrations; see male vs female 24-48 h kinetics, and female (this study) vs male (14) 36-72 h kinetics. We propose that the distinct kinetics of TSH and thyroid hormone response to fasting are in part driven by dimorphic adjustments in PVN capacity to generate TRH and in median eminence TRH release and catabolism.
We thank the technical assistance of Miguel Cisneros, Fidelia Romero, Elizabeth Mata, Graciela Cabeza, and Sergio González. Supported in part by grants from CONACYT (CB254960 and PN2015-562) to JLC. PJB and JLC conceived and designed the experiments, analyzed data, and prepared the manuscript; IL, ES, RMU, LJ and MGM generated and analyzed data. All authors approved the final version of the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
HPT
hypothalamus-pituitary-thyroid
hypothalamus-pituitary-adrenal
thyrotropin-releasing hormone
thyrotropin
thyrotropin β
thyrotropin-releasing hormone-degrading ectoenzyme
thyroxine
3,3',5-triiodo-L-thyronine
deiodinase
paraventricular nucleus
thyrotropin-releasing hormone receptor 1
corticotropin releasing hormone
corticotropin releasing hormone receptor 1
corticotropin releasing hormone receptor 2
glucocorticoid receptor
cyclophilin A
radioimmunoassay
pro-opiomelanocortin
arcuate nucleus
