Nephrotic syndrome (NS) is a common urinary system disease that carries a poor progrnosis due to nonresponsivess to current treatments. The purpose of this study was to investigate the therapeutic effects of Forsythiaside (FA) in a rat model of adriamycin-induced nephropathy (AN). Compared with control group, FA treatment significantly reduced proteinuria, serum creatinine and urea nitrogen levels and reduced the number of apoptotic cells in the kidneys. FA alleviated Adr induced renal injury, and increased the sactivity of superoxide dismutase (SOD), while decreasing malondialdehyde (MDA) and lactate dehydrogenase (LDH) in the serum. In addition, FA, in a dose-dependent manner, decreased the levels of NF-kappaBp65/MIP-2 in the kidenys, reduced the serum level of pro-inflammatory cytokines (IL-6, IL-1 β and TNF-alpha), and increased the survival rate of Adr treated rats. Taken together, the results show that FA alleviates renal dysfunction in adriamycin-induced nephropathy rats.
Nephrotic syndrome (NS) is a common disease of kidneys, which is usually caused by the increase in permeability of glomerular basement membrane. NS is characterized by excessive proteinuria, hyponatremia, high degree of edema and high blood lipid. The NS has a poor prognosis with complex treatment strategy (1).
Adriamycin (Adr) is a broad-spectrum anticancer anthracycline antibiotic which is widely used in tumor treatment. Adriamycin (Adr) induces nephropathy in rats and serves as a model for human NS (2). However, the use of Adr can cause dose-dependent toxicity to other organs such as liver, heart and kidney. Adriamycin nephropathy (AN) is often accompanied by hypoproteinemia, hypercoagulability, dyslipidemia, proteinuria, edema, ascites and other symptoms, however, the pathogenesis of these disorders is not fully understood (3-6).
Forsythiaside A (FA), chemically known as phenylethanoid glycoside, is extracted from Forsythiae Fructus (Lian Qiao). FA has a variety of biological activities, including anti-inflammatory (7), neuroprotective (8), liver protection (9, 10), anti-tumor, anti-virus, antioxidant, antipyretic, and vasodilating effects (11-14). FA reduces the levels of pro-inflammatory cytokines (TNF-α, IL-1 β and IL-6) by inhibiting NF-kappaB/MAPKs in bMEC cells stimulated by Staphylococcus aureus (7). FA also significantly alleviates peritonitis by inhibiting the activation of NF-kappaB, reducing the TNF-α, IL-6 and MCP-1 and the number of neutrophils in the peritoneal cavity (15). Furthermore, FA inhibits the production of inflammatory mediators in microglia and prevents lipopolysaccharide (LPS) induced liver injury in rats. However, the role of FA in Adr-induced nephropathy has not yet been examined (16, 17).
NF-kappaB is a transcription factor that plays reduces inflammation (18). Activation of the NF-kappaB pathway primarily increases the level of p-kappaBα and reduces the production of IkappaB by degrading I-kappaB kinase (IKK) consisting of IKKα and IKKβ subunits. With the degradation of I-kappaB, NF-kappaB complex is transported into the nucleus, triggering the transcription of inflammatory cytokines (19). Previous studies showed that FA inhibits macrophage inflammatory cytokine activation by inhibiting NF- kappa B signaling pathway (20). In addition, FA exerts neuroprotective effect on microglia by inhibiting the activation of NF- kappa B and the degradation of IkappaBα (16). However, it is not clear whether FA also attenuates Adr-induced nephropathy by regulating NF- kappa B signaling pathway.
The purpose of this study was to investigate the therapeutic effect of FA in AN and to eluciate its mechanism of action.
Adriamycin was purchased from Badatong Pharmaceutical Co., Ltd (Zhejiang, China). Forsythiaside A was from Hangzhou Dingyan Chemical Co., Ltd (Zhejiang, China). TUNEL staining kits from Roche Ltd (Shanghai, China). BCA staining kit was from Beijing Tiangen Biochemical Co., Ltd (Beijing, China), Superoxide dismutase (SOD, Shanghai, China), malondialdehyde (MDA, Shanghai, China) and LDH staining kit from Shanghai Enzyme-linked Biotechnology Co. (Shanghai, China), Rabbit antibodies to cleaved caspase-3, cleaved caspase 9, beta actin, Bcl-2, Bax, Cytochrome C, P65, p-P65 and secondary anti-rabbit IgG were from Cell Signalling Technology (Massachusetts, USA). MIP-2 was from Abcam (Cambridge, UK). ECL reagents were from Millipore Corp (MA, USA). Enzyme linked immunosorbent assay (ELISA) for analysis of cytokines was from BioTek Epoch (Winooski, VT, USA).
All animal experiments were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by The Third Affiliated Hospital of Wenzhou Medical University. A total of 70 Sprague Dawley rats (male, 6-week-old, 150-170 g) were obtained from the Animal Center of The Third Affiliated Hospital of Wenzhou Medical University and housed in a controlled environment at temperature of 25 ± 3, and humidity of 60% in a 12h light/dark cycle with free access to food and water. Rats were randomly assigned to seven groups with 10 rats in each group: Control group rats were intravenously injected with saline. Experimental animals were injected with Adr diluted in 0.9% saline in the tail vein to a final dose of 6.5 mg/kg. Adr + FA group, received received 2.5, 5 or 10 μg/ml FA and 6.5 mg/kg. Adr. After four weeks, the rats were euthanized and sera, urine and kidneys were collected. Sera were stored at – 800 C until analysis. Levels of protein in the urine, serum creatinine and urea nitrogen were quantified by an automatic chemical analyzer.
The level of proteinuria was assessed by ELISA (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) Urea nitrogen, and serum creatinine were measured by automatic biochemistry analyzer (Olympus AU2700, Japan). All experiments were carried out three time.
Renal tissues were fixed in 4% paraformaldehyde, dehydrated in escalting doses of ethanol, treated with xylene, embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E).
Paraffin sections were dewaxed in xylene in decreasing gradient of ethanol, washed with PBS, and incubated in a balanced buffer. Sections were treated with TdT enzyme and reaction was terminated with SSC. Endogenous perodxidase was quenched with H2O2 and then sections were incubated with streptavidin HRP. Staining with diaminobenzidine (DAB) was stopped by immersion of slides in distilled water, nuclei were counterastained with hematoxylin, and sections were dehydrated in ethanol, followed by xylene solution and coverslipped.
Protein content was measured by BCA kit after cell cleavage and denaturation. Proteins (30 μg) were resolved in 10% SDS-PAGE and transferred to PVDF membrane. After blocking the membrane with 5% skim milk powder at room temperature for 1 hour, membranes were inclubated with primary antibodies. Antibodies (cleaved caspase-3, cleaved caspase 9, beta actin, Bcl-2, Bax (6A7), Cytochrome C, P65, p-P65 ) were used at 1: 1000 dilution and antibody to MIP-2 was used at 1:5000 dilution. After incubation overnight at 40 C, the membranes were washed with PBS, and were then incubated for one hour at room temperature with the peroxidase labeled secondary anti-rabbit IgG at 1:2000 dilution followed by washing in PBX. Membranes were stained with ECL reagents.
MDA levels and the activity of SOD and lactate dehydrogenase (LDH) were assessed spectrophotometrically according to the manufacturer’s instructions (Vazyme, China).
IL-1 β, IL-6 and TNF-α were quantified in the sera with ELISA kit according to the manufacturer's instructions, ODs were read 450 nm and levels of cytokines were obtained from a standard curve.
Data analysis was carried out with SPSS21.0 statistic softwwere, and the data were expressed as means±standard deviation. t-test was used for comparisons of groups with the p value of <0.05 being considered as significant.
Compared with the control group, the levels of protein in the urine, serum creatinine and urea nitrogen in Adr group were significantly higher in animals treated with Adr while such levels were significantly lower in animals that received FA in a dose dependent manner (Figure 1A-C).
 Figure 1
Figure 1FA ameliorates renal dysfunction in Adr-induced nephropathy. The rats are randomly assigned to seven groups with 10 in each group: Control group, rats are injected with equivalent saline intravenously; Adr group, rats are injected with Adr diluted in 0.9% saline from the tail vein to a final dose of 6.5 mg/kg; Adr + FA (2.5 μ g / ml) group, rats injected with Adr are treated with 2.5 μ g / ml FA; Adr + FA (5 g/ml) group, rats injected with Adr are treated with 5 g/ml FA; Adr + FA (10 g/ml) group, rats injected with Adr are treated with 10 μ g / ml FA. A. Proteinuria. B. Serum creatinine. C. Urea nitrogen. **, p < 0.01 versus Control group; ##, p < 0.01 versus Adr group.
H&E showed presence of interstitial nephritis, and apoptosis in Adr group, while these changes were significantly reduced in Adr + FA (10 μg/ml) groups as compared to those treated with Adr alone (Figure 2A). TUNEL staining showed that the number of apoptotic cells in control group were significantly lower than those observed in Adr group. Treatment with FA significantly reduced the number of apoptotic cells in animals treated with Adr in a dose dependent manner (Figure 2A-B). FA also significantly inhibited the overexpression of cleaved-caspase-3 and cleaved-caspase-9 induced by Adr (Figure 2C).
 Figure 2
Figure 2FA remits pathological damage by inhibiting apoptosis in Adr-induced nephropathy. The rats are randomly assigned to seven groups with 10 in each group: Control group, rats are injected with equivalent saline intravenously; Adr group, rats are injected with Adr diluted in 0.9% saline from the tail vein to a final dose of 6.5 mg/kg; Adr + FA (2.5 μ g / ml) group, rats injected with Adr are treated with 2.5 μ g / ml FA; Adr + FA (5 g/ml) group, rats injected with Adr are treated with 5 g/ml FA; Adr + FA (10 g/ml) group, rats injected with Adr are treated with 10 μ g / ml FA. A. Adr-induced renal pathological injury is detected by H&E staining and apoptosis rate is detected by TUNEL staining. ( × 400 times) B: Statistics of TUNEL staining results; C and D. The protein levels of cleaved-caspase-3, cleaved-caspase-9, Bax (6A7), Bcl-2 and Cyto C are detected by western blotting; **, p < 0.01 versus Control group; ##, p < 0.01 versus Adr group.
The expression of Bcl-2 was significantly lower while the levels of Bax (6A7) and Cytochrome C were significantly higher in Adr group as compared to those in control group (Figure 2D). FA significantly increased Bcl-2 level, and decreased Bax (6A7, an activated pro-apoptotic protein) and Cytochrome C (Figure 2D).
To assess whether Adr induces oxidative damage in the kidenys we quantified the SOD, MDA and LDH in the kidenys. SOD was significantly lower in Adr group as compared to the control group, while MDA and LDH were significantly increased (Figure 3). FA treatment, in a dose-dependent manner, led to increase in serum level of SOD, while decreasing the serum levels of MDA and LDH (Figure 3)
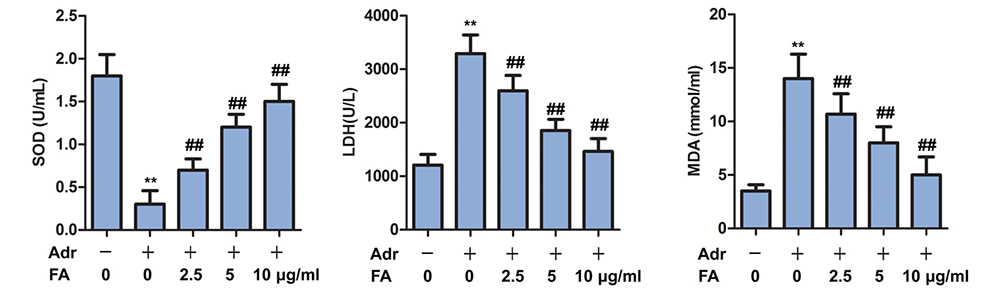 Figure 3
Figure 3FA alleviates Adr-induced oxidative stress. The rats are randomly assigned to seven groups with 10 in each group: Control group, rats are injected with equivalent saline intravenously; Adr group, rats are injected with Adr diluted in 0.9% saline from the tail vein to a final dose of 6.5 mg/kg; Adr + FA (2.5 μ g / ml) group, rats injected with Adr are treated with 2.5 μ g / ml FA; Adr + FA (5 g/ml) group, rats injected with Adr are treated with 5 g/ml FA; Adr + FA (10 g/ml) group, rats injected with Adr are treated with 10 μ g / ml FA. The contents of SOD, MDA, LDH are detected by SOD, MDA, LDH kit; **, p < 0.01 versus Control group; ##, p < 0.01 versus Adr group.
Serum levels of IL-6, IL-1 β and TNF-α were significantly higher in Adr group as compared to the control group (Figure 4) FA treatment, in a dose-dependent manner, significantly decreased the serum levels of IL-6, IL-1 β and TNF-α (Figure 4). The survival rate of animals treated with Adr was significantly increased by four weeks of treatment with FA (Figure 5A).
 Figure 4
Figure 4FA inhibits inflammatory response in Adr-induced nephropathy. The rats are randomly assigned to seven groups with 10 in each group: Control group, rats are injected with equivalent saline intravenously; Adr group, rats are injected with Adr diluted in 0.9% saline from the tail vein to a final dose of 6.5 mg/kg; Adr + FA (2.5 μ g / ml) group, rats injected with Adr are treated with 2.5 μ g / ml FA; Adr + FA (5 g/ml) group, rats injected with Adr are treated with 5 g/ml FA; Adr + FA (10 g/ml) group, rats injected with Adr are treated with 10 μ g / ml FA. The contents of inflammatory factors IL-6, IL-1β, TNF-α are detected by ELISA. **, p < 0.01 versus Control group; ##, p < 0.01 versus Adr group.
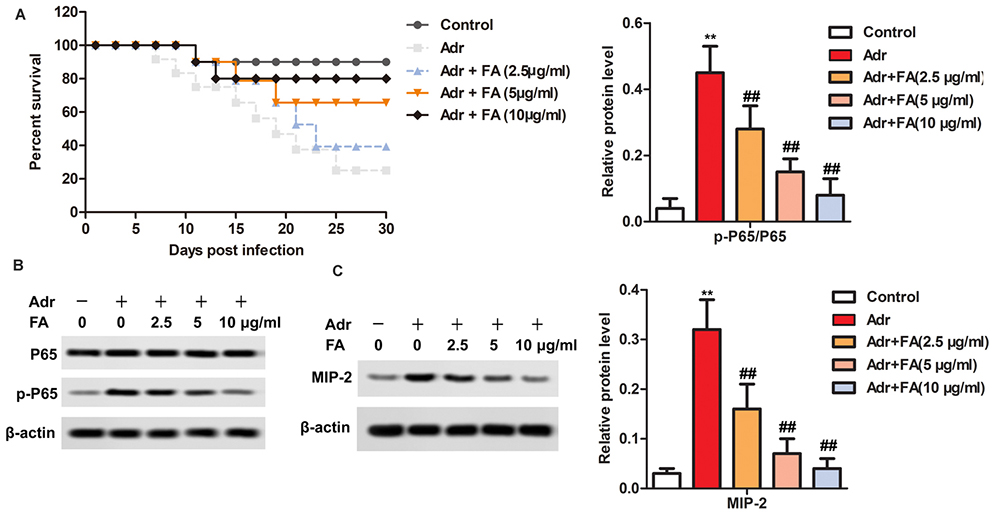 Figure 5
Figure 5FA inhibits the activation of NF-kappaB / MIP-2 pathway in Adr-induced nephropathy. The rats are randomly assigned to seven groups with 10 in each group: Control group, rats are injected with equivalent saline intravenously; Adr group, rats are injected with Adr diluted in 0.9% saline from the tail vein to a final dose of 6.5 mg/kg; Adr + FA (2.5 μ g / ml) group, rats injected with Adr are treated with 2.5 μ g / ml FA; Adr + FA (5 g/ml) group, rats injected with Adr are treated with 5 g/ml FA; Adr + FA (10 g/ml) group, rats injected with Adr are treated with 10 μ g / ml FA. A. Survival curve. B. The protein levels of p65 and MIP-2 are detected by western blotting. **, p < 0.01 versus Control group; ##, p < 0.01 versus Adr group.
To assess the involvement of the NF-kappaBp65/MIP-2 pathway which induces inflammatory response, the NF-kappa B p65 and MIP-2 were assessed by Western blotting. As compared to the control group, Treatment of animals with Adr led to the phosphorylation of p65 and MIP-2 in Adr group (Figure 5B-C) FA treatment, in a dose-dependent manner, significantly inhibited the phosphorylation of p65 and MIP-2 (Figure 5B-C).
Nephropathy bears a poor prognosis and current treatment strategies have not made drastic changes in the outcome of this disease. For his reason, there is a great interest to find proper treatment that increases the longevity of patients. For example, it has been shown that Astragaloside IV, Salvianolic acid A and Gigagliptin were effective in rats (21-23). Gigagliptin improved nephropathy by inhibiting Adr-induced apoptosis, inflammation and oxidative stress (22). FA is the main active component of Forsythiae Fructus. Forsythiae Fructus is a traditional Chinese herbal medicine with antibacterial, antioxidant, and scavenging of free radicals. Consistent with our results, Qian et al. have found that FA treatment inhibited OVA-induced asthma, significantly delayed the pathological changes and the production of inflammatory factors such as IL-4, IL-5 and IL-13 in the lung tissue induced by OVA and (24). It has also been shown that FA significantly decreased hemoglobinuria, and reduced serum creatinine and nitrogen in Adr-induced nephropathy.
Apoptosis appears to contribute to the nephropathy and for this reason, it follows that the drugs that can reduce this type of programmed cell death can lead to the improvement in neophropathy. For example, reduced apoptosis by magnolin has improvded nephropathy in rats (25). Similarly, it has been shown that atorvastatin, by inhibiting apoptosis, prevents the development of renal disease (26). Consistent with previous observation that FA inhibits the expression of apoptosis factors such as caspase-9 and caspase-3 as well as the apoptosis of hair cells (27), here we show that FA markedly reduces apoptosis in the kidenys and decreases the Adr induced nepropathy.
Oxidative stress is another factor that worsens the nephropathy and reduced oxidative stress has been shown to reduce the severity of this disease (28). Adr which induces experimental nephropathy has been shown to decrease the activity of SOD and to increase MDA level (29) Thus, it follows that treatment with agents such as lime, turmeric or FA that reduces such stress might be adjunt to reduce the severity of the disease (30-31). Consistent with these results, we showed that FA significantly, in a dose-dependent manner, increased the serum level of SOD and decreased the serum level of MDA and ADH in Adr group.
Another mediator of nephropathy is the inflammatory response that must be controlled in order to reduce the pathological damage to kidneys. For example, matrine, or Plantago major that were shown to inhibit inflammatory response reduced Adr-induced nephropathy in rats (32-33). Consistent with previous findings that FA significantly inhibited the production of endotoxin-induced inflammatory factors TNF-α, IL-1 β and PGE 2 (16), treatment with FA, significantly inhibited the phosphorylation of p65 and MIP-2 in the kidneys which are inducers of inflammation and lead to the release of inflammatory cytokines and coordinately reduced the inflammation in an Adr-induced nephropathy model. Consistent with such results, the serum FA reduced the main inflammatory cytokines including IL-6, IL-1 β and TNF-α. Consistent with our results, FA prevented LPS/GalN-induced liver injury by inhibiting the activation of NF-kappaB/ MIP-2 pathway (17). However, in this study, it is found that FA inactivates Adr-induced NF-kappaBp65 / MIP-2 signaling pathway in a dose-dependent manner.
In conclusion, FA alleviates nephropathy by inhibiting Adr-induced apoptosis, oxidative stress and inflammatory response, and by inhibiting increase of NF- kappa B p65/MIP-2 pathway. Such results suggest that FA might have a therapeutic role in patients with nephrotic syndrome.
Adr
Adriamycin
Adriamycin nephropath
Forsythoside A
Nephrotic Syndrome
