The trace element iron plays important roles in biological systems. Vital functions of both host organisms and pathogens require iron. During infection, the innate immune system reduces iron availability for invading organisms. Pathogens acquire iron through different mechanisms, primarily through the secretion of high-affinity iron chelating compounds known as siderophores. Bacterial siderophores have been used clinically for iron chelation, however synthetic iron chelators are superior for treating infection because - in contrast to siderophore-bound iron - bacteria are not able to utilize iron bound to those molecules. Additionally, utilizing siderophores-dependent iron uptake in a “trojan horse” manner represents a potential option to carry antibiotics into bacterial cells. Recently, synthetic iron chelators have been shown to enhance antibiotic effectiveness and overcome antibiotic resistance. This has implications for the treatment of infections through combination therapy of iron chelators and antibiotics.
Iron plays important roles in biological systems as a co-factor in multiple cellular pathways including: DNA synthesis and repair, electron transfer, oxygen transport and immune response (1–4). Iron exists in the 2+ (ferrous) as well as the 3+ (ferric) valence state and is absorbed by duodenal enterocytes through active, enzymatic processes, e.g. by divalent metal transporter 1 (DMT1). Iron is exported into the plasma by the iron exporter ferroportin (5). In the plasma, ferric iron is bound to transferrin in order to circulate to sites of use (6). The majority of iron is used in erythropoiesis. Erythroid precursors express cell-surface transferrin receptors, which act to take up iron by receptor mediated endocytosis (7). Finally, 65-75% of iron in the body is contained within the protoporphyrin ring of heme, associated with hemoglobin (8). Excess iron can be stored in enterocytes, hepatocytes, macrophages and other cells by binding to ferritin (9). Macrophages are able to recycle iron by phagocytosis of aged or damaged erythrocytes. In healthy individuals, the availability of free iron is limited by tight regulation of absorption, recycling and intracellular storage. Hepcidin, a peptide hormone secreted by the liver, has been shown to regulate iron homoeostasis by binding to ferroportin causing it’s internalization and degradation, preventing extracellular iron efflux (10). Transferrin receptor 1 and bone morphogenetic protein 6 receptor are able to sense transferrin saturation and tissue iron content. This allows for regulation of hepcidin expression, communicating a need for more or less iron (11).
Iron also catalyzes the production of reactive oxygen species (ROS) such as highly reactive hydroxyl radicals from the association of superoxide and hydrogen peroxide, commonly known as the Haber-Weiss reaction. The generation of ROS is one of the first responses of immune cells to an infection in order to destroy invading microbes (12). However, overproduction of ROS can lead to adverse effects on host cells by damaging DNA, lipids and proteins (13). In response to infection, the body reduces the availability of iron in both the extracellular and intracellular space. This host defense mechanism blocks iron availability for invading microorganisms, restricting their ability to proliferate and is mediated by hepcidin. In infection, increased hepcidin synthesis is induced by inflammatory mediators, such as interleukin (IL)-6 and IL-22 (10, 14, 15). Other proinflammatory stimuli such as tumor necrosis factor α (TNF-α) and interferon-γ have been linked to the upregulation of DMT1 expression, resulting in an increased uptake of iron into macrophages (16). Furthermore, IL-4, IL-10 and IL-13 increase the expression of ferritin, promoting iron storage within monocytes and macrophages (17). In summary, all of these pathophysiological mechanisms of iron retention appear to be beneficial in fighting infections by reducing levels of available iron for microbes.
Pathogens have developed various strategies to acquire iron. The majority of bacteria and fungi utilize high-affinity chelating compounds to bind iron, so called siderophores. This area has been extensively studied and hundreds of siderophores have been discovered. Siderophores are classified into three main structural families, carboxylates, catecholates and hydroxamates (18). Catecholate siderophores have the highest affinity for ferric iron relative to carboxylate and hydroxamate siderophores under physiological conditions. Enterobactin, a catecholate siderophore, exhibits the highest known affinity for ferric iron, higher than that of the host iron binding protein transferrin (18). Hydroxamate siderophores are the most common group of siderophores in nature (19). Some siderophores from bacteria are used clinically for iron chelation. Desferrioxamine (DFO) is a clinically approved iron chelator originating from Streptomyces pilosus (20). DFO has three bidentate hydroxamic acid groups along the DFO backbone. The chain-like molecules wrap around iron in a 1:1 Fe (3+)/DFO complex manner (21). DFO has been used clinically to mobilize excess iron to excretion in patients with hemochromatosis and beta-thalassemia (22). However, DFO is not ideal as an iron chelator in bacterial infections as many bacterial species are able to utilize the iron sequestered within DFO (23). Growth of Microbacterium spp. has been shown to essentially require DFO while the growth of other bacteria (e.g., Gordonia, Paenibacillus and Burkholderia) is significantly promoted by DFO presence (24).
Synthetic iron chelators typically contain oxygen, nitrogen or sulfur-donor atoms that form bonds with iron. They are chemical diverse, allowing for different binding capacities and preference for ferric or ferrous iron. In order to be used therapeutically, chelators must successfully compete with biological iron binding substances. Although DFO has been shown to effectively treat iron overload, it requires prolonged infusions five to seven days per week. Research has therefore focused on developing superior, synthetic iron chelators. A summary of different iron chelator properties can be found in Table 1 (25).
| Properties | Deferoxamine | Deferiprone | Deferasirox |
|---|---|---|---|
|
Binding capacity |
Hexadentate (1:1) | Tridentate (3:1) | Bidentate (2:1) |
| Route of administration | Subcutaneous, intravenous | Oral tablet | Oral tablet |
| Side Effects |
Local skin reaction |
Agranulocytosis Musculoskeletal and joint pains Gastrointestinal |
Gastrointestinal |
| Half-life | 47-134 minutes | 3-4 hours | 8-16 hours |
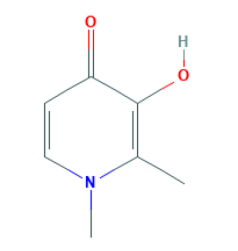
| Structure |  |
 |
 |
Deferiprone (Ferriprox®; DFP) is an FDA-approved oral iron chelator. DFP has comparable efficacy to DFO but is more effective in removing iron from the heart. It has a plasma half-life of 47-134 minutes and should therefore be taken three times daily. Major toxic side effects of DFP include agranulocytosis, musculoskeletal and joint pains, gastric intolerance, and zinc deficiency. However, these are considered to be reversible (26).
Deferasirox (Exjade®; DFX) is an FDA-approved oral iron chelator with similar efficacy to that of DFO in reducing liver iron levels. DFX has a plasma half-life of 8 to 16 hours and should therefore be taken once daily. Gastrointestinal disturbances are the most common side effect of DFX but can be improved by changing time of DFX administration. Rare reports of hepatic failure and serum creatinine increase have led to the suggestion that liver and kidney function should be monitored monthly (27).
Iron withholding is a host defense mechanism against invading pathogens. Synthetic iron chelation intensifies the host defense strategy, possessing antimicrobial potential. Recent studies suggest a potential role for iron chelators in treating infection. The impact of a novel iron chelator, DIBI, on bacterial proliferation in a murine model of sepsis found a significant decrease in bacterial counts in blood and peritoneal lavage fluid (PLF) when DIBI was used in combination with imipenem (28). Iron chelators have also been shown as an effective therapy against biofilms formed by Pseudomonas aeruginosa. The combination of DFO or deferasirox (DFX) with tobramycin reduced established biofilms and viable bacteria, as well as prevented the formation of new biofilm on cystic fibrosis airway cells (29). Lastly, combination therapy of DFX and vancomycin was shown to reduce viability of methicillin-resistant and vancomycin-intermediate strains of Staphylococcus aureus in vitro (30).
Further strategies for iron acquisition have been extensively studied in Gram-negative bacteria, few examples are known in regard to Gram-positive bacteria (31). Several pathogenic bacteria secrete hemolysins to lyse erythrocytes, and hemoglobin proteases to degrade the protein (32). The expression of hemolysins and other virulence factors is under control of the accessory gene regulatory (Agr) quorum-sensing two-component system (33). The small molecule savarin has been shown to alter binding of Agr to DNA and thereby prevent virulence gene upregulation. Studies by Sully et al. found savarin to be an effective treatment in murine S. aureus skin infection models by inhibiting Agr quorum sensing (34).
Gram-negative pathogens are able to uptake heme directly through TonB-dependent outer membrane (OM) receptors. Transport through the periplasmic and inner membrane is then facilitated by ABC transport system. Once in the cytoplasm heme is degraded and the iron is stored (31). Gram-negative bacteria are also able to acquire iron through the secretion of hemophores which have the ability to bind heme and heme-containing proteins. These hemophores then deliver heme to OM receptors (e.g., HasR) which allow heme to be internalized (35). Pseudomonas aeruginosa acquires heme via the hemophore, heme acquisition system A (HasA) protein. Studies by Shirataki et al. showed the crystal structure of the heme-loaded HasA and concluded that other metal complexes could also fit within the binding site. This knowledge allowed them to use Fe-phthalocyanine (Fe-Pc), a synthetic metal complex, to bind HasA and inhibit growth of P. aeruginosa, even in the presence of heme-bound HasA (36).
In Gram-positive bacteria, the iron regulated surface determinant (Isd) system of Staphylococcus aureus is a widely studied mechanism of iron acquisition from heme and hemoglobin. The Isd system consists of nine iron regulated proteins, IsdA, IsdB, IsdC, IsdH/HarA, IsdD/E/F, IsdG and IsdI. Together, these proteins interact with heme proteins to extract the heme molecule and internalize it into the cytoplasm of bacteria (37). Bacterial pathogens are also able to acquire iron through sources of iron such as transferrin, lactoferrin and ferritins through OM receptors that directly recognize these proteins. Neisseria utilizes transferrin-binding protein A (TbpA), a Ton-B dependent receptor, for the uptake of iron from human transferrin (38). It has been demonstrated that E. coli of intestinal origin is capable of using catecholamines in order to acquire iron from lactoferrin and transferrin, stimulating the growth of bacteria (39).
Bacteria utilize the ferric uptake regulator (Fur) family of transcriptional activators in order to control iron metabolism (40). Fur is a DNA-binding repressor that uses ferrous iron as a co-factor. Under normal iron conditions Fur binds to promotor regions of iron-regulated gene to repress their expression. During iron starvation, the complex dissociates from DNA allowing transcription of iron-regulated genes (32). However, fur has a higher affinity for Zn2+. Consequently, studies by Klemm and colleagues impaired the fur system by treating urinary tract E. coli and K. pneumonia isolates with high levels of Zn2+. This created competition for fur and effectively reduced biofilm formation (41).
Bacteria with stronger iron acquisition strategies have a growth advantage. Klebsiella pneumoniae is known to cause a significant number of community-acquired (CA) infections. Studies by Holt et al. found isolates from CA invasive infections to have additional siderophore and iron-metabolism genes. They concluded this enhanced ability to sequester host iron allowing K. pneumoniae to cause disease in immunologically competent human hosts (42, 43). Targeting the nutritional immunity of invading microbes (i.e. iron metabolism) has been shown to have impact on virulence. Therefore, targeting the range of different proteins involved in bacterial iron uptake presents as a possible novel target for new antibiotics. However, uptake mechanisms and proteins vary across pathogens. This requires a therapy with a carefully chosen target protein or pathway, in contrast to a broad-range treatment which may not be suitable.
Bacterial structure plays a key role in antibiotic resistance. The bacterial envelope, consisting of an inner and outer membrane, decreases antibiotic penetration. Utilizing siderophore-dependent iron uptake in a “Trojan horse” manner has been used as a strategy to circumvent this barrier. This strategy assembles the siderophore, linker and antibiotic into one conjugate in order for the bacteria to attempt to make use of the siderophore and in doing so, transport the antibiotic across the outer membrane. Most conjugates are unable to be transported across the inner membrane, therefore the most successful utilize antibiotics with periplasmic targets (44). Siderophore-antibiotic conjugates have been developed for use against Pseudomonas aeruginosa. Budzikiewicz and colleagues conjugated PVD to ampicillin and tested its activity against P. aeruginosa. The conjugate had high levels of antibacterial activity against ampicillin resistant strains. When conjugated with PVD, ampicillin was able to utilize the iron-uptake pathway in order to reach its target in the periplasm and inhibit synthesis of the peptidoglycan (45).
The rapid appearance of antibiotic resistance is termed by the Centers for Disease Control and Prevention (CDC) as a global crisis requiring the development of new antibiotics and novel approaches to the treatment of infections (46, 47). Recent evidence suggests iron chelators as potential treatment to overcome antibiotic resistances in both fungi and bacteria. Growth of Candida albicans, an opportunistic pathogen with developing resistance to antifungals, has been shown to be inhibited when treated with DIBI at low concentrations and in combination with fluconazole (FLC) in vitro. Furthermore, combination treatment of DIBI/FLC resulted in total growth inhibition in vitro of FLC resistant C. albicans strain LP1158-07, demonstrating the ability of iron chelation to overcome antibiotic resistance (48). The ability of iron chelators to overcome antibiotic resistance has also been shown in Acinetobacter baumannii, a major cause of hospital-acquired pneumonia. Mice infected with ciprofloxacin (CIP)-resistant A. baumannii strain LAC-4 were treated with CIP/DIBI combination in vivo. DIBI was found to improve the treatment efficacy of CIP and reduce bacterial burdens in the lung, spleen and blood. In addition, DIBI enhanced the efficacy of CIP for CIP-resistant LAC-4 isolates in vitro (23). Results of these studies demonstrate the potential of iron chelators to work in conjunction with antibiotics, in order to improve their effectiveness of and overcome antibiotic resistance.
Decreased systemic iron levels reduce erythropoiesis and are responsible for the so-called anemia of chronic disease (ACD). ACD is considered the second most prevalent anemia worldwide, after iron deficiency anemia, and is linked to a wide range of chronic infections and autoimmune diseases (Table 2). In recent literature it remains unclear whether ACD is marker of disease severity and progression or a causative factor (49). In patients suffering from ACD, the natural response of increasing erythropoietin with decreasing levels of hemoglobin is weakened and proliferation and differentiation of erythroid precursors is diminished. The resulting reduced hemoglobin levels can impact morbidity and mortality (50). ACD results from the bodies defense mechanism against invading bacteria, therefore the best treatment is curing the underlying inflammatory/infectious disease. In other clinical conditions, low plasma iron levels characteristic of anemia are treated with oral or intravenous iron supplementation. However, this treatment is of concern in critically ill patients as iron potentiates bacterial growth (51). Furthermore, studies found intravenous administration of iron to be associated with an increased risk of all cause infection (52).
| ● Disease Groups |
| ● Immune-mediated diseases |
| ● Cancer |
| ● Infections |
| ● Inflammatory diseases |
| ● Chronic kidney diseases |
| ● Anemia of critical illness |
| ● Congestive heart failure |
| ● Obesity |
| ● Anemia of the elderly |
| ● Chronic pulmonary disease |
In conclusion, iron balance is necessary in order to treat acute infections. Iron is crucial during the host’s innate immune response (i.e. ROS production). However, iron restriction proves effective in reducing bacterial proliferation. Bacteria with enhanced iron uptake mechanisms (e.g., Klebsiella pneumoniae) have been shown to have increased virulence. Therefore, methods for targeting bacterial iron uptake present as a strategy to provide bacterial iron starvation without depriving the host. Synthetic iron chelators enhance the hosts innate iron withholding mechanisms and have been shown to reduce viability of bacteria. Recent studies demonstrate the ability of iron chelators, in combination with antibiotics, to not only improve the effectiveness of antibiotics but to overcome antibiotic resistance. Future studies could use this knowledge to create combination therapies, using synthetic iron chelators, to treat life-threatening infections.
