Atherosclerosis (AS) is one of the leading causes of death, with variable frequencies in different populations. To this end, we examined whether AS is associated with the miR-145 rs353291 polymorphism and specific rs353291 alleles. The distribution of the rs353291 C allele and CC genotypes was significantly higher in AS patients than in controls. Compared to individuals with the rs353291 TT genotype, carriers of the rs353291 C allele showed a significant increase in the plasma levels of sCD40L, IL-6 and MCP-1 and a decrease in miR-145 expression. After ox-LDL administration, the peripheral blood mononuclear cells (PBMCs) of rs353291 C allele carriers showed significantly increased CD40 and MCP-1 expression compared with those of individuals with the rs353291 TT genotype. These data suggest that the rs353291 C allele may increase susceptibility to atherosclerosis.
Atherosclerosis (AS) is a chronic inflammatory disease characterized by the formation of AS plaques. AS is also the common pathologic basis of coronary artery disease (CAD), peripheral artery disease (PAD), and ischemic stroke. To date, atherothrombosis is still the leading cause of cardiovascular morbidity and mortality (1).
Some important single nucleotide polymorphisms (SNPs) have been associated with the process of AS. For example, many SNPs in the IL1RL1 gene are involved in regulating the serum concentration of soluble suppression of tumorigenicity 2 (sST2). Among different genotype carriers, individuals with the IL1RL1 rs950880 AA genotype show appreciably lower levels of sST2, and IL1RL1 rs950880 AA homozygous genotype has been identified as an independent predictor of all-cause mortality in CAD and PAD (2). Another study in AS patients has also indicated that the combined peroxisome proliferator-activated receptor-γ (PPARγ) C1431T, liver X receptor-α (LXRα) G482S, and PPARγ co-activator-1α (PGC-1α) -115G/A polymorphisms significantly increase the susceptibility to CAD and may be used to predict the severity of CAD in the Thai population (3). Furthermore, our previous studies showed that two important SNPs, CD40 rs1883832 and aldehyde dehydrogenase 2 (ALDH2) rs671, are associated with susceptibility to atherosclerotic cerebral infarction and poststroke epilepsy (4-6).
microRNAs (miRNAs) are a class of small noncoding RNAs of 18-25 nucleotides in length that inhibit translation and/or promote mRNA degradation by interacting with a specific mRNA sequence at the 3’-untranslated region (3’-UTR) of target mRNA (7, 8). miRNAs are derived from a stem-loop structured RNA predecessor spanning 70-90 nucleotides, and their synthesis is conducted by the Dicer enzyme. miRNAs are highly conserved among different species throughout evolution, and their expression is characteristic of rigorous organizational specificity and timing specificity. Emerging evidence has demonstrated that miRNAs play important roles in many cellular processes and pathological processes, including cardiovascular diseases and tumorigenesis (9-11). Oxidized low-density lipoprotein (ox-LDL) leads to the abnormal expression of miR-9, miR-125a-5p, miR-146, and miR-155 in human primary monocytes. Restoring the normal levels of these miRNAs can reduce lipid uptake in monocytes and decrease the secretion of many inflammatory cytokines, including IL-2, IL-6, tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) (12). miR-1 (13), miR-21 (14), miR-143/145 (15, 16), miR-221 (17), and miR-322 (18) have also been reported to be involved in the process of AS by regulating the phenotype of vascular smooth muscle cells (VSMCs).
miR-145 is among the most studied miRNAs associated with AS, and it is generally believed that miR-145 exerts protective effects against proliferative vascular diseases. However, previous research has mainly focused on its role in regulating the phenotype of VSMCs by targeting specific mRNAs (19, 20). However, in a recent study, we reported that miR-145 exerts an anti-AS function by suppressing the expression of CD40 (15). CD40 is a type I transmembrane glycoprotein receptor that belongs to the tumor necrosis factor superfamily and has a relative molecular weight of 50 kDa. CD40 is widely expressed on different cells, such as immunity cells, platelets, fibroblasts, endothelial cells (ECs), and VSMCs. When combined with its ligand CD40L, the CD40/CD40L signaling can stimulate T and B lymphocytes and induce the release of IL-1, IL-2, IL-6, and TNF-α (21). Through the specific interaction with CD40, miR-145 may participate in the regulation of inflammation levels and monocyte activation during AS.
Accumulated research has revealed that the SNPs in miRNAs and their target mRNAs can significantly demolish the normal interactions between miRNAs and mRNAs, leading to various cardiovascular diseases (22, 23). Although the role of miR-145 has been reported in many studies concerning AS, there is limited research on the association of certain miR-145 SNPs with susceptibility to AS, and the complete anti-AS mechanism controlled by miR-145 in the regulation of inflammation and monocyte activation remains to be fully elucidated. Here, we report a novel functional SNP in miR-145 (rs353291) that is associated with AS susceptibility. We also present evidence that the miR-145 rs353291 C allele affects the expression of miR-145 and CD40 in peripheral blood mononuclear cells (PBMCs) from AS patients and regulates the plasma levels of sCD40L, monocyte chemotactic protein 1 (MCP-1) and IL-6. Thus, the miR-145 rs353291 C allele may be used as a new predictor for AS.
The study cohort consisted of 328 AS patients and 374 gender- and age-matched control subjects; all subjects were enrolled in The Third Xiangya Hospital from September 2014 to December 2017. AS was defined according to the “The early detection technology of vascular diseases in China” issued by the Disease Control and Prevention Committee for Vascular Diseases in 2011: brachial-ankle pulse wave velocity (BaPWV) < 1400 cm/s is normal, and BaPWV >1400 cm/s is arteriosclerosis. This study was approved by the Ethics Committee of the Third Xiangya Hospital of Central South University, and written informed consent was obtained from all subjects.
Genomic DNA was extracted from whole blood samples by using standard phenol/chloroform protocols and stored at -20°C. A polymerase chain reaction (PCR) was performed on a personal thermal cycler (Biometra®, Germany). PCR products were amplified from genomic DNA with the following primers: 5’- CCTGTGAGGCTGGATGTG-3’ (sense); 5’-GGGAAGAGCCATAGTGTT-3’ (antisense). A 20 μl PCR was performed containing 10 μl 2× PCR buffer, 1 μl of each primer (10 pmol), 2 μl genomic DNA template and 6 μl double-distilled water (ddH2O). The PCR amplification conditions were as follows: a denaturation step at 95°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s, with a final incubation at 72°C for 10 min. Genotype analysis was measured by DNA sequencing completed by Sangon Biological Company.
Plasma levels of sCD40L, IL-6 and MCP-1 were measured with an immunoassay (Quantikine CD40 ligand, R&D Systems). Briefly, 40 μl dilution solution was added to the microplate wells, and then 10 μl of sample was added to each well. After incubation at 37°C for 30 min, the optical density (OD) of each sample was recorded by a microplate reader at 450 nm. The intra-assay and interassay coefficients of variation were 5% and 6%, respectively.
PBMCs were obtained from buffy coats after centrifugation at 2000 g for 30 min over 5 mL Ficoll-Hypaque density gradients, washed twice with ice-cold PBS, and suspended in RPMI 1640 containing 10% fetal calf serum (FCS), 50 U/mL penicillin and 50 μg/mL streptomycin (5).
Fragments containing the rs353291T allele were synthesized by PCR, and the primers were designed to incorporate Kpn I and Hind III restriction sites for cloning into the pGL3-basic vector (Promega, Madison, WI). Subsequently, the rs353291C allele was obtained by using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). After construction of the rs353291T and rs353291C allele plasmids, the accuracy of the inserts was confirmed by sequencing. The two constructed plasmids were named pGL3-rs353291T and pGL3-rs353291C, respectively.
Real-time PCR was performed using the ABI ViiA7 real-time PCR system with the SYBR Green method according to the manufacturer’s instructions. The relative abundance of miR-145 was normalized to the expression level of U6. All amplification reactions were performed in triplicate (15).
Protein was extracted from cultured PBMCs with RIPA lysis buffer (containing 0.1% PMSF) (Beyotime Biotech, China) according to the manufacturer's instructions. An equal amount (100 μg) of total protein was separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (Beyotime Biotech, China) and then transferred onto a polyvinylidene difluoride (PVDF) (Pell, USA) membrane after electrophoresis. The membranes were immunoblotted with Abs against CD40 (human monoclonal antibody, Abcam, UK), MCP-1 (human polyclonal antibody, CST, USA), p-p65 (human polyclonal antibody, CST, USA), p65 (human polyclonal antibody, CST, USA) or GAPDH (Abcam, UK), followed by a horseradish peroxidase-conjugated secondary antibody. The immunoblots were detected by a Bio-Rad calibrated densitometer.
Human THP-1 cells were first loaded with 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM; Beyotime Biotechnology, China) and then allowed to attach to human amniotic epithelial cells (HAECs) in a 24-well plate for 1 h at 37°C. Then, the supernatant was discarded, and the plate was washed twice with PBS. Finally, the 24-well plate was observed under fluorescence microscopy with an excitation wavelength at 480 nm and an emission wavelength at 530 nm to count the adhesive THP-1 cells.
Statistical analyses were performed using SPSS 16.0. The significance was based on contingency tables and calculated using the χ2 test. Differences among groups were analyzed using the t test. All descriptive results of continuous variables are expressed as the mean ± SD. P-values less than 0.05 were considered significant.
The demographic characteristics and distribution of risk factors in both the patients and controls are shown in Table 1. No differences in age, gender, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP) or plasma lipid levels were found between the two groups. Therefore, the susceptibility to AS is comparable between these two groups.
| Parameter | AS (n=328) | control (n=374) | P |
|---|---|---|---|
| Age, year | 48.14±4.27 | 49.82±5.32 | NS |
| BMI, kg/m2 | 22.96±2.13 | 23.12±2.61 | NS |
| SBP, mmHg | 138.51±10.25 | 137.58±9.36 | NS |
| DBP, mmHg | 85.62±7.31 | 87.25±8.47 | NS |
| Creatinine (Cr), μmol/L | 86.55±12.38 | 87.42±11.50 | NS |
| HDL-C, mmol/L | 1.30±0.39 | 1.28±0.55 | NS |
| LDL-C, mmol/L | 2.92±0.43 | 2.78±0.74 | NS |
| Triglyceride (TG), mmol/L | 1.89±0.39 | 1.91±0.48 | NS |
| Total cholesterol (TC), mmol/L | 4.86±0.77 | 4.84±0.82 | NS |
The distribution of genotypes was consistent with the Hardy-Weinberg equilibrium in both AS patients and controls. The miR-145 rs353291 polymorphism sequencing showed three genotypes: TT, CT and CC (Figure 1). As shown in Table 2, the frequency of the CC genotype was significantly higher in the AS patient group than in the control group (22.3% vs 15.0%, χ2=12.505, P=0.002), and the distribution of rs353291 C allele carriers in AS patients was also higher than that in controls (75.0% vs 63.9%, χ2=10.075, P=0.002, odds ratio (OR) = 1.695, 95% confidence interval (95%CI): 1.222–2.350).
 Figure 1
Figure 1Gene sequencing diagram of the miR-145 rs353291 polymorphism (T C). (a) TT genotype. (b) CT genotype. (c) CC genotype.
| Genotype | AS (n=328) | Control (n=374) | χ2 | P |
|---|---|---|---|---|
| TT | 82 (25.0%) | 135 (36.1%) | 12.505 | 0.002 |
| TC | 173 (52.7%) | 183 (48.9%) | ||
| CC | 73 (22.3%) | 56 (15.0%) | ||
| TC+CC | 246 (75.0%) | 239 (63.9%) | 10.075 | 0.002 |
To explore the relationship between the miR-145 rs353291 polymorphism and the plasma levels of sCD40L, IL-6 and MCP-1, blood samples from both AS patients and controls were collected and determined by ELISA. As shown in Figure 2 a, c and e, compared with controls, AS patients showed significant increases in the plasma levels of sCD40L, IL-6 and MCP-1. When AS patients were divided according to the rs353291 C allele, we observed a significant increase in the plasma levels of sCD40L, IL-6 and MCP-1 in rs353291 C allele (TC+CC) carriers compared with those in rs353291 TT genotype carriers (Figure 2 b, d and f).
 Figure 2
Figure 2Association of the rs353291 polymorphism with plasma levels of sCD40L, IL-6 and MCP-1. The levels of sCD40L, IL-6 and MCP-1 in AS patients and controls, n=328 in AS patients, n=374 in control. (a, c, e) and in AS patients according to the rs353291 C allele, n=82 in TT genotype, n=246 in CT+CC genotype (b, d, f). Values are presented as the means±SD, **P< 0.01 vs control, ##P< 0.01 vs TT genotype.
We used the stem-loop primers of miR-145 synthesized by Guangzhou RiboBio Co. to amplify miR-145 in the PBMCs from AS patients and controls. As shown in Figure 3a, compared with control subjects, the PBMCs from AS patients showed decreased miR-145 expression . We further investigated the association of the miR-145 rs353291 polymorphism with miR-145 expression in all subjects. We observed that miR-145 rs353291 C allele carriers generally showed lower miR-145 expression than that of rs353291 TT genotype carriers (Figure 3b), and this difference was more significant in AS patients (Figure 3c).
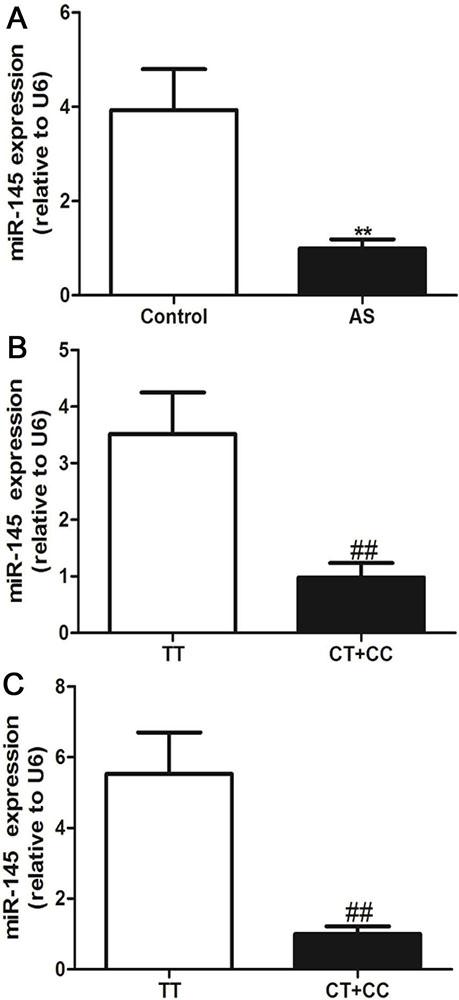 Figure 3
Figure 3Effects of the miR-145 rs353291 polymorphism on miR-145 expression in PBMCs. The levels of miR-145 expression in AS patients and controls, n=302 in AS patients, n=340 in control (a), in all subjects according to the rs353291 C allele, n=201 in TT genotype, n=441 in CT+CC genotype (b) and in AS patients according to the rs353291 C allele, n=80 in TT genotype, n=222 in CT+CC genotype (c). Values are presented as the means±SD, **P< 0.01 vs control, ##P< 0.01 vs TT genotype.
To analyze the underlying mechanism by which the rs353291 polymorphism affects miR-145 expression, we constructed two plasmids, pGL3-rs353291C and pGL3-rs353291T, and transfected these molecules into VSMCs and THP-1 cells. As shown in Figure 4, after transfection with the same amount of plasmid (2 μg), the transcriptional activity, as indicated by a remarkable decrease in fluorescence intensity, was significantly lower in cells transfected with the pGL3-rs353291C plasmid than in cells transfected with the pGL3-rs353291T plasmid. This result suggests that the rs353291C allele has reduced transcriptional activity in vitro, which may in turn cause a decrease in the expression of miR-145.
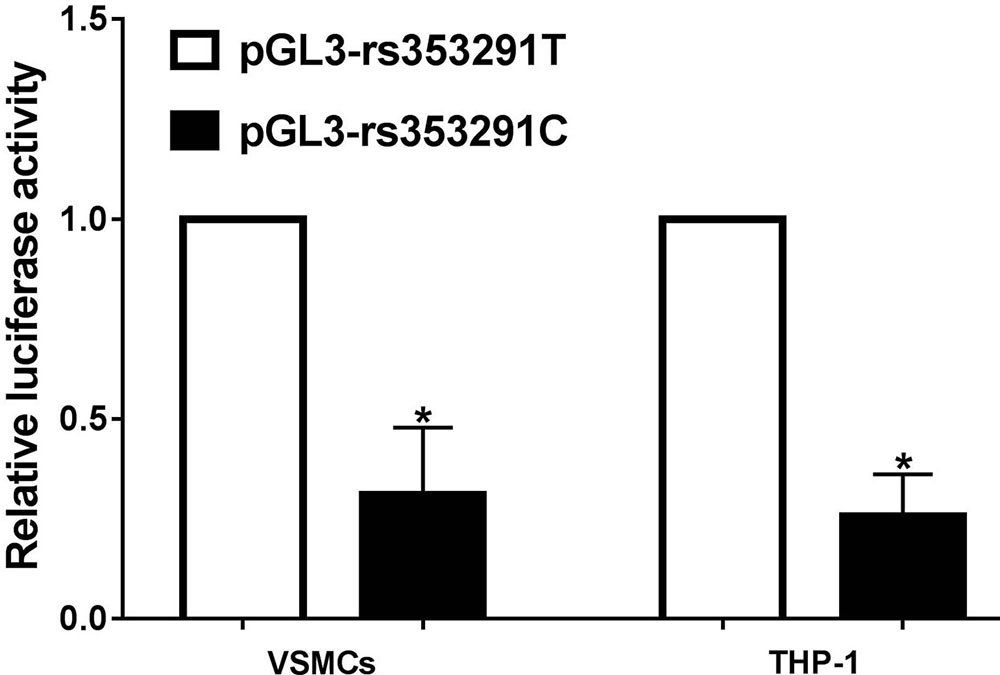 Figure 4
Figure 4Relative luciferase activity in VSMCs and PBMCs. The transcriptional activity was significantly lower in cells transfected with pGL3-rs353291C plasmid than in cells transfected with pGL3-rs353291T plasmid. Each experiment was performed in triplicate, and the data are presented as the means ± SD. *P< 0.05 vs pGL3-rs353291T.
In a previous study, we found that many AS stimulants can result in a significant decrease in miR-145 expression and a subsequent increase in inflammatory cytokines in VSMCs (15, 16). Here, we collected PBMCs from healthy individuals with different genotypes to evaluate whether ox-LDL-induced increases in inflammatory factors in PBMCs were associated with different rs353291 alleles. As shown in Figure 5, the treatment of PBMCs with 100 μg/mL ox-LDL significantly decreased miR-145 expression, and the PBMCs from rs353291 C allele carriers showed a more significant decrease in miR-145 expression than that in those from rs353291 TT genotype carriers. PBMCs from rs353291 C allele carriers also showed a more significant increase in the expression of CD40 and MCP-1 than that in PBMCs from rs353291 TT genotype carriers after ox-LDL administration (Figure 6).
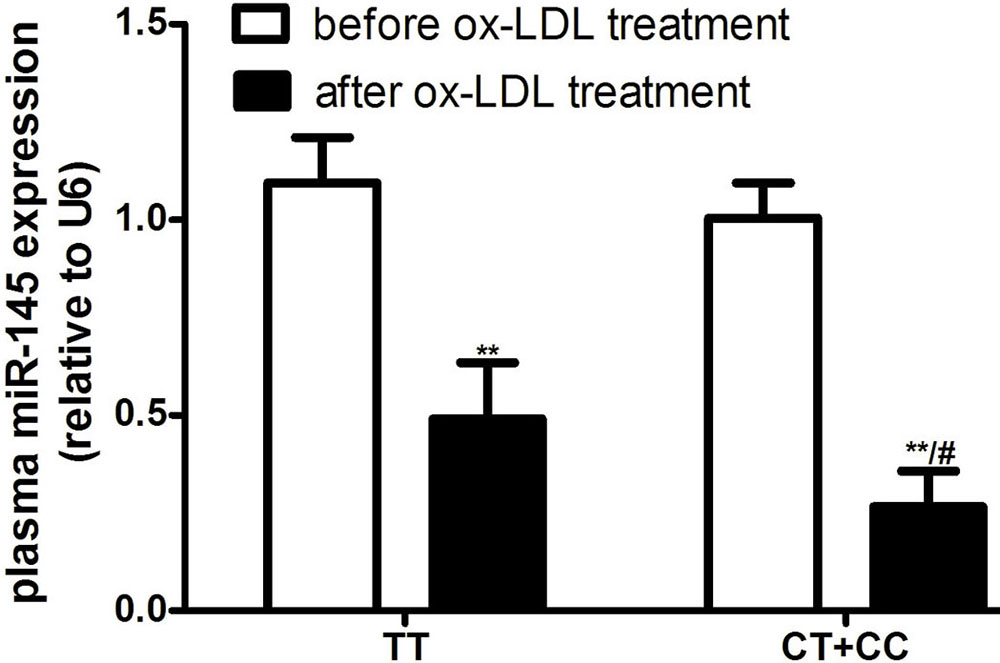 Figure 5
Figure 5Effects of the miR-145 rs353291 polymorphism on miR-145 expression in PBMCs induced by ox-LDL. The levels of miR-145 expression in PBMCs. n=3. **P< 0.01 vs before ox-LDL treatment, #P< 0.05 vs TT genotype.
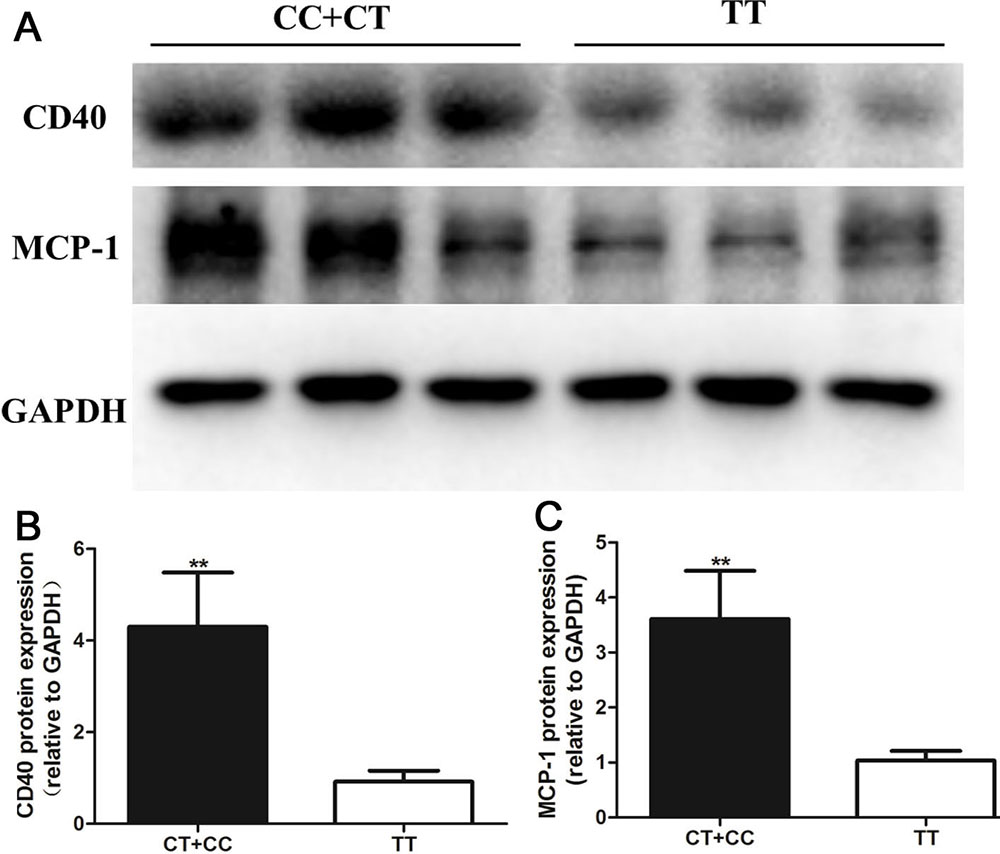 Figure 6
Figure 6Effects of the miR-145 rs353291 polymorphism on CD40 and MCP-1 expression in PBMCs induced by ox-LDL. a) The protein levels of CD40 and MCP-1 were determined by Western blotting. b) Statistical analyses of CD40 protein. c) Statistical analyses of MCP-1 protein. Values are presented as the means±SD; n=3. Experiments were performed 3 times with similar results. **P< 0.01 vs TT genotype.
The pGL3-rs353291C or pGL3-rs353291T plasmids were also used to transfect THP-1 cells to explore the effects of the rs353291 polymorphism on the NF-κB pathway. As expected, when compared with THP-1 cells transfected with the pGL3-rs353291T plasmid, cells transfected with the pGL3-rs353291C plasmid showed enhanced activation of the NF-κB pathway upon 100 μg/mL ox-LDL stimulation (Figure 7). In addition, although ox-LDL induced a significant increase in monocyte binding, TPH-1 cells transfected with either the pGL3-rs353291C plasmid or the pGL3-rs353291T plasmid showed decreased levels of monocyte adhesion, but the extent of the decrease in monocyte binding was higher in THP-1 cells transfected with the pGL3-rs353291T plasmid than in those transfected with the pGL3-rs353291C plasmid (Figure 8). These results suggest that the rs353291 T allele attenuates the activation of the NF-κB pathway and lowers ox-LDL-induced monocyte adhesion.
Although miR-145 functions as a biological repressor of vascular growth in AS, the exact mechanisms that regulate miR-145 expression in AS patients and how these mechanisms affect the AS process are not fully known. The strategy to predict AS in an early stage is limited, especially for individuals without significant clinical symptoms. Efforts to uncover the control of miR-145 expression and to define the potential target for predicting AS susceptibility in the early state are extremely needed. In the present study, we identified the functional SNP rs353291 in miR-145 as a novel AS risk factor, and this polymorphism is also associated with elevated plasma levels of sCD40L, IL-6, and MCP-1 and increased CD40 expression from PBMCs in AS patients. In an in vitro study, we established different genotype cell models by collecting PBMCs from individuals with different miR-145 rs353291 genotypes and then treated these PBMCs with ox-LDL. We found that ox-LDL induced significantly higher CD40 and MCP-1 expression in PBMCs from carriers with the rs353291 C allele than in those with the rs353291 T allele. In addition, a dual luciferase reporter assay revealed that the rs353291 C allele influenced the transcriptional activity of miR-145. These findings make a significant conceptual advance by linking the miR-145 polymorphism with AS susceptibility and plasma cytokine levels and reveal the impacts of the miR-145 polymorphism on the pathogenesis of AS.
miR-145 is a 23-nucleotide noncoding RNA transcribed from the conserved imprinted miR-143/miR-145 gene cluster located on human chromosome 5q33.1. Recent studies have reported that several miR-145 polymorphisms are associated with diseases. Fang Yuan and colleagues demonstrated that the functional SNP rs353292 in the flanking region of miR-143/145 is associated with increased susceptibility to colorectal cancer and that the rs353292 T allele is a risk factor (24). Another study reported that the rs4705342 T>C polymorphism in the promoter of the miR-143/145 gene cluster is associated with a decreased risk of ischemic stroke (25). However, to our knowledge, there is no research focusing on the association of the miR-145 rs353291 polymorphism with AS. In this study, we observed the distribution of the miR-145 rs353291 C allele in AS patients and controls to determine whether the C allele of the miR-145 rs353292 polymorphism can be used as a predictor for AS. Our results indicate that the frequency of the rs353291 C allele was significantly higher in AS patients than in controls, which suggests that individuals with the rs353291 C allele may be regarded as a high-risk population for AS. In a further strategy to prevent AS, screening out carriers of rs353291 C and giving these individuals stricter drug therapy may be a better anti-AS method. However, a large-scale study is still needed to identify this feasibility.
Given that the miR-145 rs353291 polymorphism was associated with AS susceptibility, we then asked how this polymorphism influences the AS process. miR-145 is highly expressed in the cardiovascular system and immune system, and the aberrant expression of miR-145 is associated with many diseases. miR-145 is regarded as a phenotypic marker and regulator of VSMCs, and the exogenous overexpression of miR-145 by miR-145 precursor packaged adenovirus or miR-145 mimics significantly increased the expression of VSMC differentiation marker genes, including SM α-actin, calponin and SM-MHC (15, 16, 26). The abnormal expression of miR-145 also accounts for the TGF-β1-induced endothelial-to-mesenchymal transition through the metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-145-TGFBR2/SMAD3 signaling pathway and the migration and invasion of colorectal cancer cells via a PAK4-dependent pathway (27, 28). Therefore, in this study, we collected PBMCs from both AS patients and controls to determine whether there is a difference in miR-145 expression and whether this difference is associated with different miR-145 rs353291 genotypes. Our results indicate that compared with the controls, the PBMCs from AS patients showed decreased levels of miR-145 and that rs353291 C allele carriers also showed lower miR-145 expression than that in rs353291 TT genotype carriers. Consistent with this finding, several studies have also revealed the effect of miRNA polymorphisms on miRNA expression. For example, Fang Yuan et al. reported that the functional variant rs353292 in the miR-143 gene is associated with altered miR-143 expression in colorectal cancer patients, and the rs353292 T allele is associated with lower miR-143 expression (24). Because rs353291 is located 450 bp upstream from the miR-145 gene inside the miRNA 143 host gene transcript, the rs353291 polymorphism may affect the transcriptional activity of the host gene. However, no studies have investigated the possible link between the miR-145 rs353291 polymorphism and miR-145 expression, especially under AS conditions. To this end, we constructed two plasmids, pGL3-rs353291C and pGL3-rs353291T, and transfected these molecules into VSMCs and THP-1 cells to test the influence of the rs353291 polymorphism on transcriptional activity. As expected, the transcriptional activity was significantly lower in cells transfected with the pGL3-rs353291C plasmid than in cells transfected with the pGL3-rs353291T plasmid. Therefore, our observation suggests that the higher prevalence of the rs353291 C allele may lead to the suppression of miR-145 expression in AS patients and thus affect the susceptibility of different individual to AS.
As described in our results, although the PBMCs from AS patients showed decreased miR-145 expression compared with that of controls, the difference in miR-145 expression among genotypes was more significant in AS patients. We hypothesized that the elevated inflammatory cytokines in the circulation of AS patients may enhance the effect of the rs353291C allele on miR-145 expression. Monocytes are an important cell type that participates in multiple pathological and physiological pathways, such as lipid metabolism, coagulation, apoptosis, oxidative stress, angiogenesis, and immunoreaction. Therefore, in this study, we collected PBMCs from healthy donors with different rs353291 genotypes and treated the cells with ox-LDL to mimic AS in a cell model. The results obtained in the present study demonstrated that after treatment with ox-LDL, the PBMCs collected from C allele carriers showed a dramatic decrease in miR-145 expression compared with that in those from TT genotype carriers, although the basic levels of miR-145 were comparable between different genotypes.
The CD40/CD40L system serves as a pivotal link to inflammation, immunity, and coagulation, and we demonstrated that CD40 is a direct target of miR-145 in a previous study (15). Therefore, we detected the CD40 and sCD40L levels both in vivo and in vitro and analyzed the influence of the rs353291 polymorphism on the CD40/CD40L system. Our findings indicated that AS patients with the rs353291 C allele showed significantly higher levels of sCD40L than those in AS patients with the rs353291 T allele. Consistent with the in vivo data, the PBMCs from C allele carriers also showed significantly increased CD40 expression after ox-LDL treatment. The data in our study showed a clear correlation between the rs353291 polymorphism and CD40/CD40L system that has never been addressed in previous studies. In support of this observation, some studies have previously demonstrated that the elevated CD40 expression by this anomaly partly accounts for the pathogenesis of AS, and the inhibition of CD40 expression either by specific siRNA or drugs may be an effective therapeutic strategy for AS (29-31). Therefore, the findings in this research suggest that individuals with different miR-145 rs353291 genotypes may exhibit varying degrees of CD40 and CD40L expression in response to the altered internal environment in AS patients. By using a dual luciferase reporter assay, we showed that the rs353291 C allele influences the transcriptional activity of miR-145, which may be the driving force for the decreased miR-145 expression in rs353291 C allele carriers. However, the rs353291 polymorphism in the miR-143/miR-145 gene cluster may result from altered posttranslational processes, including acetylation/deacetylation, and RNA-binding protein modifications, which can change the expression of miR-145. These possibilities will be actively investigated in our ongoing experiments.
The results reported here also demonstrate that the miR-145 rs353291 polymorphism is involved in the altered levels of inflammatory cytokines in AS patients. The pro-inflammatory role of the rs353291 C allele in AS is not surprising because in previous studies, we also found that the miR-145/CD40 pathway is associated with inflammation status, and the CD40/CD40L system plays a crucial role in the activation of the NF-κB pathway and the maturation of dendritic cells (32). This notion is further supported by our in vitro studies showing that the PBMCs from rs353291 C allele carriers showed elevated CD40 and MCP-1 expression. More importantly, THP-1 cells transfected with the miR-145 pGL3-rs353291C plasmid showed enhanced NF-κB activity compared with that in THP-1 cells transfected with the miR-145 pGL3-rs353291T plasmid. Therefore, maintaining the normal level of miR-145 in AS patients, especially for rs353291 C allele carriers, may be a therapeutic strategy for AS. However, the off-target effects of this strategy should also be considered.
In summary, the miR-145 rs353291 C allele functions as a risk factor for AS in the Chinese population. From in vivo and in vitro experiments, we demonstrated that the rs353291 C allele leads to a decrease in miR-145 expression and an increase in CD40, sCD40L, IL-6, and MCP-1 expression. Because of the pivotal role of the CD40/CD40L system in inflammation and the immune system, our results suggest that miR-145 rs353291 polymorphism-based miR-145 repression contributes to the increased inflammation levels in AS patients and increased susceptibility to AS, representing a novel therapeutic target for AS patients with the miR-145 rs353291 C allele. However, the sample size and the source of AS patients in our study were limited, and further large-scale multicenter trials and in-depth mechanism studies are still needed to confirm our observation.
This work was supported by grants from Chinese National Science Foundation (No.81000120), The Natural Science Foundation of Hunan Province (2015JJ3160), The Jiyizhupao of Third Xiangya Hospital (JY201520) and The Independent Exploration and Innovation Project for Postgraduates of Central South University (2018zzts945). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
AS
atherosclerosis
peripheral blood mononuclear cells
enzyme-linked immunosorbent assay
monocyte chemotactic protein 1
coronary artery disease
peripheral artery disease patients
single nucleotide polymorphisms
soluble suppression of tumorigenicity 2
peroxisome proliferator-activated receptor-γ
liver X receptor-α
PPARγ co-activator-1α
aldehyde dehydrogenase2
microRNAs
3’-untranslated region
oxidized low-density lipoprotein
tumor necrosis factor-α
transforming growth factor-β
vascular smooth muscle cells
endothelial cells
brachial-ankle pulse wave velocity
polymerase chain reaction
optical density
fetal calf serum
SDS-polyacrylamide gel electrophoresis
polyvinylidene difluoride
body mass index
systolic blood pressure
diastolic blood pressure
metastasis associated lung adenocarcinoma transcript 1.
