Gastric cancer (GC) is the most common cancer with a poor prognosis and the third leading cause of cancer death in the world, for which no effective therapeutic target exists. We tested the hypothesis that FAM46C might be involved in regulation of proliferation and apoptosis in GC. FAM46C was down-regulated and its expression negatively correlated with the expression of β-catenin that drives proliferation and apoptosis. Overexpression of FAM46C inhibited cell proliferation, induced G1 phase arrest and promoted apoptosis. Activation of Wnt/β-catenin signaling pathway in GC cell lines quenched the effect of FAM46C overexpression. On the other hand, FAM46C silencing attenuated DKK1-mediated inhibition of G1 phase, cessation of proliferation and induction apoptosis. Together, these data show that FAM46C shows tumor suppressor properties and such effects are mediated, at least in part, by Wnt/β-catenin in GC.
Gastric cancer (GC) is the fifth most common cancer in the world. According to the World Health Organization, 952,000 cases of GC were diagnosed in 2012 (1). In China alone, approximately 679,100 GC cases were reported, resulting in nearly 498,000 deaths in 2015 (2). Due to treatment resistance, metastasis and late recurrence, the prognosis of GC is very poor, with a 5-year survival rate lower than 30% (3, 4). Neither conventional treatments, such as chemoradiotherapy and perioperative chemotherapy, nor currently used biomarkers have prolonged lifespan (5, 6). Hence, understanding the underlying molecular mechanisms and identifying novel biomarkers are central to the development of effective treatment for GC.
The Wnt/β-catenin pathway plays a pivotal role in several human cancers, including GC (7). Wnt/β-catenin is known to regulate diverse biological activities in cells, including proliferation, polarity, apoptosis, invasion and migration (8). Aberrant Wnt/βcatenin signaling is frequently observed in human cancers (9-12). As widely described in the literature, Wnt signaling consists of the canonical (Wnt-dependent) and non-canonical (Wnt-independent) pathways. In the absence of Wnt, β-catenin is degraded by a destruction complex consisting of Axin (the scaffolding protein), adenomatous polyposis coli gene product (APC, the tumor suppressor), glycogen synthase kinase 3 (GSK3) and casein kinase 1 (CK1). In the presence of Wnt, β-catenin cannot be degraded and can then bind TCF/LEF (T cell factor/lymphoid enhancer factor) in the nucleus to activate expression of target genes (13).
Family with sequence similarity 46 (FAM46), whose function remains largely unknown, consists of four members (FAM46A, FAM46B, FAM46C and FAM46D), of which FAM46C has been implicated in diseases (14). Specifically, FAM46C has been reported as a potential marker for multiple myeloma (15, 16) and tumor suppressor in hepatocellular carcinoma (17). Recently, transcriptome analysis has revealed that FAM46C may be a potential predictor of recurrence in GC (18). However, the precise function of FAM46C in GC has yet to be elucidated. In the present study, we investigated the function of FAM46C in GC. Interestingly, we found that FAM46C was downregulated in GC tissues and its expression negatively correlated with β-catenin. Using overexpression and knockdown approaches, we then demonstrated tumor suppressive role of FAM46C in GC and showed FAM46C-dependent regulation of cell proliferation, cell cycle and apoptosis via modulation of Wnt/β-catenin pathway.
A total of 60 GC tissues and 30 non-tumor tissues were obtained from Changzhou No.2 People's Hospital Affiliated to Nanjing Medical University. All patients provided written informed consent for the research.
Human GC cell lines MKN45, MKN74 and AGS were obtained from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) and penicillin/ streptomycin (Invitrogen, Carlsbad, CA, USA). Cells were incubated at 37°C under 5% CO2.
FAM46C lentiviral constructs that were used for overexpression and knockdown studies were synthesized by Genechem company (Shanghai, China). VECTOR (control empty vector) and NC lentiviral vectors were synthesized at the same time. To ensure knockdown efficiency, three lentiviral constructs for knockdown were used. The sequences of constructs for FAM46C knockdown are listed in Table 1.
| Gene Name | Forward primer 5’-3’ | Reverse primer 5’-3’ |
|---|---|---|
| Quantitative RT-PCR | ||
| FAM46C | CGGAATTCATGGCAGAGGAGAGCAGCTG | CGGGATCCTTAGTTACAGGGCAGCCAGG |
| Sequences for construction of lentivirus for FAM46C knockdown | ||
| shFAM46C-1 | CCAGGGATTGCATGTCCTT | |
| shFAM46C-2 | GGACGAGGCAACTTTCCAA | |
| shFAM46C-3 | GCAACTTCAGCAACTACTA | |
| Sequences for construction of lentivirus for FAM46C overexpression | ||
| Gene Name | Forward primer 5’-3’ | Reverse primer 5’-3’ |
| FAM46C primers | CGGAATTCATGGCAGAGGAGAGCAGCTG | CGGGATCCTTAGTTACAGGGCAGCCAGG |
| FAM46C-CDS | ATGGCAGAGGAGAGCAGCTGTACCAGGGATTGCATGTCCTTCAGCGTGCTCAACTGGGATCAGGTTAGCCGGCTGCA | |
| TGAGGTCCTCACTGAAGTTGTACCTATCCACGGACGAGGCAACTTTCCAACCTTGGAGATAACTCTGAAG | ||
| GACATCGTCCAGACCGTCCGCAGTCGGCTGGAGGAGGCAGGCATCAAAGTGCACGACGTCCGGCTGAATG | ||
| GCTCCGCAGCTGGCCACGTTTTGGTCAAAGACAATGGCTTGGGCTGCAAAGACCTGGACCTAATCTTCCA | ||
| TGTGGCTCTTCCAACAGAGGCAGAATTTCAGCTGGTTAGAGATGTGGTTCTGTGTTCCCTTCTGAACTTC | ||
| CTGCCAGAGGGTGTGAACAAGCTCAAAATCAGTCCAGTCACTCTGAAGGAGGCATATGTGCAGAAGCTAG | ||
| TGAAGGTTTGCACGGACACTGACCGCTGGAGCCTGATCTCCCTCTCCAACAAGAACGGGAAGAACGTGGA | ||
| GCTGAAGTTTGTCGACTCCATTCGGCGTCAGTTTGAGTTCAGTGTGGACTCTTTCCAAATCATCCTGGAT | ||
| TCTTTGCTTTTCTTCTATGACTGTTCCAATAATCCCATCTCTGAGCACTTCCACCCCACCGTGATTGGGG | ||
| AGAGCATGTACGGGGACTTTGAGGAAGCTTTTGACCATCTGCAGAACAGACTGATCGCCACCAAGAACCC | ||
| AGAAGAAATCAGAGGCGGGGGACTTCTCAAGTACAGCAACCTTCTTGTGCGGGACTTCAGGCCCACAGAC | ||
| CAGGAAGAAATCAAAACTCTAGAGCGCTACATGTGCTCCAGGTTCTTCATCGACTTCCCGGACATCCTTG | ||
| AACAGCAGAGGAAGTTGGAGACTTACCTTCAAAACCACTTCGCTGAAGAAGAGAGAAGCAAGTACGACTA | ||
| CCTCATGATCCTTCGCAGGGTGGTGAACGAGAGCACCGTGTGTCTCATGGGGCATGAACGCAGGCAGACT | ||
| CTGAACCTCATCTCCCTCCTGGCCTTGCGTGTGCTGGCGGAACAAAACATCATCCCCAGTGCCACCAACG | ||
| TCACCTGTTACTACCAGCCGGCCCCTTACGTCAGTGATGGCAACTTCAGCAACTACTACGTTGCCCATCC | ||
| TCCAGTCACCTACAGCCAGCCTTACCCTACCTGGCTGCCCTGTAACTAA | ||
Cells were infected with FAM46C lentiviruses (overexpression or knockdown) according to manufacturer’s instruction. After 48h transduction, parts of the cells were used to measure infection efficiency by quantitative RT-PCR and western blot, and the remaining cells were cultured for other experiments.
LiCI, an agonist for Wnt/β-catenin, was purchased from Aladdin (Aladdin, China). The working concentration of LiCl was 10 mmol/L. DKK1, a Wnt/β-catenin inhibitor, was purchased from Aladdin (Aladdin, China). The working concentration of DKK1 was 50 ng/ml.
Total RNA of GC tissues and cell lines was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). Quantitative RT–PCR was used to measure relative mRNA expression of target genes. Reverse Transcription System (TaKaRa, Dalian, China) and SYBR Green qPCR Mixes ( (TaKaRa, Dalian, China) were used to synthesize cDNA and perform qRT–PCR, respectively. GAPDH was used as a housekeeping gene. The sequences of primer are listed in Table 1.
Fresh tissue samples were fixed with formalin and embedded with paraffin. The sections were deparaffinized, rehydrated and boiled for 5 min in 10 mM citrate buffer (pH 6.0) followed by incubation with 3% H2O2 for 15 min. After washing with PBS, slides were blocked with 10% normal blocking serum for 30 minutes, then incubated with FAM46C primary antibody and biotin-labelled secondary antibodies. Localization of peroxidase conjugates was determined using diaminobenzidine tetrahydrochloride solution as chromogen and hematoxylin for counterstaining.
RIPA lysis buffer (Beyotime, Shanghai, China) was used to extract protein from cultured cells. Protein was collected by centrifugation and concentration was measured by BCA kit (Bio-Rad, Hercules, CA, USA). Target protein was analyzed according to manufacturer’s instructions. GAPDH was used as an internal control. The following primary antibodies were used: FAM46C, c-Myc and active caspase3 (all from Abcam); β-catenin, Bcl-2, GAPDH and Cyclin D1 (all from Cell Signaling Technology (CST)). Secondary antibodies were purchased from Beyotime.
Cell Counting Kit-8 (CCK-8, Beyotime, Shanghai, China) and BrdU ELISA Kit were used to analyze cell proliferation according to manufacturers’ instructions. Briefly, same number of cells (~5000) were plated into 96-well plates and cultured overnight. After treatment, 10 μL CCK-8 was added into 90 μl FBS-free medium at each time point and cultured for 1 h. Cell proliferation was analyzed by measuring absorbance at 450 nm. For cell proliferation analysis using BrdU, cells were cultured with BrdU (10 μM) for 1, 2 and 3 h. At each time point, 200 μL of cells were collected and incubated with peroxidase conjugated anti-BrdU antibody for 30 min. Absorbance was measured at 370 nm.
Propidium iodide (PI) staining and Annexin V/PI Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) were used to measure cell cycle and apoptosis respectively. For cell cycle analysis, cells were collected and fixed in 70% ethanol. Cells were then washed twice with PBS and stained with PI and RNase A for 20 min at room temperature. For analysis of apoptosis, cells were collected and stained with Annexin V and PI according to manufacturer’s protocol. Flow cytometry (BD Biosciences, San Jose, CA, USA) was used to analyze cell cycle and apoptosis.
GSEA was used to explore the enrichment score of specific signatures in the gene sets that correlated with FAM46C expression. The genes identified to be on the leading edge of the enrichment profile were subjected to pathway analysis. Fold-change values were exported for all genes and analyzed with version 2.2.0. of GSEA. A gene set with nominal P ≤ 0.05 was considered to be significantly enriched in genes.
BALB/c nude mice of 4-6 weeks were obtained from the Shanghai Laboratory Animal Company. MKN45 cells (2 × 106) with FAM46 overexpression or VECTOR were subcutaneously injected into the right flank of each mouse. After 7 weeks, all mice were sacrificed and tumors were harvested for Western blot.
All data were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) and Student’s unpaired t-test were used for comparison between groups. A P value of <0.05 was considered statistically significant.
To examine the expression pattern of FAM46C, quantitative RT-PCR was used to measure FAM46C mRNA in 60 GC tissues and 30 non-tumor tissues. Our results revealed FAM46C downregulation in GC tissues compared with non-tumor tissues (Figure 1A), which was consistent with the analysis on data from TCGA dataset (https://cancergenome.nih.gov/) (Figure 1B). To determine FAM46C protein expression, immunohistochemistry and Western blot were employed. Compared with non-tumor tissues, FAM46C expression was reduced in 65% (39 out of 60) of GC tissues as shown by immunohistochemistry and 68.3% (41 out of 60) of GC tissues as shown by Western blot (Figure 1C and 1D). Interestingly, gene set enrichment analysis (GSEA) revealed that FAM46C was negatively correlated with Wnt signaling pathway (Figure 1E). Indeed, in examining β-catenin mRNA expression in GC and non-tumor tissues using qRT-PCR, we found that β-catenin was upregulated in GC tissues (Figure 1F). Furthermore, Pearson correlation analysis similarly determined negative correlation between FAM46C and β-catenin in GC tissues (Figure 1G).
 Figure 1
Figure 1FAM46C was downregulated and negatively correlated with -catenin in GC tissues. (A) Quantitative RT-PCR was used to measure mRNA expression of FAM46C in GC tissues (n=60) and non-tumor tissues (n=30) using two housekeeping genes GAPDH (left panel) and β- actin (right panel) as internal control. (B) FAM46C expression was decreased in GC tissues (n=249) compared with non-tumor tissues (n=33) from TCGA database. (C) FAM46C protein expression was detected by immunohistochemistry in GC tissues (n=60) and non-tumor tissues (n=30). (D) FAM46C protein levels were detected by Western blot in GC tissues (n=60) and non-tumor tissues (n=30). (E) GSEA demonstrated that FAM46C was negatively related with the Wnt pathway. (F) qRT-PCR was used to measure expression of -catenin in GC tissues (n=60) and non-tumor tissues (n=30). (G) Pearson correlation analysis revealed negative correlation between FAM46C and -catenin in GC tissues (r = - 0.6704).
As reported, FAM46C expression is lower in MKN45 and MKN74 cell lines and higher in AGS cell line (18). To explore the function of FAM46C in GC, we altered the expression levels of FAM46C using lentivirus. First, MKN45 and MKN74 cells were transduced with FAM46C overexpression and VECTOR lentiviruses. Confirming the efficiency of overexpression, cells transduced with FAM46C lentivirus indeed displayed elevated expression of FAM46C compared with cells transduced with lentivirus VECTOR in both MKN45 (Figure 2A) and MKN74 cells (Figure 3A). Next, we measured cell proliferation using CCK-8 and BrdU assays. We found that FAM46C overexpression suppressed cell proliferation in MKN45 (Figure 2B and 2C) and MKN74 cells (Figure 3B and 2C). Our earlier data indicated negative correlation between FAM46C and β-catenin. Hence, we tested whether FAM46C may regulate Wnt/β-catenin signaling. To do so, we used LiCl, an agonist for Wnt/β-catenin, together with FAM46C lentivirus. Interestingly, cells treated with 10 mmol/L LiCl and FAM46C lentivirus displayed higher proliferation than VECTOR alone, but showed lower proliferation than cells only treated with 10 mmol/L LiCl in MKN45 (Figure 2B and 2C) and MKN74 (Figure 3B and 2C) cells. Next, we analyzed cell cycle and apoptosis by flow cytometry. Our results showed that FAM46C overexpression induced G1 phase arrest compared with VECTOR, but treatment with LiCl and FAM46C lentivirus attenuated arrest in MKN45 cells (Figure 2D, F). In addition, FAM46C overexpression promoted apoptosis of MKN45 cells compared with VECTOR, while treatment with LiCl and FAM46C lentivirus reduced apoptosis compared to FAM46C alone (Figure 2E, G). Similarly, in MKN74 cells, FAM46C overexpression arrested cells in G1 phase and promoted apoptosis compared with VECTOR, but cells treated with LiCl and FAM46C lentivirus weakened these effects (Figure 3D, E, F and G). Together, these results suggested that FAM46C may play a tumor suppressor role in MKN45 and MKN74 cell lines.
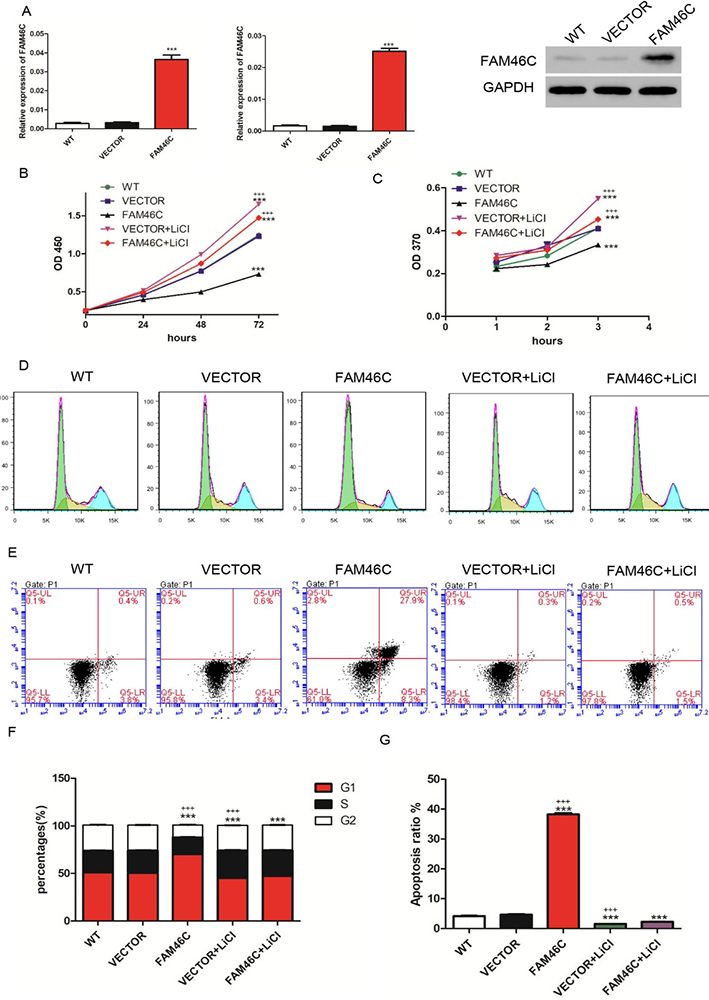 Figure 2
Figure 2Cell proliferation, cell cycle and apoptosis were measured after treatment with FAM46C lentivirus and/or LiCl in MKN45 cells. (A) qRT-PCR (left panel: using GAPDH as internal control; middle panel: using β- actin as internal control) and Western blot were employed to measure FAM46C expression after transduction with FAM46C lentivirus. (B, C) CCK-8 (B) and BrdU (C) assays were used to analyze cell proliferation. (D, E) cell cycle (D) and apoptosis (E) after treatment with FAM46C lentivirus and/or LiCl. (F) Statistical analysis of cell cycle. (G) Statistical analysis of cell apoptosis. WT: wide type cell; VECTOR: transduced with lentivirus VECTOR; FAM46C: transduced with FAM46C lentivirus; VECTOR+ LiCl: treated with VECTOR lentivirus and LiCl; FAM46C+ LiCl: treated with FAM46C lentivirus and LiCl. (***, P < 0.001 vs VECTOR; +++, P<0.001 vs FAM46C+ LiCl).
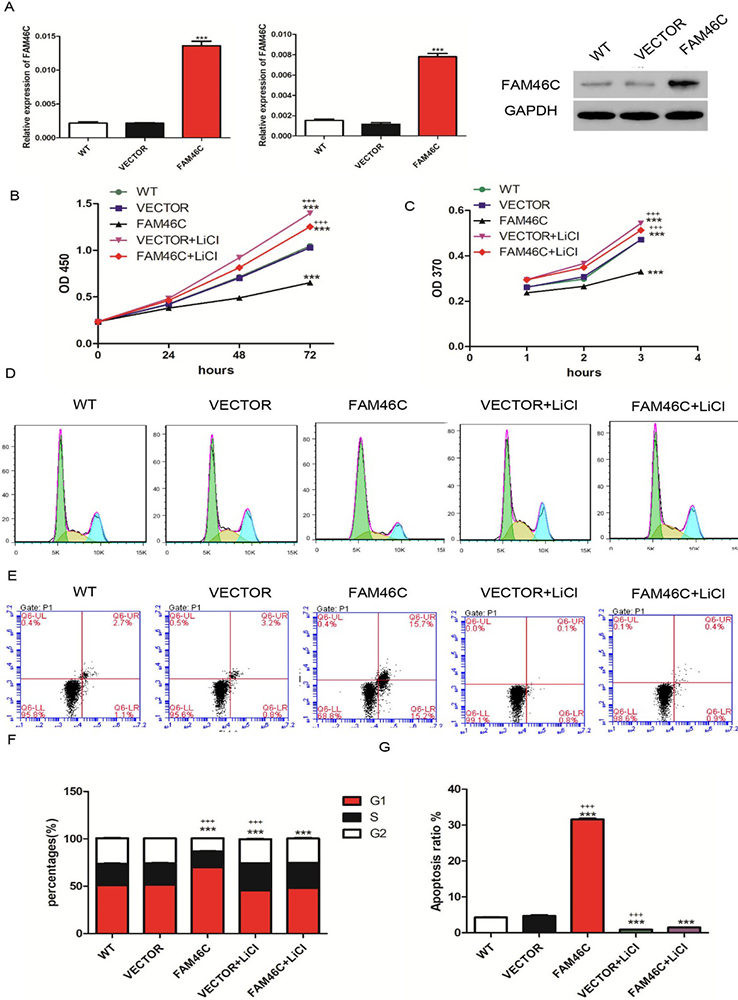 Figure 3
Figure 3Cell proliferation, cell cycle and apoptosis were assessed after treatment with FAM46C lentivirus and/or LiCl in MKN74 cells. (A) qRT-PCR (left panel: using GAPDH as internal control; middle panel: using β- actin as internal control) and Western blot were used to measure FAM46C expression after transduction with FAM46C lentivirus. (B, C) CCK-8 (B) and BrdU (C) assays were used to analyze cell proliferation. (D, E) cell cycle (D) and apoptosis (E) after treatment with FAM46C lentivirus and/or LiCl. (F) Statistical analysis of cell cycle. (G) Statistical analysis of cell apoptosis. WT: wide type cell; VECTOR: transduced with lentivirus VECTOR; FAM46C: transduced with FAM46C lentivirus; VECTOR+ LiCl: treated with VECTOR lentivirus and LiCl; FAM46C+ LiCl: treated with FAM46C lentivirus and LiCl. (***, P < 0.001 vs VECTOR; +++, P<0.001 vs FAM46C+ LiCl).
According to GSEA, cell cycle and apoptosis were demonstrated to have significant association with FAM46C (Figure 4A). Based on our previous data in which FAM46C induced G1 phase arrest and apoptosis, we determined the expression of genes involved in these cellular processes, including Ki67, cyclin D1, CDK4, CDK6, Bcl-2 and active caspase 3. As shown in Figure 4B, FAM46C overexpression diminished the expression of Ki67, CDK4, CDK6, Bcl-2 and cyclin D1 but induced active caspase3 compared with VECTOR. In contrast, treatment with VECTOR and the Wnt/β-catenin agonist LiCl induced Ki67, CDK4, CDK6, Bcl-2 and Cyclin D1 but suppressed active caspase 3 compared with VECTOR alone. Moreover, cells treated with FAM46C and LiCl showed higher levels of Ki67, CDK4, CDK6, Bcl-2 and Cyclin D1, and inhibition of active caspase3 compared with FAM46C alone. However, compared with MKN45 cells treated with LiCl, cells treated with both FAM46C and LiCl showed reduced expression of Ki67, CDK4, CDK6, Bcl-2 and Cyclin D1, but increased expression of active caspase 3. Furthermore, FAM46C overexpression inhibited β-catenin and c-Myc, whereas LiCl treatment induced β-catenin and c-Myc. Lastly, the expression level of β-catenin and c-Myc in MKN45 cells treated with both FAM46C and LiCl was higher than FAM46C-treated cells but lower than LiCl-treated cells (Figure 4B). Similar results were observed in MKN74 cells (Figure 4C).
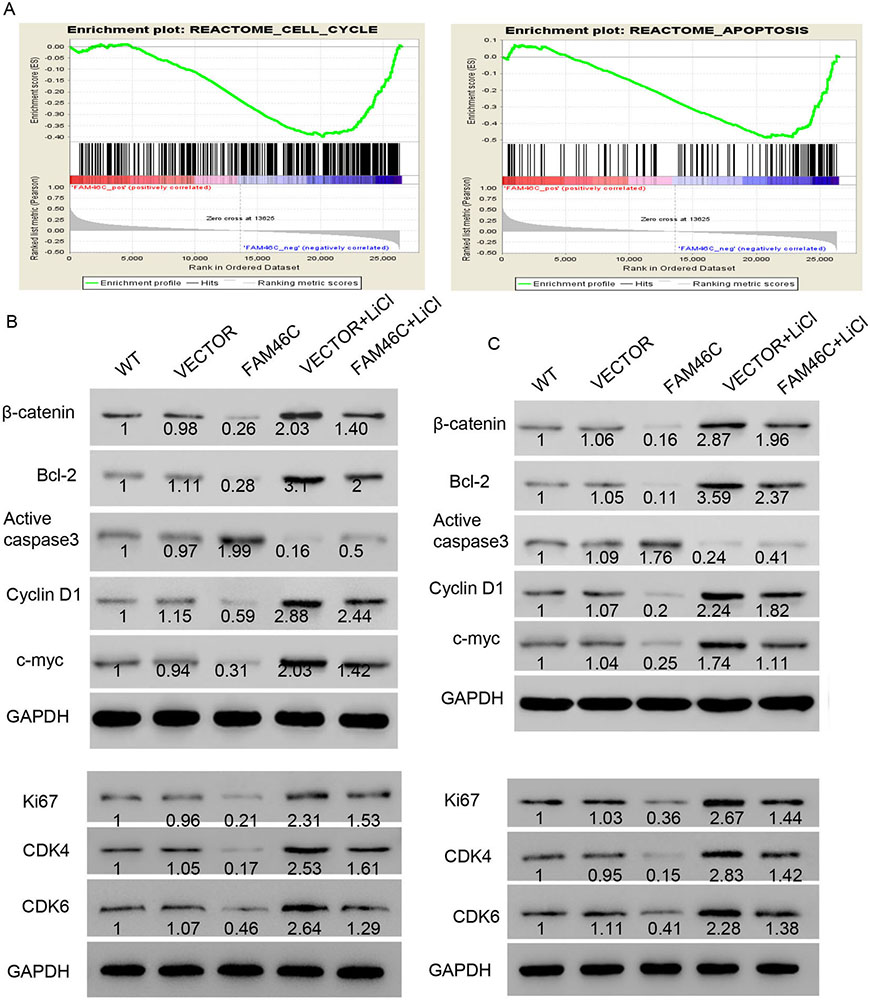 Figure 4
Figure 4FAM46C overexpression inhibited Wnt/-catenin signaling pathway. (A) Cell cycle and apoptosis were associated with FAM46C expression using GSEA. (B, C) Western blot was used to detect protein levels of several biomarkers of relative pathways. WT: wide type cell; VECTOR: transduced with lentivirus VECTOR; FAM46C: transduced with FAM46C lentivirus; VECTOR+ LiCl: treated with VECTOR lentivirus and LiCl; FAM46C+ LiCl: treated with FAM46C lentivirus and LiCl.
It has been reported that AGS cells display high expression of FAM46C. Hence, we transduced AGS cells with FAM46C knockdown lentivirus. As shown in Figure 5A, all three lentiviruses significantly suppressed FAM46C mRNA and protein expression, with shFAM46C-1 exhibiting the most robust knockdown efficiency and thereby used in subsequent experiments. Again, we analyzed cell cycle and apoptosis by flow cytometry and found that cells treated with 50 ng/ml DKK1, which inhibited Wnt/β-catenin, showed higher ratio of G1 stage cells and increased apoptosis compared with NC. Cells transduced with shFAM46C displayed lower ratio of G1 stage cells and decreased apoptosis compared with NC. Interestingly, cells treated with both DKK1 and shFAM46C exhibited higher ratio of G1 stage cells and more apoptosis than shFAM46C but to less extent than DKK1 (Figure 5 D, F, E, G). Collectively, these results indicated that silencing FAM46C weakened DKK1-induced G1 phase arrest and apoptosis.
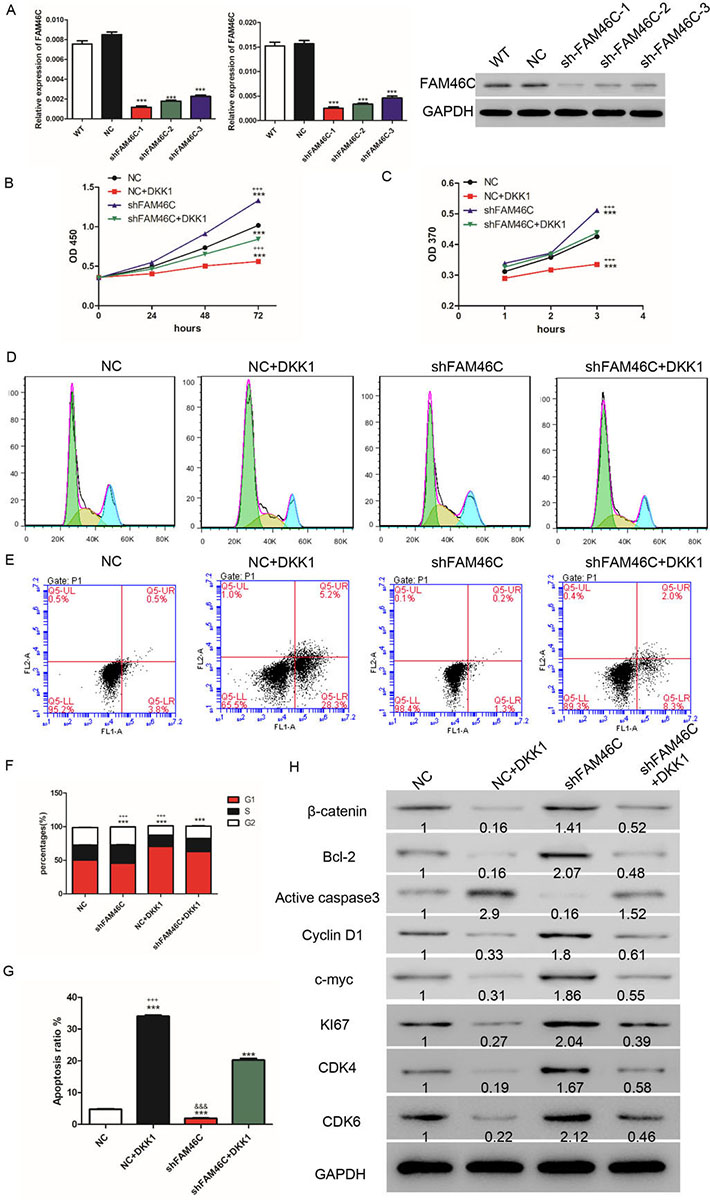 Figure 5
Figure 5FAM46C silencing promoted GC cell proliferation through Wnt/β-catenin pathway. (A) qRT-PCR (left panel: using GAPDH as internal control; middle panel: using β- actin as internal control) and Western blot were used to measure FAM46C expression after transduction with FAM46C knockdown lentivirus. (B, C) CCK-8 (B) and BrdU (C) assays were used to analyze cell proliferation. (D, E) cell cycle (D) and apoptosis (E) after treatment with shFAM46C lentivirus and/or DKK1. (F) Statistical analysis of cell cycle. (G) Statistical analysis of cell apoptosis. (H) Western blot was used to detect protein levels of several biomarkers of relative pathways. NC: transduced with negative control lentivirus; NC+ DKK1: treated with negative control lentivirus and DKK1; shFAM46C: transduced with FAM46C knock-down lentivirus; shFAM46C+ DKK1: treated with FAM46C knock-down lentivirus and DKK1. (***, P < 0.001 vs NC; +++, P<0.001 vs shFAM46C+ DKK1).
To further understand the function of FAM46C in GC, AGS cells were treated with shFAM46C and DKK1 and cell proliferation was assessed using CCK-8 and BrdU kits. As shown in Figure 5B, C, FAM46C knockdown promoted cell proliferation whereas DKK1 inhibited proliferation compared with NC. Cells treated with both DKK1 and shFAM46C exhibited higher proliferation than DKK1 but lower than shFAM46C (Figure 5B, C). In addition, silencing of FAM46C induced Ki67, CDK4, CDK6, Bcl-2 and Cyclin D1 at the protein level but diminished active caspase 3 compared with NC. In contrast, cells treated with DKK1 showed reduction of Bcl-2 and Cyclin D1 but induction of active caspase 3 compared with NC. Likewise, AGS cells treated with both shFAM46C and DKK1 displayed higher levels of Bcl-2 and Cyclin D1 and lower level of active caspase 3 than cells treated with DKK1 alone, although to less extent than cells treated with shFAM46C alone. Silencing of FAM46C induced β-catenin and c-Myc while DKK1 inhibited β-catenin and c-myc. Lastly, treatment with shFAM46C and LiCl in AGS cells elevated the expression of β-catenin and c-Myc compared to DKK1, but to less extent than treatment with shFAM46C alone (Figure 5H).
To validate the mechanism of FAM46C in GC, we performed xenograft experiments in BALB/c nude mice. We found that FAM46C overexpression resulted in reduction of Ki67, CDK4, CDK6, Bcl-2 and Cyclin D1 but induction of active caspase 3 compared with VECTOR.
FAM46, which consists of four members, has been implicated in various diseases (14), but its precise function, particularly that of FAM46C, is largely unknown. It has been reported that mutation of FAM46C is observed in myelomas (19, 20). One study has shown that FAM46C is a poly (A) polymerase and its loss of function drives multiple myelomas by destabilizing ER response transcripts (21). Another study has demonstrated that overexpression of FAM46C significantly suppresses cell proliferation and increases cell population in G2/M phase as well as apoptotic rates by decreasing Ras expression, MEK1/2 phosphorylation and ERK1/2 phosphorylation (17). Moreover, FAM46C has been reported as a predictor of hepatic recurrence in patients with resectable GC, yet the molecular function of FAM46C is not fully understood (18). In the present study, we sought to understand the role of FAM46C in GC. To do so, we evaluated transcription in tumor specimens and found downregulation of FAM46C in GC (Figure 1), which is consistent with previous study (18). β-catenin plays a pivotal role in diverse human cancers, including GC (7). Likewise, we observed reduced transcription of β-catenin in GC, as well as negative correlation between β-catenin and FAM46C (Figure 1). Interestingly, FAM46C overexpression in GC cell lines inhibited proliferation, and induced G1 phase arrest and apoptosis (Figure 2 and 3), suggesting that FAM46C may act as a potential tumor suppressor in GC, which has been hinted in other studies (17, 18, 21).
Mechanistically, the relation between FAM46C and β-catenin had not been studied. To investigate this, FAM46C was ectopically expressed in cells with or without LiCl (Wnt/β-catenin agonist) treatment. As shown in Figure 4, FAM46C suppressed GC cell proliferation. In addition, FAM46C knockdown decreased DKK1-induced G1 phase arrest and apoptosis (Figure 5). As DKK1 is a well-known inhibitor of Wnt/β-catenin signaling (22-26), these results suggest that FAM46C plays tumor suppressive functions in GC, at least in part, through Wnt/β-catenin. Furthermore, we uncovered similar results of biomarkers of relative pathway in vivo (Figure 6).
 Figure 6
Figure 6Western blot was used to detect protein levels of several biomarkers of relative pathways in tumor samples from BALB/c nude mice. VECTOR: BALB/c nude mice were injected with cells that have been transduced with VECTOR lentivirus; FAM46C: BALB/c nude mice were injected with cells transduced with FAM46C lentivirus.
In summary, our study demonstrated that FAM46C suppressed GC cell proliferation through inhibition of Wnt/β-catenin pathway. How FAM46C regulates β-catenin and their precise interaction in GC are not known and invite exciting future directions for investigation. Given our findings, silencing of FAM46C may serve a protective role and potential therapeutic target in GC. However, extensive validation in vivo is needed to establish relevance of these findings in the clinic.
All the authors contributed to this article. No authors report any conflict of interest.
GC
Gastric cancer
adenomatous polyposis coli gene product
glycogen synthase kinase 3
casein kinase 1
T cell factor/lymphoid enhancer factor
Family with sequence similarity 46
fetal bovine serum
Gene set enrichment analysis
