Loss of pancreatic beta-cells by apoptosis appears to play an important role in the development of insulin deficiency and the onset and/or progression of the diabetes mellitus. To this end, we report that curcumin prevents high glucose (HG) induced oxidative stress and apoptosis in mouse pancreatic beta cells. Moreover, curcumin prevents HG induced increase in expression of CHOP, decrease in PCG-1α and phosphorylation of ERK1/2 (pERK1/2) without any effect on the phosphorylation levels of p38 and JNK. Moreover, similar to curcumin, blockade of pERK1/2 reduced the HG induced apoptosis in pancreatic beta cells. Overexpression of CHOP or siRNA knockdown of PCG-1α counteracted the effect of curcumin on HG induced apoptosis and oxidative stress. These results suggest that curcumin acts through CHOP/PCG-1α and ERK1/2 signaling to block the HG induced oxidative stress and apoptosis.
Glucose is a physiological stimulator of insulin secretion by pancreatic islet cells. However, Type 2 diabetes mellitus (T2DM) leads to oxidative stress and apoptosis of beta cells, which leads to further hypoinsulinemia and hyperglycemia (1-4). C/EBP homologous protein (CHOP), and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) are involved in oxidative stress (11-12). CHOP, an enhancer binding protein, is involved in endoplasmic reticulum stress-induced apoptosis and inhibits peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), which is a master regulator of mitochondrial biogenesis (4, 13-14). PGC-1α is a master regulator of ROS scavenging enzymes that appears to contribute to cell survival (15). In present study, we examined whether curcumin, which is extracted from rhizomes of curcuma longa, rhizome curcumae, rhizoma curcumae and acorus calamus, might reduce the apoptosis through, its anti-oxidative, anti-inflammatory, anti-apoptotic, and angiogenic properties (5-10). We also examined whether the effect of curcumin is mediated through CHOP, PGC-1α and ERK1/2 mediated regulation of oxidative stress and apoptosis in pancreatic beta cells that were subjected to a high glucose environment.
Dulbecco's Modified Eagle Medium F-12 (DMEM/F12) and fetal bovine serum (FBS) were obtained from Hyclone (Hyclone, Logan, UT, USA). Curcumin and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) apoptosis assay kit was from KeyGEN BioTECH (KeyGEN, Shanghai, China). The antibodies to CHOP, PGC-1αlpha, p-JNK, pERK1/2, pP38 and beta -actin were obtained from Abcam (Abcam, MA, USA). Cell-counting kit-8 assay (CCK-8) was obtained from DOJINDO molecular technologies (DOJINDO, Kumamoto, Japan). Oxidative stress assay kit was obtained from Beyotime Biotechnology (Beyotime, Shanghai, China).
Min-6 mouse pancreatic beta cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). These cells were cultured in Dulbecco's Modification of Eagle's medium (DMEM; Gibco, Gaithersburg, MD) containing 10% fetal bovine serum (Gibco, CA, USA) in 5% CO2 at 37°C. Min-6 cells were treated with curcumin for 4 h prior to incubation in 5 mM or 25 mM glucose.
The adenovirus of CHOP used in this study was purchased from Genekey Co., Ltd .(Genekey, Shanghai, China). Cells were transfected with Lipofectamine 2000 (Invitrogen-Thermo Fisher Scientific, Carlsbad, CA) with PGC-1 using the target sequences shown in Table 1. 48 h after each transfection, the efficiencies of CHOP overexpression and PGC-1α knockdowns were assessed using Western blotting.
| PCR primers | ||
|---|---|---|
| Gene Name | Sense 5′-3′ | Anti-sense5′-3′ |
| CHOP | TGTCCTTCTTCGGGTCTCAG | GTACACTGCCCTTGTGGAC |
| PGC-1α | GGGCCCTTATTTTTGATCA | GTCCCCGTTCCCTGTAACTC |
| GAPDH | CTTCCCTACCGGTACGG | GCCCAGATGGGCTCTGT |
| siRNA | ||
| PGC-1α | TTGACCAGATTCGAGTTCTTGG | |
| siRNA control | CCGAATTGCATCGCCGGTAAC | |
Total RNA was extracted from Min-6 cells using TRIzol reagent (Thermo Fisher Scientific, MA, USA). The purity of RNA was assessed by measuring the absorbance ratio of 260/280 nm using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, MA, USA). Reverse transcription of RNA was carried out using a PrimeScript™ RT reagent Kit with gDNA eraser (RR047A; Takara, Tokyo, Japan). The cDNAs were quantitated by qRT-PCR using SYBR® Premix Ex Taq™ (RR420A; Takara, Tokyo, Japan). The data were normalized using levels of GAPDH as a housekeeping control and were further analysed by the 2−ΔΔCT method. The primers used for qPCR are listed in the Table 1.
Total protein was isolated from Min-6 cells and solubilized using RIPA lysis buffer containing proteinase inhibitor (Sigma, USA). Concentrations of proteins were assessed by BCA assay kit (Pierce, Rockford, IL, USA). Proteins (20 μg) were resolved in 10% SDS-PAGE gel (120 V, 120 mins) and were transferred to polyvinylidene difluoride (PVDF) membrane (400 mA, 90 mins). Blots were blocked for 2 hours with BSA, and were incubated with at 4 °C overnight with primary antibodies against PCG-1α (1:1000, Abcam, MA, USA), CHOP (1:1000, Abcam, MA, USA), pERK1/2 (1:1000, Abcam, MA, USA), pJNK (1:1000, Abcam, MA, USA), Bax (1:1000, Abcam, MA, USA), Bcl-2 (1:1000, Abcam, MA, USA), Tubulin (1:2000, Abcam, MA, USA) or beta-actin (1:5000, Abcam, MA, USA ). Immunopositive bands were analyzed using a FluorChem M system (Protein Simple, San Jose, CA, USA).
Oxidative stress was examined by assessing ROS generation, MDA level and SOD activity using commercial kits from Beyotime Biotechnology (Beyotime, Shanghai, China).
Apoptosis was assessed using terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling assay (TUNEL) kit (KeyGEN, Shanghai, China). Images were captured at 100× using a fluorescence microscope ((LSM510META, Carl Zeiss, Jena, Germany). The apoptotic pancreatic beta cells were counted in at least ten representative microscopic fields and expressed as percentages of positive staining cells relative to total number of cells. In this experiment, each group randomly selected 10 different areas of fluorescence, and counted the total number of cells and apoptotic cells, Then, the number of positive cells counted in each slide and the total number of cells were analyzed and compared. A total of 72331 cells and 5065 positive cells were counted in present study, the specific total number of cells and the number of positive cells in each experiment were shown in the figure legends. In addition, the apoptotic protein Bcl-2 / Bax ratio was further assessed.
Means ± standard error of the mean (SEM) were determined using SPSS 17.0 software. Statistical analyses were performed using Student's t test. P Values of less than 0.05 were considered as being significant (*p<0.05; ** p<0.01 and ***p<0.001). The p values of treatments are cited in the Table 2.
| Treatment | ROS | MDA | SOD |
|---|---|---|---|
| NG | |||
| HG | P<0.001 VS NG | P<0.001 VS NG | P<0.001 VS NG |
| HG+ Ad con | NS VS HG | NS VS HG | NS VS HG |
| HG+ Ad Chop | NS VS HG | NS VS HG | NS VS HG |
| HG+ siRNA con | NS VS HG | NS VS HG | NS VS HG |
| HG+ PGC-1α siRNA | NS VS HG | NS VS HG | NS VS HG |
To explore the influence of high glucose on oxidative stress and apoptosis, pancreatic beta cells were cultured with normal glucose (NG 5 mM) or high glucose (HG, 25 mM) in presence and absence of curcumin. The treatment of cells with HG and not NG led to oxidative stress as evidenced by increase in ROS, MDA and SOD activity and apoptosis as evidenced by increased in TUNEL positivity (Figure 1A-B). Treatment of cells with curcumin alone at 1-10 μM did not change the viability of cells after 24 hr of treatment, however, the viability of cells decreased with 50 μM (Figure 2A). The treatment of cells with curcumin at a concentration of 10 μM led to a significant reduction in ROS, and MDA and decreased SOD activity inducible with HG (Figure 2B-2D). Moreover, curcumin (10 μM) significantly inhibited HG (25 mM) induced apoptosis in a dose dependent manner (Figure 2E).
 Figure 1
Figure 1HG increases oxidative stress and apoptosis in pancreatic beta cells. A. ROS generation, B. MDA level and C. SOD activity. D. TUNEL immunofluorescence staining (total cells: 8028, positive cells: 591). Scale bar, 100 μm. Each experiment was repeated 3 times. * P < 0.05, ** P < 0.01, *** P < 0.001. NG: normal glucose (5 mM), HG: high glucose (25 mM), ROS: reactive oxygen species, MDA: malondialdehyde, SOD: superoxide dismutase.
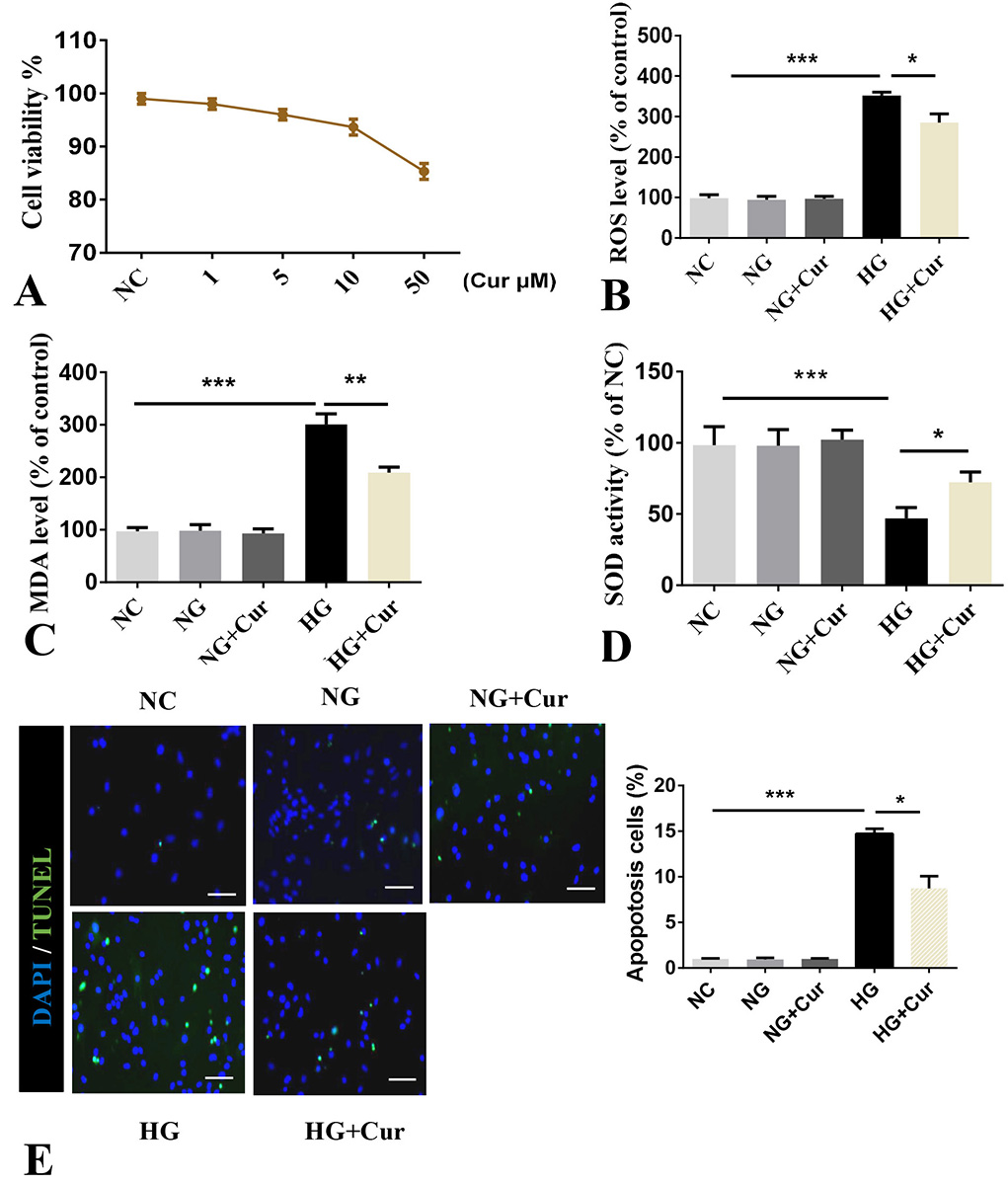 Figure 2
Figure 2Curcumin suppresses HG (25 mM) induced oxidative stress and apoptosis in pancreatic beta cells. Pancreatic beta cells were treated with 10 μM of curcumin for 24 h and were used to perform relevant experiments. A. Cell viability assessed by CCK-8 assay. B. ROS. C. Level of MDA. D. Activity of SOD. E. TUNEL (total cells: 14244, positive cells: 892). Scale bars, 25 μm . Data represent the mean±SD of 3 independent measurements. *P< 0.05. **P< 0.01. ***P< 0.001. NC: normal control, NG: normal glucose (5 mM), HG: high glucose, Cur: curcumin.
In order to assess how curcumin might reduce oxidative stress and apoptosis, we examined its effects on the regulators of oxidative stress namely, CHOP and PGC-1α. We first assessed the effect of 10 μM of curcumin on temporal changes in the expression of CHOP and PGC-1α. These analyses showed that curcumin, in a time dependent manner, led to the significant decrease in the expression of CHOP while it simultaneously increased the expression of PGC-1α (Figure 3A-B). Treatment of cells for 24 hours with curcumin at 0, 1, 5 and 10 μM showed its effects on CHOP and PGC-1α to be dose dependent (Figure 3C-D).
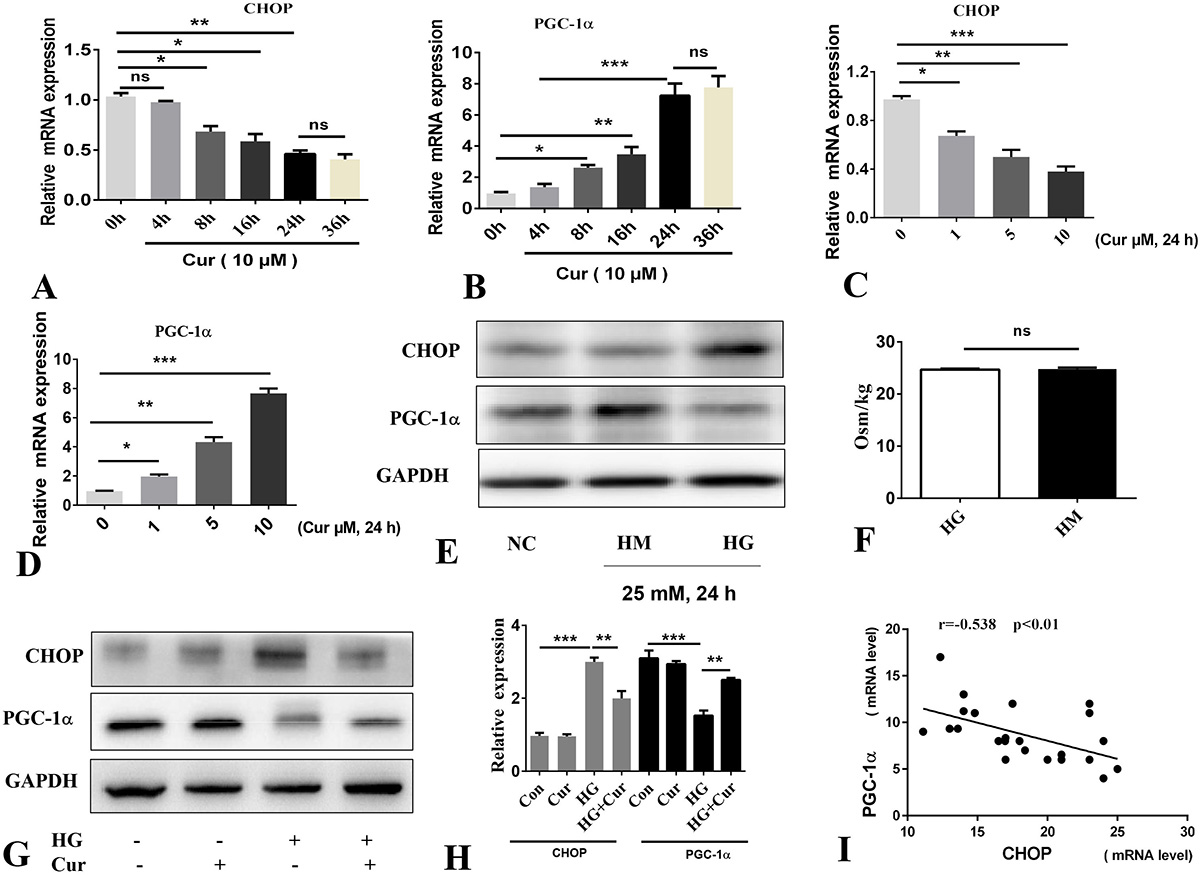 Figure 3
Figure 3Curcumin regulates CHOP and PGC-1α expression. A-D. qPCR. E. Western blotting; cells were treated by HM (25 mM) or HG (25 mM). F. Osmolality of 25 mM mannitol and HG. G-H. Western blotting and quantification of values. I. Expression of CHOP and PGC-1α mRNA in cells treated with HG (25 mM). Data represent the mean±SD of 3 independent measurements. *P< 0.05. **P< 0.01. ***P< 0.001. Cur: curcumin (10 μM), HM: mannitol group, HG: high glucose. ns: not significant.
We, then, carried out the analysis on cells that were treated with HG in presence and absence of curcumin. Treatment of cells with 25 mM HG led to increased CHOP while PGC-1α was decreased (Figure 3E). These effects were not due to increased osomotic pressure by HG since the cells that were treated with 25 mM mannitol (HM, 25 mM mannitol and HG have the same osmotic pressure Figure 3F) failed to show any effect on the expression of CHOP or PGC-1α.
Treatment of pancreatic beta cells with curcumin for 4 h prior to incubation with HG 25 mM for 24 hours showed that curcumin counteracted the effect of HG (Figure 3G-H). The inverse relationship of CHOP and PGC-1α expression in curcumin treated cells induced by HG is shown in Figure 3I. To further validate the existence of an inverse relation between expression of CHOP and PGC-1α, CHOP was overexpressed by an adenoviral vector (Ad-CHOP). The overexpression of CHOP led to inhibition of PGC-1α, at the mRNA and protein level (Figure 4A-B). These results were further validated by the siRNA knockdown of PGC-1α, which also showed that this knockdown markedly upregulated the mRNA and protein expression of CHOP (Figure 4C-D). Moreover, We found that overexpression of CHOP or knockdown of PGC-1α can induce oxidative stress injury of pancreatic beta cells and further aggravate the level of oxidative stress induced by HG (Figure 4E-G).
 Figure 4
Figure 4Chop and PGC-1α mediates oxidative stress injury in pancreatic beta cells. Transfection efficiency of chop and PGC vectors and their regulatory relationship were shown as A-D. A. qPCR. B. Western blotting. C. qPCR. D. Western blotting. E-G. The effect of Chop and PGC-1α on oxidative stress injury of pancreatic beta cells. *P< 0.05, ***P< 0.001.
CHOP and PGC-1α appear to inversely regulate oxidative stress and apoptosis in the pancreatic beta cells that are treated with HG. To show this, we examined the apoptosis and oxidative stress as evidenced by cellular levels of ROS, MDA and SOD acitvity in cells that were treated with HG, or HG and curcumin in cells that were either tranduced with empty adenoviral vector or CHOP or control siRNA and PGC-1α siPNA (Figure 5A-C).
 Figure 5
Figure 5Curcumin alleviates HG induced oxidative stress and apoptosis via CHOP and PGC-1α. A. Level of ROS. B. Activity of SOD. C. Level of MDA. D and F. TUNEL(total cells: 33625, positive cells:2395). Scale bar = 25 μm. E and J. Western blotting.Data represent the mean±SD of 3 independent measurements. *P< 0.05. **P< 0.01. ***P< 0.001. HG: high glucose (25 μM), Cur: curcumin (10 μM), Ad-CHOP: Adenovirus-CHOP (50 MOI), Ad-con: Adenovirus-control (50 MOI).
These experiments confirmed that HG leads to a significant increase in oxidative stress as evident by increased ROS, and MDA while reducing the activity of SOD whereas treatment of cells with curcumin significantly reduced the level of ROS and MDA and increased the activity of SOD. Overexpression of CHOP or knockdown of PGC-1α partially blocked the inhibitory role of curcumin on HG induced oxidative stress (Figure 5A-C).
We then examined the effect of curcumin on HG induced apoptosis in pancreatic beta cells transduced with CHOP adenoviral vector or PGC-1α siRNA. The HG induced apoptosis attenuated by curcumin was partially blocked by adenoviral overexpression of CHOP or by virtue of siRNA knockdown of PGC-1α (Figure 5D-J).
We considered that the effect of curcumin in inhibition of HG induced oxidative stress and apoptosis might not be exclusively through regulation of expression of CHOP and PGC-1α. Phosphorylation of ERK 1/2 is known to induce apoptosis (16-18). Thus, we examined effects of curcumin on phosphorylation of ERK1/2 in cells that were subjected to NC and HG. Treatment of cells with curcumin inhibited the phosphorylation of HG induced ERK1/2 (Figure 6A). To show that this response is specific, we examined the effct of curcumin on the phosphorylation levels of c-Jun N-terminal kinases (JNK)1/2 and p38. The expression of p38 and p-JNK induced by HG was not significantly inhibited by curcumin showing that the effect of curcumin is specific to ERK1/2 (Figure 6B-C). To validate whether the inhibition of ERK1/2 phosphorylation could reduce HG induced oxidative stress and apoptosis, the phosphorylation of ERK1/2 was inhibited by its inhibitor, PD98059 (Figure 6D). Similar to the effect of curcumin, inhibition of p-ERK1/2 significantly inhibited HG induced oxidative stress and apoptosis (Figure 6A, 6E-I).
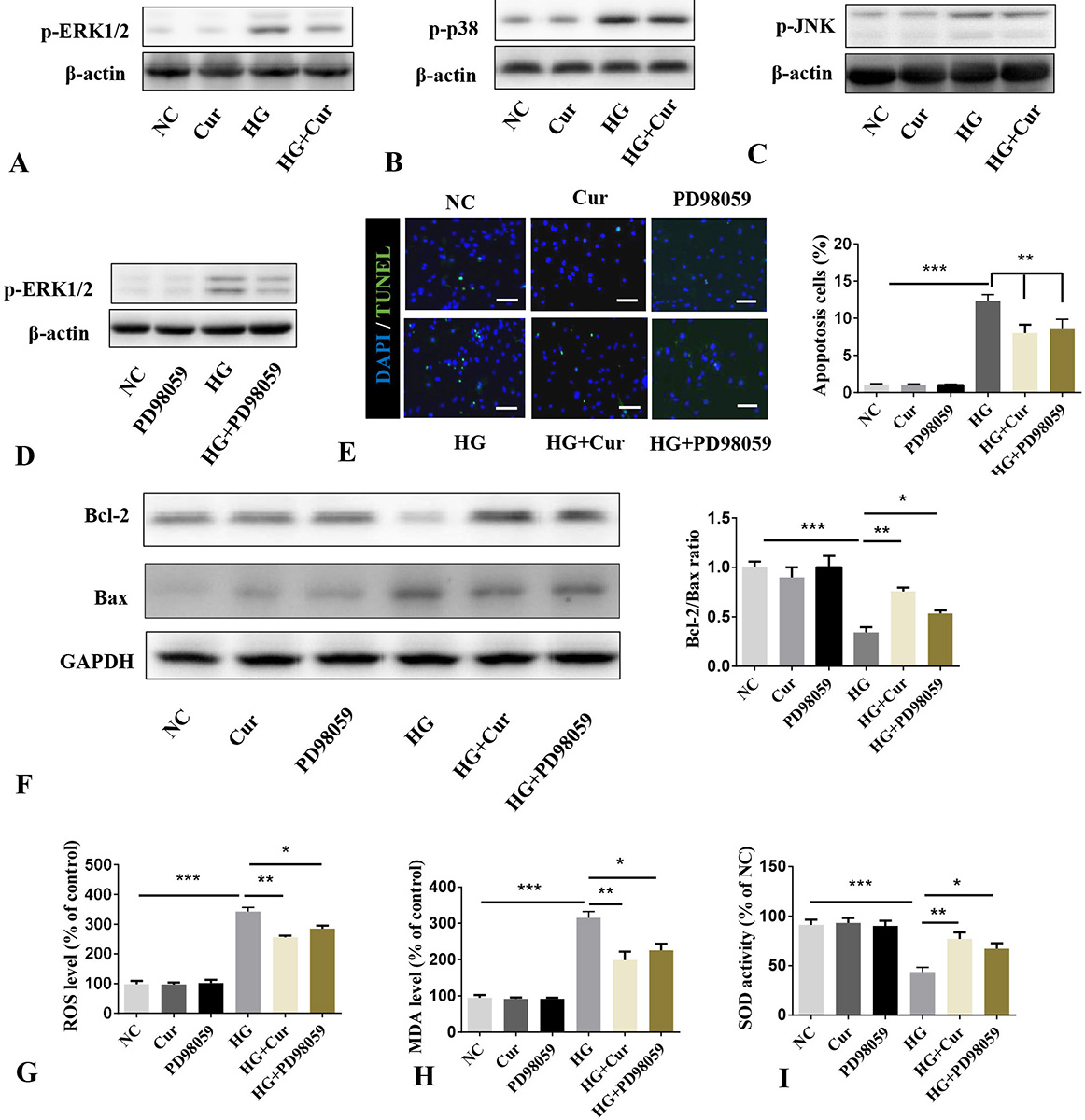 Figure 6
Figure 6Curcumin alleviates HG induced oxidative stress and apoptosis via ERK1/2 pathway. A-C. Cells induced by HG (25 mM) were treated with or without curcumin (10 μM) for 24 h. Western blotting of phosphorylation levels of ERK1/2, JNK, and p38. D. Cells induced by HG (25 mM) were treated with or without p-ERK1/2 inhibitor PD98059 (20 μM). Western blotting of phosphorylation levels of ERK1/2 was dtermined. E. TUNEL in cells treated with curcumin (10 μM) treatment prior 4 h to incubation with p-ERK1/2 inhibitor (20 μM) (total cells: 16434, positive cells: 1187). Scale bar = 25 μm. F. Western blotting was used to detect apoptotic protein. G. Level of ROS. H. Level of MDA level. I. Activity of SOD. *P< 0.05. **P< 0.01. ***P< 0.001. NG: normal glucose; HG: high glucose; Cur: curcumin; Ad-CHOP: Adenovirus-CHOP (50 MOI); Ad-con: Adenovirus-control (50 MOI), ROS: reactive oxygen species, MDA: malondialdehyde, SOD: superoxide dismutase.
In conclusion, consistent with other published reports, we show that HG treatment of beta islet beta cells induced pERK1/2 expression (19-21). Yet, in some studies, HG decreased the phosphorylation of ERK1/2 and also increased the phosphorylation of p38 and JNK (22-23). In our analysis, we failed to see a similar effect of HG on phosphorylation of p38 and JNK (21, 23). We also show here that curcumin relieves oxidative stress and opposes apoptosis induced by HG in pancreatic beta cells jointly by modulating the expression of CHOP and PGC-1αlpha as well as regulation of ERK 1/2 phosphorylation without any effect on the phosphorylation levels of p38 and JNK. Furthermore, overexpression of CHOP or siRNA knockdown of PCG-1alpha counteracted the effects of curcumin on HG induced oxidative stress and apoptosis. Finally, blockade of pERK1/2 reduced the HG induced apoptosis in pancreatic beta cells. Together, these data show that curcumin might have clinical application in reducing the negative impact of hyperglycemia in diabetic patients (Figure 7).
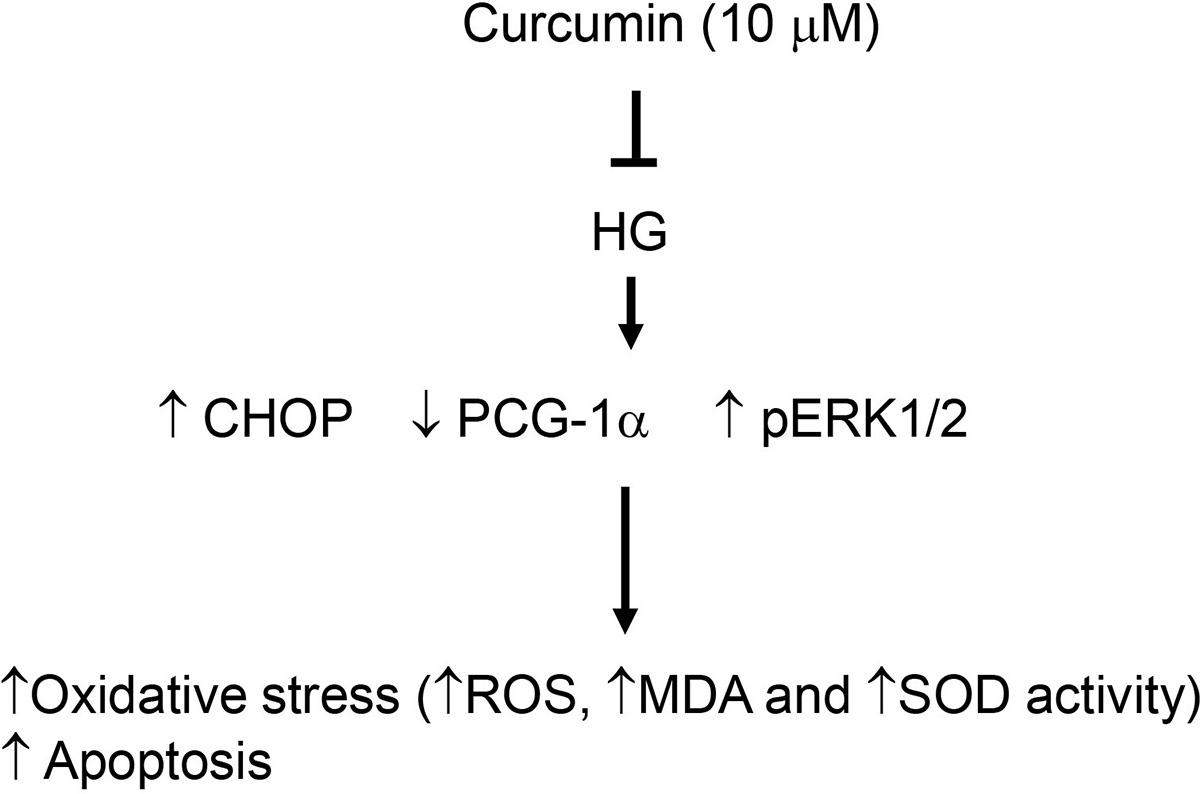 Figure 7
Figure 7Mode of actions of curcumin in prevention of oxidative stress and apoptosis . HG: high glucose; CHOP: C/EBP homologous protein; PCG-1α: peroxisome proliferator-activated receptor-γ coactivator-1α; ROS: reactive oxygen species, MDA: malondialdehyde, SOD: superoxide dismutase.
#Hou Kaijian, Yongru Chen contributed equally to this work. This study was supported by Shantou science and technology project (No:180828094013938).
Real-time quantitative polymerase chain reaction
RT-qPCR
ROS
MDA
SOD
DM
HG
T2DM
DMEM/F12
FBS
BSA
