Gastric cancer is the fourth most common malignancy world-wide that bears a high mortality by invasiveness and metastases. To this end, we examined the role of miR-1 in mobility and migration of gastric cancer cells. miR-1 was down-regulated and Sorcin, which supports invasion, was highly expressed in gastric cancer cell lines as compared to the control. The overexpression of miR-1 significantly inhibited the mobility and migration of gastric cancer cells, while, its knockdown exerted an oppoiste effect. In addition, while overexpression of miR-1 suppressed the expression of Sorcin, the siRNA knockdown of Sorcin significantly counteracted the effect of miR-1 inhibitor on cell invasion and migration of gastric cancer cells. A miR-1 mimic decreased while its inhibitor increased the MMP-7 and VEGF required for invasion. Taken together, the findings support the view that miR-1 controls the mobility and migration of gastric cancer cells and might be a therapeutic target for blocking gastric cancer invasion.
Gastric cancer, which accounts for 10% of malignant tumors, is the fourth most common malignancy in the world and second leading cause of cancer death (1-2). Although, gastric cancer is treated with surgery and chemotherapy, there is as yet no cure due to cancer invasion, metastasis and development of resistance to chemotherapy (3-5). To invade, cancer cells undergo an Epithelial to Mesenchymal Transition (EMT), by which, cancer cells lose their epithelial cell lineage and acquire mesenchymal cell characteristics capable of displaying invasive behavior and drug resistance (6-9).
Sorcin is a class of drug-resistant calcium-binding proteins that is highly expressed in gastric, colorectal, breast and lung cancers (10-12). Sorcin participates in EMT and its silencing inhibits EMT and metastasis of breast and rectal cancers (12-13). miR-1 is a short-chain non-coding RNA that is known to regulate Sorcin through Ca2+ channel, to control the development of diverse forms of cancer and to restrain epithelial-mesenchymal transition and metastasis (14-22). In the present study, we examined the expression of both miR-1 and Sorcin in gastric cancers and examine how they jointly regulate the mobility and migration of cancer cells in vitro.
Normal gastric epithelial cell line (GES-1), gastric cancer cell lines (HGC-27, SGC-7901, MGC-803, MKN-28) were purchased from the ATCC (Maryland, American). GES-1 cells were cultured in DMEM-H (Gibco, Rockville, MD) medium with 10% fetal bovine serum and cancer cell lines were cultured in RPMI-1640 with 10% fetal bovine serum (Gibco, Rockville, MD). Cells were kept at 37°C in a 5% CO2 atmosphere.
Cells were seeded in six-well plates at a density of 1×05 cells/ml. After incubation for 24 h, cells were treated with miR-1 mimic, miR-1 inhibitor or with small interfering RNA (siRNA) in presence of lipofectamine 3000 (InVitrogen, Carlsbad, CA) according to the manufacturer’s instructions for 24 h. RNA fragments were synthesized by Shanghai JiMa Pharmaceutical Technology Co., Ltd. (Shanghai, China).
Total protein in protein extracts was measured using a BCA kit. Proteins were resolved in SDS-PAGE and transferred onto PVDF membranes. Membranes were blocked in 5% skim milk powder at room temperature for 2h. These were then incubated with the primary antibodies, followed by washing and incubation with HRP-conjugated secondary antibodies. Bands were revealed by use of enhanced luminol-based chemiluminescent (ECL) substrate and analyzed by ChemiDoc XRS imaging system. In each assay GAPDH was used as housekeeping control.
Total RNA was extracted according to the manufacturer’s instruction (TRIzol kit, InVitrogen). The cDNA was synthesized using the reverse transcription kit (InVitrogen). Quantitative analysis of cDNA was performed according using Fluorescence Quantitative PCR Kit (Thermo Fisher Scientific, Waltham, USA).
For the transwell invasion assays, SGC-7901 cells pre-transfected with miR-1 mimic or miR-1 inhibitor (2×104 cells per well) were placed in the upper chamber of each Transwell dish (8 mm pore size; Merck Millipore Corp, Billerica, MA, USA) that were coated with Matrigel (Becton-Dickinson, New Jersey, USA). The lower chamber was filled with complete medium (containing 20% FBS). Cells were incubated at 37°C for 24 h and non-invading cells on the upper membranes were removed and the invasive cells entering the lower chamber were fixed in 95% ethanol, stained with hematoxylin, photographed and quantified. Briefly, the invasive cells in the lower chamber were counted by blindly by three individuals.
For wound-healing assays, cells were seeded at 1×05/ml in a 6-well plate coated with Matrigel and were cultured until reached a 90% confluent. Then, the dishes were scored by creation of a straight scratch line using a sterile pipette tip. Dishes were gently washed to removed detached cells. Cells migrated to the scratch line were counted in 24 h with a digital camera (Leica DFC300FX).
Cells were transfected with 100 nM pGL4 vector (Promega, Madison, Wisconsin)with miR-1 mimic, wild type or mutant form of Sorcin alone or simultaneously (Figure 4A). Forty-eight hours after transfection, cells were collected and luciferase activity was measured by Dual-Luciferase Reporter Assay kit (Promega, Madison, Wisconsin) in accordance with the manufacturer’s instructions.
Experiments were carried out at least in triplicates. Data were analyzed using SPSS 10.0. Statistical software and the results were expressed as means ± standard deviations. T test was used to determine differences between two groups. The one-way-ANOVA test was used and the variances were analyzed when the variances followed a normal distribution. Results with p values < 0.05 were considered significant.
The relative expression of miR-1 in gastric cancer cell lines (HGC-27, SGC-7901, MGC-803 and MKN-28) was significantly lower than that in normal gastric epithelial cells. Among these cells, GES-1 and SGC-7901 showed the lowest expression level of miR-1 (Figure 1A, *p < 0.0.5., **p < 0.0.1.). The expression of Sorcin was significantly up-regulated in in gastric cancer cell lines as compared with the normal gastric epithelial cells GES-1 (Figure 1B). The low expression of miR-1 and the high expression of Sorcin in gastric cell lines suggest that miR-1 and Sorcin are inversely related.
 Figure 1
Figure 1
MiR-1 was lowly expressed and Sorcin was highly expressed in gastric cancer cells. (A) The expression of miR-1 in gastric cancer cell lines (HGC-27, SGC-7901, MGC-803 and MKN-28) and normal gastric epithelial cells GES-1 was detected by qRT-PCR, (B) Western blot was used to detect the expression of Sorcin in gastric cancer cell lines and gastric epithelial cells. All these experiments were repeated at least three times. * p < 0.0.5., ** p < 0.0.1. versus control group.
The expression of miR-1 was manipulated by increasing or decreasing its expression by transfecting the SGC-7901 cells, respectively with miR-1 mimic or inhibitor (Figure 2A, * p < 0.0.5., *** p < 0.0.0.1). As shown in Figure 2B and 2C, the number of invasive cells was decreased by miR-1 mimic and was increased by miR-1 inhibitor as compared with the control group. The wound healing assay also showed that the repair of the in vitro wounds closer rate was lowered by miR-1 mimic and was increased by miR-1 inhibitor as compared with the control group (Figure 2D, 2E, * p < 0.0.5.). The matrix metalloproteinase-7 (MMP-7) and vascular endothelial growth factor (VEGF) were also down-regulated by miR-1 mimic and up-regulated by miR-1 inhibitor (Figure 2F, 2G, * p < 0.0.5.). These results suggest that overexpression of miR-1 inhibits the invasive behavior and migrating potential of gastric cancer cells.
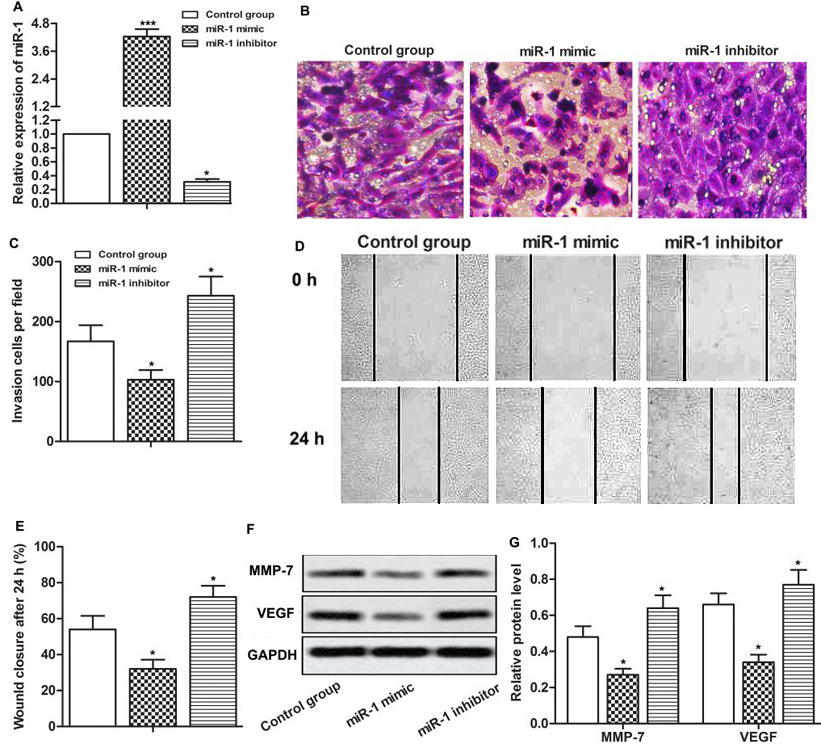 Figure 2
Figure 2
Up-regulated miR-1 inhibited the invasion and migration of gastric cancer cells. The cells were divided into control group, miR-1 mimic group and miR-1 inhibitor group. MiR-1 mimic and miR-1 inhibitors were synthesized and transfected into SGC-7901 cells, respectively. (A) The expression of miR-1 was detected by qRT-PCR. (B) Transwell invasion assay was used to detect the invasive ability of gastric cancer cells. (C) Quantitative analysis of invasive cells. (D) The migration ability of gastric cancer cells was detected through wound healing assay. (E) Quantitative analysis of wound healing assay. (F) Western blot was used to detect the expression of MMP-7 and VEGF. (G) Quantitative analysis of MMP - 7 and VEGF protein expression levels. GAPDH was used as internal reference. * p < 0.0.5., *** p < 0.0.0.1 versus control group.
We argue that since miR-1 appear to inhibit Sorcin expression in tumor cells (22) and down-regulation of Sorcin inhibits Epithelial to Mesenchymal Transition (EMT) and metastasis (12), that miR-1 might also regulate these features in tumor cells. To test this, we, therefore, first tested the effects of miR-1 in Sorcin expression. As shown in Figure 3A and 3B, the suppression of Sorcin was suppressed by miR-1 mimic and was significantly elevated by miR-1 inhibitor (*p < 0.0.5.). Consistent with an effect on phenotype, the overexpression of miR-1 significantly increased the expression of E-cadherin and inhibited the expression of N-cadherin while miR-1 inhibitor exerted an opposite role and inhibited the expression of E-cadherin and promoted the expression of N-cadherin (Figure 3C, 3D, * p < 0.0.5.). These findings show that miR-1 enforces the epithelial phenotype, an effect that potentially resists EMT.
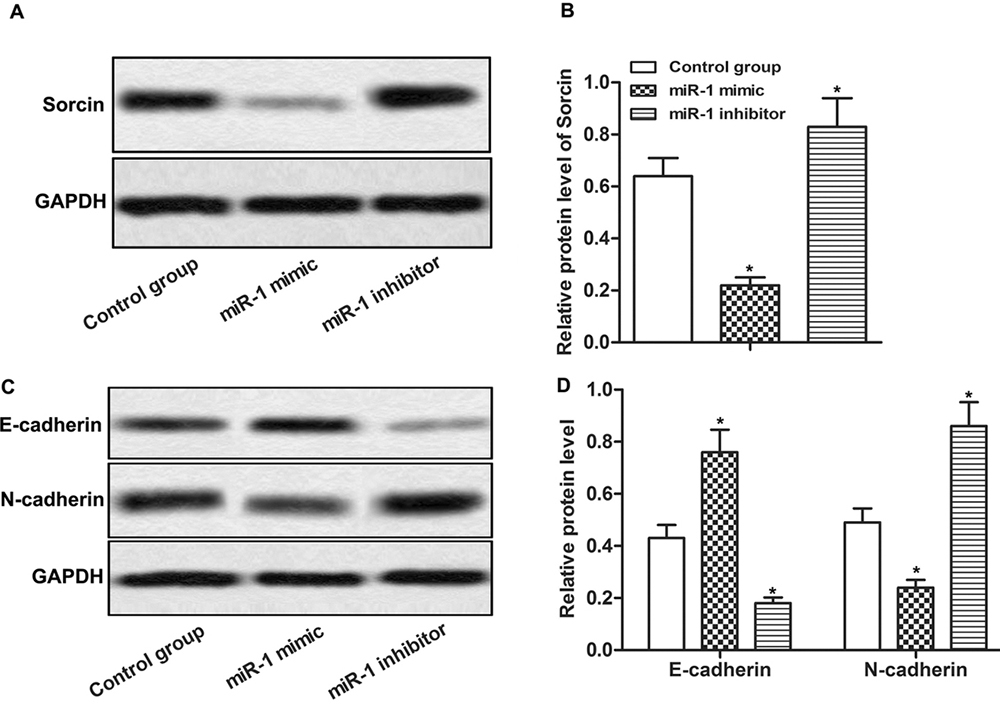 Figure 3
Figure 3
Up-regulated miR-1 inhibited Sorcin expression and EMT. The cells were divided into control group, miR-1 mimic group and miR-1 inhibitor group. MiR-1 mimic and miR-1 inhibitors were synthesized and transfected into SGC-7901 cells, respectively. (A) Western blot was used to detect the expression of Sorcin. (B) Quantitative analysis of Sorcinthe expression level of Sorcin. (C) Western blot was used to detect the expression of E-cadherin and N-cadherin. (D) Quantitative analysis of E-cadherin and N-cadherin protein expression levels. GAPDH was used as internal reference. * p < 0.0.5., versus control group.
The TargetScan showed that Sorcin has a miR-1-complementary binding site, suggesting existence of interaction between miR-1 and Sorcin (Figure 4A). The existence of such an interaction was examined by transfecting cells alone or simultaneously with 100 nM miR-1 mimic and wild type or mutant form of Sorcin and then carrying out luciferase assay. The results showed a distinct downtrend when cells were co-transfected with miR-1 mimic and wildtype Sorcin 1. However, and no effect was observed when the cells were transfected with the mutated form of Sorcin (Figure 4B , ** p < 0.0.1.). These findings establish of existence of a negative feedback loop comprised of miR-1 andSorcin.
To demonstrate that interaction of miR-1 and Sorcin, we transfected Sorcin siRNA into gastric cancer cells (Figure 4C, 4D, ** p < 0.0.5.). This transfection led to decreased expression of E-cadherin and increased expression of N-cadherin in cells that were also transfected with miR-1 inhibitor (Figure 4E, 4F, * p < 0.0.5., # p < 0.0.5., ## p < 0.0.1.).
 Figure 4
Figure 4
Sorcin siRNA abolished the effect of miR-1 inhibitor on EMT and the invasion and migration of gastric cancer cells. The cells were divided into control group, miR-1 inhibitor group and inhibitor + Sorcin siRNA group. MiR-1 inhibitor and Sorcin siRNA were synthesized and transfected into SGC-7901 cells, respectively. (A) The binding site of Sorcin and miR-1 was predicted by TargetScan. (B). Relationship between Sorcin and miR-1was tested by Luciferase assay (**p < 0.0.1.) (C) Western blot was used to detect the expression of Sorcin. (D) Quantitative analysis of Sorcin protein expression. (E)Western blot was used to detect the effect of Sorcin siRNA on the expression of E-cadherin and N-cadherin in gastric cancer cells. (F) The quantitative analysis of E-cadherin and N-cadherin expression levels. (G) Transwell invasion was used to detect the effect of Sorcin siRNA on gastric cancer cell invasion. (H)The migration ability of gastric cancer cells was detected by wound healing assay. (I)Western blot was used to detect the expression of MMP-7 and VEGF. (J) Quantitative analysis of MMP-7 and VEGF protein expression levels. GAPDH was used as internal reference. *p < 0.0.5. versus control group, # p < 0.0.5., ## p < 0.0.1. versus miR-1 inhibitor group.
The invasive and migratory ability of cancer cells in miR-1 inhibitor group cells were also significantly enhanced by suppressing Sorcin by transfecting cells with Sorcin siRNA (Figure 4G-H). On the other hand, the expression of MMP-7 and VEGF in gastric cancer cells which was significantly increased by inhibition of miR-1 was further enhanced by transfecting the gastic cancer cells with Sorcin siRNA (Figure 4I-J, * p < 0.0.5., # p < 0.0.5.).
Together, such results suggest that miR-1 suppresses cell migration and mobility and enforces the epithelial phenotype by down-regulating the expression of Sorcin.
A large number of studies show that miRNAs are involved in the development of various cancers, including gastric cancer, and act either as tumor suppressors or tumor oncogenes. For example, miR-194, miR-34A, miR-410 and miR-1 are recognized as tumor suppressors and inhibit cell proliferation as well as EMT and migration of gastric cancer cells (23-25). On the other hand, miR-362 and miR-630 act as oncogenes and promote the proliferation and migration of gastric cancer cells (26-27).
The prognosis of gastric cancer appears to be directly related to the expression level of miR-1. For example, in one study, the 5-year survival rate of patients with gastric cancer was higher when the expression of miR-1 was found to be high while a low miR-1 expression correlated with a poor prognosis (28). To find how miR-1 might be involved in gastric cancer behavior, we used cell lines that inherently show a low level of miR-1. Then, the miR-1 was overexpressed in these cells and motility and migration ability of gastric cancer cells were assessed. This showed that such an over-expression inhibited the mobility and migration of SGC-7901 cells. This treatment also reduced the protein levels of MMP-3 and VEGF that participate in tissue remodeling and angiogenesis and which are required for invasive behavior (26, 28). The knock down of miR-1 exerted an opposite effect. These findings are consistent with previous results that show that miR-1 has a tumor supressive role by regulating the expression of downstream oncogenes including c-MET, MALAT1, KRAS, etc (29-31).
Sorcin is a common calcium-binding protein which unfrt physiolohic conditions it regulates myocardial contractility (22). Recently, it has been shown that Sorcin over-expression in cancer cells, including colon, breast, and nasopharyngeal cancers, confers drug resistance to tumor cells (13, 32-34). Consistent with such reports, we find that the expression of Sorcin in gastric cancer cell lines was significantly increased (35). Given that it was recently shown that miR-1 affects myocardial contractility by down-regulating the expression of Sorcin, we considered whether similar interaction occurs in gastric cancer cells (22). Consistent with such thesis, the expression of Sorcin was elevated by miR-1 inhibitor and was suppressed by miR-1 mimic. Moreover, inhibition of Sorcin by Sorcin siRNA significantly reversed the effect of miR-1 inhibitor on mobility and migration behavior of gastric cancer cells, suggesting Sorcin as a downstream target for the effects of miR-1 in gastric cancer cells.
EMT is an important process which is closely associated with cancer cell metastasis. In the EMT process, tumor cells lose epithelial cell characteristics. Up-regulated MMP-7, MMP-3 and VEGF accelerate the degradation of extracellular matrix proteins, which increase the metastasis ability of cancer cells and promote tumor cells to obtain stem cell characteristics (9). Lijun Xu et al. found that miR-1 was able to inhibit EMT by regulating MAPK and PI3K / Akt pathway in rectal cancer cells (21). Y-N Liu et al. have shown that miR-1 regulates the expression of Slug which is the downstream target of EMT to inhibit EMT in prostate cells (36). Our investigation showed that overexpressed miR-1 elevated the expression of E-cadherin and suppressed the expression of N-cadherin. Combine the experimental results mentioned above we know that overexpressedmiR-1 inhibited cell invasion and migration by inhibiting the occurrence of EMT in gastric cancer cells. Sorcine and long non-coding RNAs are key regulators of EMT and tumour drug resistance of cancer stem cells (6-9, 37-38). Sorcin might be involved in such processes by regulation of E-cadherin and VEGF expression (12). Here, we showed that overexpression of miR-1 suppressed the expression of Sorcin and coordinately increased E-cadherin and VEGF expression. Moreover, knockout of Sorcin abolished the effect of miR-1 inhibitor on cell migration and the expression of MMP-7 and VEGF. Taken together, the findings support the view that miR-1 controls the mobility and migration of gastric cancer cells.
We sincerely appreciate the technical support from The F.irst affiliated hospital of Wenzhou medical University.
EMT
epithelial-mesenchymal transition
matrix metalloproteinase-7
vascular endothelial growth factor
