Overactivation of renin-angiotensin system (RAS) is one of the main pathophysiological features in the evolution of chronic heart failure (CHF). The (pro)renin receptor ((P)RR) represents an important player in a tissue renin-angiotensin system (tissue RAS), which mediates tissue injury through fibrosis and hypertrophy of the affected organs in CHF patients. In our study we used plasma samples from 556 elderly subjects with CHF and 198 healthy participants in order to evaluate prognostic and diagnostic potential of s(P)RR in setting of CHF. The patients with CHF showed significantly higher plasma levels of s(P)RR than the healthy volunteers (p=0.0005). We observed association between higher s(P)RR plasma concentrations and lower left ventricular ejection fraction and higher degree of left ventricular dilatation on baseline echocardiography examination of the CHF patients. Elderly CHF patients with higher baseline s(P)RR plasma concentration were at same risk for death, stroke and hospitalization due to heart failure worsening at mean follow-up from forty-eight months in comparison to low s(P)RR counterparts.
Chronic heart failure (CHF) is a highly prevalent disease in general population, with a profound impact on quality of life and high mortality (1). Following findings from previous studies, throughout the past decades reduced left ventricular ejection fraction was recognized as a hallmark of chronic heart failure (HFrEF). However data on temporal trends suggest increased incidence of elderly patients with signs and symptoms of heart failure with the preserved left ventricular ejection fraction (HFpEF), underlining necessity for more comprehensive understanding of pathophysiological mechanisms involved in the evolution of CHF (1). Chronic activation of the renin-angiotensin system (RAS) has a major role in development and progression of CHF (2). In addition to its circulatory effects, recent discovery of multiple essential autocrine and paracrine functions of angiotensin II, also revealed the presence of the tissue renin-angiotensin system (tissue RAS), as an important contributing factor in the organ specific angiotensin II mediated injury in setting of the CHF (3).
The (pro)renin receptor ((P)RR) was first identified by Nguyen and his group in 2002. (P)RR is known to be an important player in the tissue RAS (4). Three variants of (P)RR are discovered: (1) a full-length integral transmembrane protein, (2) a soluble (P)RR fraction (s(P)RR), present in extracellular department and (3) a truncated form composed of the transmembrane and cytoplasmic domains (5, 6). The evidence from multiple studies performed on animal experimental model have showed that activation of the (P)RR by renin and prorenin results in increased catalytic activity of renin, induction of intracellular signaling pathways including MAP kinases, as well as release of s(P)RR in extracellular space (6-8). Conditional (P)RR knock-out mice developed lethal heart failure with numerous myocyte morphological changes. This finding specifically, lead to an assumption, that (P)RR also regulates the assembly and function of vacuolar H+-ATPase (V-ATPase), which has essential function in the intracellular pH homeostasis (9, 10). Although some clinical studies found up-regulation of (P)RR in setting of heart failure, definitive information on quantity and distribution patter of s(P)RR in elderly CHF population, are still insufficient and scarce (11). In addition to this, data concerning relevance of s(P)RR as prognostic marker, as well as it’s association with structural myocardial remodeling in CHF patients are almost nonexistent.
Thus, the aims of this study were to determine 1) whether the s(P)RR is dysregulated in elderly patients with chronic heart failure 2) to evaluate potential relationship between s(P)RR plasma concentration and degree of left ventricular remodeling and dysfunction 3) to explore, whether s(P)RR is suitable for diagnostic and prognostic purposes in CHF patients.
In this study we used plasma samples of elderly CHF patients, previously investigated in the Cardiac Insufficiency Bisoprolol Study in Elderly Study (CIBIS-ELD) Trial. The CIBIS-ELD represents an investigator-initiated, randomized, double-blind, parallel-group multicenter trial, which compared the tolerability and therapeutic effects of two proven beta-blockers in patients with clinically manifested heart failure (12). For the purposes of our study we used plasma sample from baseline examination of CHF patients, prior to any main-study intervention. To be enrolled, patients had to be clinically stable, without signs or symptoms of the heart failure worsening in previous two weeks. Only patients on regular, guideline’s compliant heart failure medical therapy, without dosage changes in past two weeks, were included in our study.
The patients were recruited between April 2005 and April 2008. Eligible participants for CIBIS-ELD trial were elderly patients (age equal or more than 65 years), with symptomatic chronic heart failure in previous three months, New York Heart Association (NYHA) functional class equal or more than class II at time of enrolment, or with an left ventricular ejection (LV-EF) fraction equal or less than 45% (12). At the baseline examination the patients underwent medical history taking, clinical examination, blood samples collection, electrocardiography and echocardiography. The office blood pressure was measured through the local study personal blinded to study end-points, by patients being comfortably seated in a quiet surrounding and relaxed for 5- 10 minutes, at least one hour after taking prescribed antihypertensive medication. The readout was repeated after one minute and an average of two readouts was used to represent the patient’s blood pressure.
All the patients provided written informed consent and the trial was in compliance with the principles outlined in the Declaration of Helsinki.
In order to provide control group, we examined healthy volunteers according their clinical history and examination, measurement of blood pressure and heart rate, anamnestic assessment of eventual symptoms and signs of heart disease, evaluation of concomitant medication, laboratory tests, electrocardiography and echocardiography. Upon an informed consent of 198 healthy subjects and an exclusion of any relevant heart diseases, we used these plasma samples as a control probes for our analysis.
Role of the funding sources and conflict of interest for the CIBIS-ELD study, inclusion and exclusion criteria, as well as study results have been previously published (12). We report that no commercial entity provided financial support for the purposes of our subanalysis.
N-terminal pro-brain natriuretic peptide (NT-pro-BNP) was determined using commercially available assay (Elecsys, Roche Diagnostics, Basel, Switzerland).
We measured s(P)RR using a sandwich ELISA (CellTrend GmbH Luckenwalde, Germany). All tests were done in duplicate. Briefly, the microtiter 96-well polystyrene plates were coated with an anti human s(P)RR capture-antibody (CellTrendGmbHLuckenwalde, Germany). Conformational epitopes of the antibody were maintained by addition of 1 mM calcium chloride to every buffer. Duplicate samples of a 1:25 plasma dilution were incubated at 4°C for 2 hours. After washing steps, plates were incubated for 60 minutes at room temperature with a 1:2.000 dilution of an anti human s(P)RR antibody, labeled with biotin (CellTrend GmbH Luckenwalde, Germany), used for detection. After further washing steps, plates were incubated for 60 minutes at room temperature with a 1:250 dilution of streptavidin, labeled with horseradish-peroxidase (streptavidin-HRP, R&D Systems, Minneapolis, USA). Following further washing steps, plates were incubated for 30 minutes at room temperature protected from light with 3,3`, 5,5` tetramethylbenzidine (TMB, SeramunDiagnostica GmbH, Wolzig, Germany). Color develops in proportion to the amount of streptavidin-HRP. The absorption at 450 nm is proportional to the s(P)RR concentration. In order to obtain a standard curve, plates were incubated with test serum from s(P)RR positive index donor. The inter-assay variability was 11,7%; the intra-assay variability was 9,4%. All samples were blinded for diagnosis during analyses by the laboratory personnel. The values of s(P)RR are presented as Units/ml ± mean standard error. The manufacturer of protein assay, CellTrend GmbH, had no influence on the study design, statistical analysis, or manuscript preparation.
After completion of CIBIS-ELD study and measurements of s(P)RR plasma concentration secondary statistical analysis was conducted. Due to lack of normal distribution, for the purposes of our statistical analysis log transformd plasma concentrations of s(P)RR were used. The baseline variables were presented as frequencies and percentages for binary variables, or as the means and standard errors (SE), i.e. in combination with log-transformed s(P)RR. Data were presented overall and separately in the respective groups. Comparisons of all groups were performed by Fisher’s exact test or Wilcoxon test, respectively. Quantitative measures were analyzed by Kandall’s rank correlation and adjusted for multiple comparisons. For these purposes statistical software R (R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.) was used.
Baseline characteristics of study participants are presented in Table 1. We evaluated 556 CHF patients from CIBIS-ELD study, as well as 198 healthy volunteers. Mean age of CHF patients and healthy controls was 73±5 and 58±7 years respectively. Out of total number of 556 patients, 34% of patients with chronic heart failure and 67 % in a group of healthy controls were females.
| Clinical variables | CHF- cohort | Controls | p |
|---|---|---|---|
| Number | 556 | 198 | - |
| Age (mean, years ± SD1) | 73±5 | 58±7 | p<0.05 |
| Female ( %) | 34 | 67 | p<0.05 |
| Height ( mean, cm ± SD) | 168±9 | 168±9 | NS |
| Weight (mean, kg ± SD) | 78±14 | 73±14 | NS |
| Blood pressure (mean diastolic, mmHg ± SD) | 81±11 | 78±10 | NS |
| Blood pressure (mean systolic, mmHg ± SD) | 138±21 | 129±15 | NS |
| Hypertension (%) | 83 | - | - |
| Diabetes Mellitus ( Typ I and II, %) | 26 | - | - |
| Hyperlipidemia (%) | 64 | - | - |
| Smoking ( %) | 8,3 | 3.6 | p<0.05 |
| Primary etiology of heart failure | |||
| Coronary artery disease (%) | 46 | - | - |
| Hypertension (%) | 35 | - | - |
| Cardiomyopathy (%) | 8 | - | - |
| Valvular disease (%) | 7 | - | - |
| Uncertain etiology (%) | 4 | - | - |
| Heart rate (heartbeats per minute, mean ± SD ) | 73±13 | 72±10 | NS |
| 6-Minute-Walktest (meters, mean ± SD) | 321±117 | 583±80 | p<0.05 |
| HbA1C ( %)2 | 6.4 | - | - |
| Plasma creatinine (mean, μmol/l ± SD) | 101±33 | 78±14 | p<0.05 |
| NT-pro-BNP (mean in pg/ml) | 1385 | - | - |
| Echo-parameters | |||
| LV-EF3 (mean, % ± SD) | 42±13 | 61±7 | p<0.05 |
| LVEDD4 (mean, mm ± SD) | 56±10 | 47±5 | p<0.05 |
| LVESD5 (mean, mm ± SD) | 42±11 | 29±5 | p<0.05 |
| IVSED6 (mean, mm ± SD) | 11±2 | 10±1 | NS |
| PWDED7 (mean, mm ± SD) | 10±2 | 10±1 | NS |
There were no significant differences in mean systolic and diastolic blood pressure between both groups of study participants (138±21 mmHg vs. 129±15 mmHg for systolic, and 81±11 mmHg vs. 78±10 mmHg for diastolic blood pressure respectively). The leading cause of heart failure in CHF group were coronary artery diseases (46%), followed by hypertension (35%) and cardiomyopathy (8%).
In line with the current ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure, the patients with heart failure with reduced ejection fraction (HFrEF) were defined as patients with the ejection fraction lower than 50%. The patients with the heart failure with preserved ejection fraction (HFpEF) were required to have ejection fraction equal or more than 50% and NT-pro-BNP more than 125 pg/ml (1). According to these criteria, 412 individuals were ranked into the HFrEF and 120 patients into the HFpEF group. In 24 patients with LV ejection fraction more than 50% we did not conduct NT-pro-BNP plasma quantification and these patients were excluded from comparison analysis between HFrEF and HFpEF group.
Table 2 features the clinical parameters for patients with HFrEF and HFpEF. The patients in the HFpEF group had numerically higher blood pressure compared to the HFrEF (133±20 mmHg vs. 127±18 mmHg). At study enrollment, the HFrEF patients were more frequently treated with mineralocorticoid-receptor antagonists than HFpEF patients (40% vs. 24% respectively).
| Clinical variables | HFrEF | HFpEF | p |
|---|---|---|---|
| Number (n) | 412 | 120 | |
| Age (mean, years ± SD1) | 72±5 | 72±5 | NS |
| Female gender (%) | 22 | 46 | p<0.05 |
| NYHA class | II-66% | II-65% | NS |
| III-30% | III-35% | ||
| Height (mean, cm ± SD) | 169±9 | 166±9 | NS |
| Weight (mean, kg ± SD) | 78±13 | 79±17 | NS |
| Blood pressure (mean diastolic, mmHg ± SD) | 77±10 | 80±10 | NS |
| Blood pressure (mean systolic, mmHg ± SD) | 127±18 | 133±20 | NS |
| Hypertension (%) | 80 | 83 | NS |
| Diabetes Mellitus (Typ I and II, %) | 28 | 27 | NS |
| Hyperlipidemia (%) | 62 | 66 | NS |
| Smoking (%) | 9 | 11 | NS |
| Coronary artery disease (%) | 65 | 62 | NS |
| Heart rate (mean, heartbeats per minute) | 75±10 | 70±11 | NS |
| 6-Minute-Walktest (mean, meters ± SD) | 318±120 | 327±115 | NS |
| Echo-parameters | |||
| LV-EF2 (mean, % ± SD) | 37±8 | 60±6 | p<0.05 |
| LVEDD3 (mean, mm ± SD) | 59±10 | 54±7 | NS |
| LVESD4 (mean, mm ± SD) | 49±10 | 36±6 | NS |
| IVSED5 (mean, mm ± SD) | 10±2 | 12±2 | NS |
| PWDED6 (mean, mm ± SD) | 10±2 | 11±2 | NS |
| LV-mass by Devereux (mean, g ± SD) | 240±70 | 271±86 | NS |
| E/E`7 | 10±1 | 13±1 | p<0.05 |
| Medical therapy | |||
| ACE inhibitor (%) | 81 | 80 | NS |
| ARBs (%) | 5 | 6 | NS |
| MRAs (%) | 40 | 24 | p<0.05 |
| Diuretics (thiazide and/or loop diuretics, %) | 64 | 65 | NS |
| Beta-Blockers (%) | 63 | 68 | NS |
The plasma s(P)RR concentrations showed an asymmetric distribution pattern (for CHF group - mean s(PRR) plasma concentration of 1.52 Units/ml, with standard error 1.01 Units/ml; for healthy volunteers - mean s(P)RR plasma concentration 1.37 Units/ml, standard error 1.02 Units/ml (Figure 1).
 Figure 1
Figure 1Distribution pattern of plasma s(P)RR concentrations in healthy individuals A) concentration of s(P)RR are presented as units per milliliter (mean s(P)RR concentration 1.33 Units/ml, standard error 1,03 Units/ml) B) s(P)RR distribution profile after logarithmic transformation (log s(P)RR plasma concentration 0,32 Units/ml, standard error 0,02 Units/ml).
Initially we compared s(P)RR plasma concentrations between CHF patients (mean log s(P)RR=0.42±0.01 Units/ml, n=556) and healthy controls (mean log s(P)RR=0.32±0.02 Units/ml, n=198, Figure 2). In the chronic heart failure group significantly higher s(P)RR plasma levels were observed (p=0.0005). Statistical analysis have showed that the s(P)RR distribution in our CHF cohort was not affected by age (age ≥ 73 years vs. age < 73 years, p=0.12), sex (male vs. female, p=0.11), or NYHA class (NYHA class > II vs. NYHA class ≤ II, p=0.85).
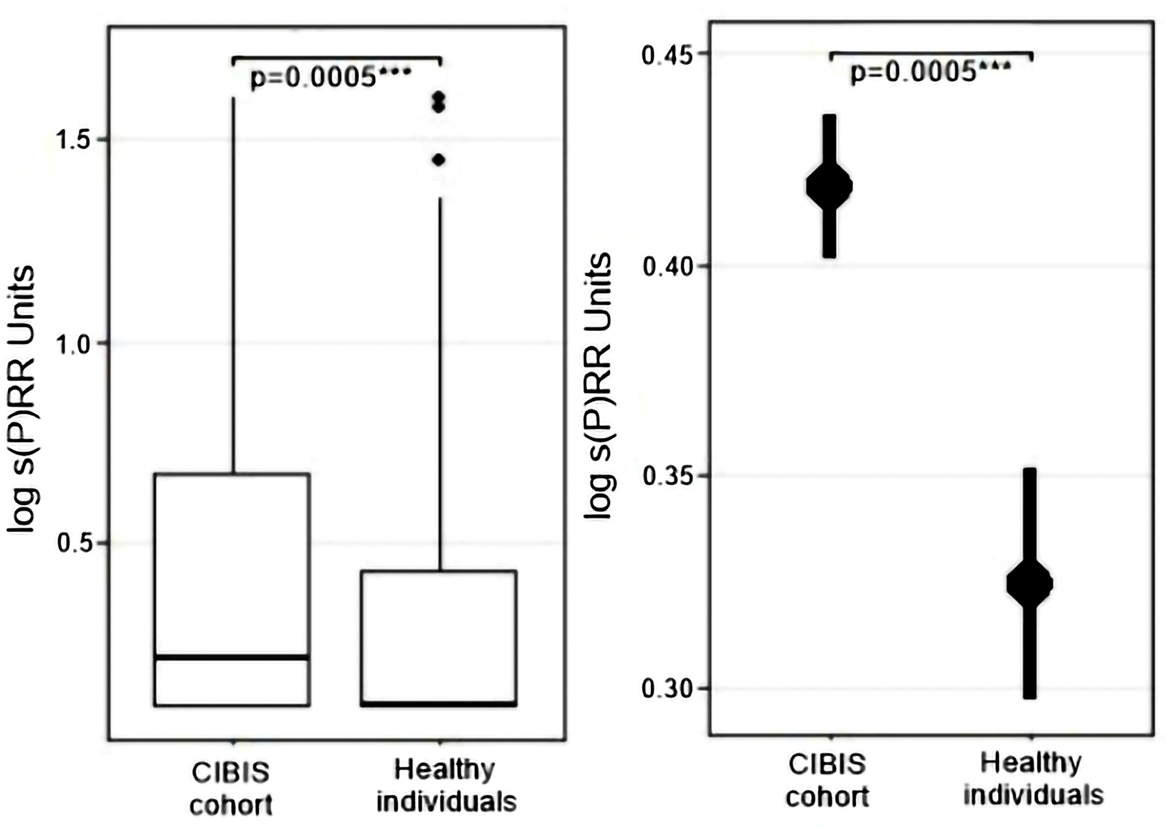 Figure 2
Figure 2Differences in plasma s(P)RR concentrations between patients with chronic heart failure (n=556) and control group of healthy individuals (n=198).
Comparing plasma levels of s(P)RR between HFrEF group (mean log s(P)RR=0.42±0.02 Units/ml, n=412) and HFpEF group (mean log s(P)RR=0.41±0.03 Units/ml, n=120, Figure 3), non-significantly elevated levels of s(P)RR in HFrEF patients were found (p=0.91). Similar results were observed, when s(P)RR plasma concentration was analyzed in a setting of advanced left ventricular dilatation, where patients with left ventricular end diastolic diameter (LVEDD) of more than 55 mm (mean log s(P)RR=0.45±0.02 Units/ml, n=268) presented higher s(P)RR than patients without left ventricular dilatation (LVEDD≤ 55mm, mean log s(P)RR=0.39±0.01 Units/ml, n=280, p=0.08, Figure 4).
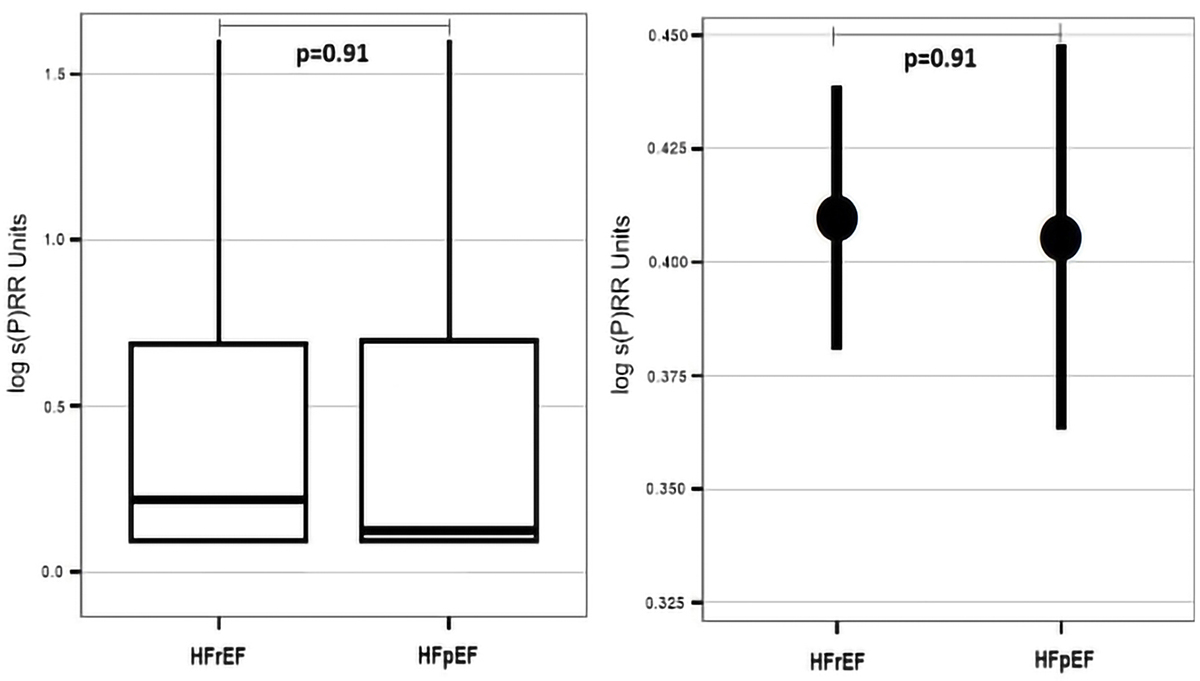 Figure 3
Figure 3Differences in plasma s(P)RR concentrations between chronic heart failure patients with reduced ejection fraction ( HFrEF, n=412) and chronic heart failure patients with preserved ejection fraction (HFpEF, n=120).
 Figure 4
Figure 4Differences in plasma s(P)RR concentrations between HFrEF patients with (LVEDD>55mm, n=268) and without presence of left ventricular dilatation (LVEDD<55mm, n=280).
Next, we investigated correlation between echocardiographic parameters of left ventricular remodeling in CHF patients and plasma s(P)RR concentration (Table 3). We observed a negative correlation between s(P)RR concentrations and left ventricular ejection fraction, and positive correlation of plasma s(P)RR concentration and left ventricular end diastolic diameter, end systolic diameter, as well as left atrial diameter. At the same time circulatory s(P)RR concentration did not show clinically relevant correlation with left ventricular shortening fraction and walking distance in six minute walk test at baseline.
| Clinical variables | r1 | p2 |
|---|---|---|
| Left ventricular ejection fraction (%) | -0.1 | 0.003 |
| Left ventricular shortening fraction (%) | -0.02 | 0.09 |
| Six-minute walk test (meters) | -0.2 | 0.77 |
| Left ventricular end diastolic diameter (mm) | 0.2 | 0.001 |
| Left ventricular end systolic diameter (mm) | 0.2 | 0.004 |
| Left atrial diameter (mm) | 0.1 | 0.05 |
The influence of concomitant medical treatment of CHF patients on plasma sPRR levels is presented on Table 4.
| Concomitant medication | log sPRR plasma concentration (mean Units/ml±SE) | ||
|---|---|---|---|
| Drug classes | Yes | No | p1 |
| ACE inhibitor | 0.41±0.01 | 0.37±0.02 | 0.10 |
| ARBs | 0.42±0.05 | 0.39±0.01 | 0.43 |
| Beta-Blockers | 0.40±0.01 | 0.42±0.02 | 0.45 |
| MRAs | 0.44±0.03 | 0.38±0.01 | 0.13 |
| Diuretics (Hydrochlorothiazide and/or loop diuretics) | 0.43±0.02 | 0.37±0.01 | 0.02 |
CHF patients under treatment with angiotensin-converting enzyme (ACE) inhibitor (n=429), angiotensin II receptor (ARBs) blocker (n=28), and mineralocorticoid-receptor (MRAs) antagonists (n=194) showed a tendency towards higher s(P)RR plasma concentrations compared to the patients naïve to these medication. Patients on diuretic-therapy (loop and/or thiazide; n=341) exhibited significantly increased s(P)RR level (p=0.02).
We used receiver operating characteristics curve analysis (ROC) to examine whether s(P)RR in addition to NT-pro-BNP, known as gold standard biomarker of heart failure, can facilitate identification of the CHF patients with reduced left ventricular ejection fraction or left ventricular dilatation. In these terms plasma concentration of s(P)RR provided no additional diagnostic benefit adding to NT-pro-BNP (Figure 5).
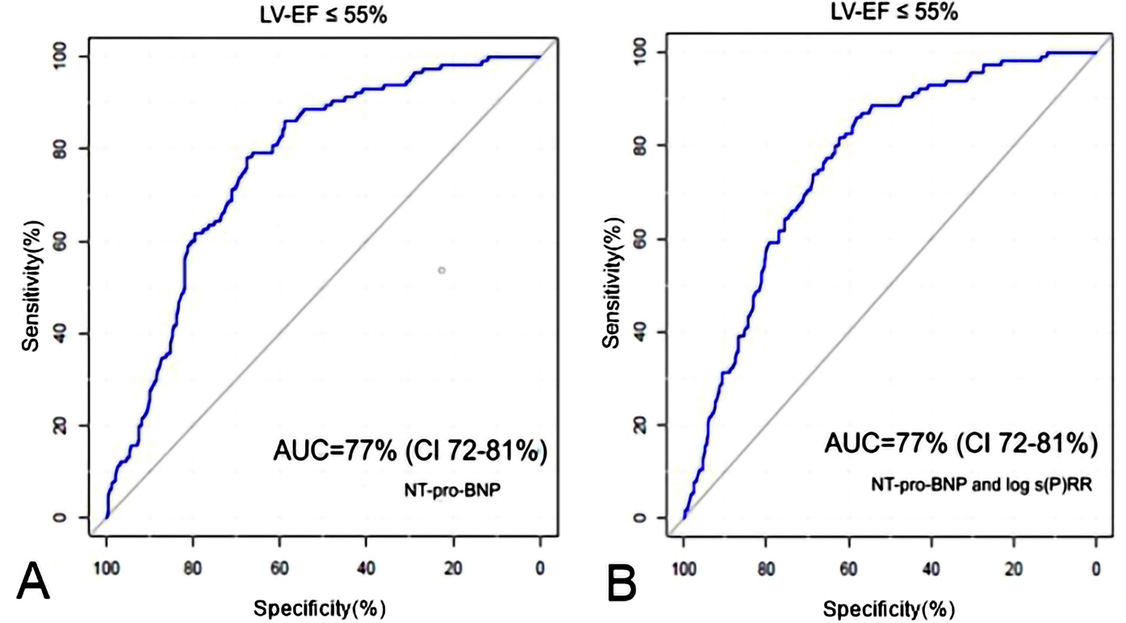 Figure 5
Figure 5Receiver operating characteristics curve analysis (ROC) for identifying chronic heart failure patients with reduced ejection fraction (HFrEF) A) for NT-pro-BNP B) binary logistic regression prognostic model composed from NT-pro-BNP and log s(P)RR plasma concentration. AUC- area under the curve, CI-confidential index.
Clinical characteristics of study collective involved in a follow-up are presented in Table 5.
| Clinical variables | Frequency |
|---|---|
| Number (n) | 430 |
| Alive, clinic visit (n) | 189 |
| Alive, follow-up by telephone (n) | 148 |
| Alive, doesn’t want follow-up (n) | 93 |
| Weight (mean, kg ± SD1) | 79±15 |
| Diastolic blood pressure (mean, mmHg ± SD) | 77±12 |
| Systolic blood pressure (mean, mmHg ± SD) | 137±22 |
| Heart rate (heartbeats per minute, mean ± SD) | 68±13 |
| 6-Minute-Walktest (mean, meters ± SD) | 337±125 |
| HbA1C2 ( %) | 8 |
| Plasma creatinine (mean, μmol/l ± SD) | 110±30 |
| NT-pro-BNP (mean in pg/ml) | 1485 |
| Echo-parameters | |
| LV-EF3 (mean, % ± SD) | 50±10 |
| LVEDD4 (mean, mm ± SD) | 51±10 |
| LVESD5 (mean , mm ± SD) | 38±10 |
In order to evaluate prognostic performance of baseline plasma level of s(P)RR on clinical course of chronic heart failure we divided all patients into a group with high s(P)RR concentration and group with low s(P)RR baseline concentration, irrespective of etiology of heart failure (cut off for geometric mean s(P)RR plasma concentration of 1.52 Units/ml, log s(P)RR=0.42 Units/ml). No statistically significant differences were observed between patients with higher and lower baseline plasma s(P)RR concentration concerning left ventricular ejection fraction, left ventricular end systolic and end diastolic diameter by echocardiographic assessment in the course of a 48 month follow-up examination (Figure 6).
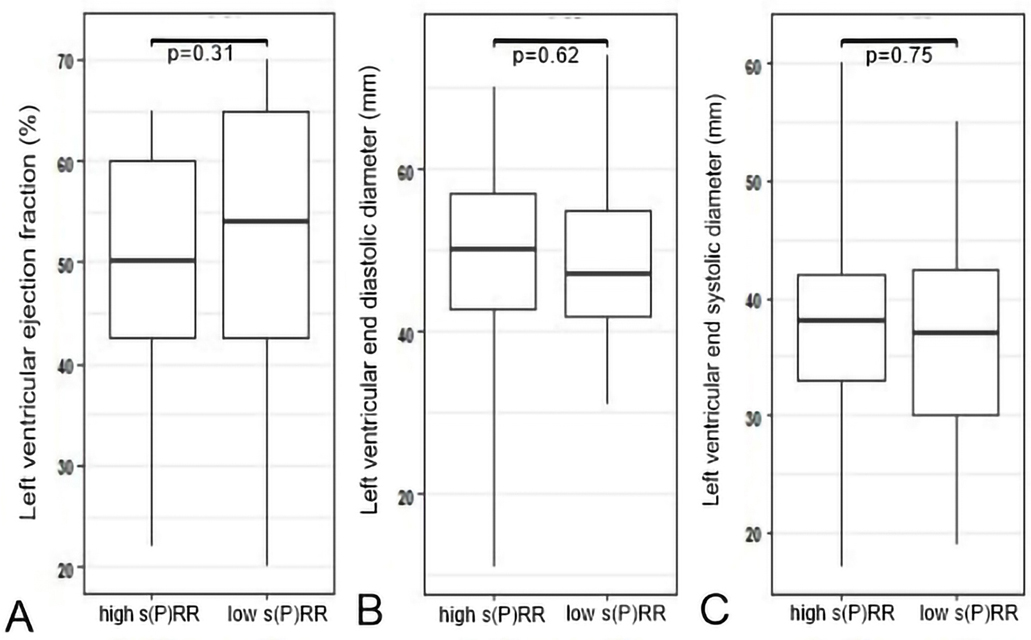 Figure 6
Figure 6Differences in echocardiography findings of patients with chronic heart failure at mean follow up of 48 months in respect of baseline plasma s(P)RR concentration A) left ventricular ejection fraction in percents at follow-up between patients with high and low plasma s(P)RR baseline concentration, B) left ventricular end diastolic diameter in millimeter at follow up between patients with high and low plasma s(P)RR baseline concentration C) left ventricular end systolic diameter in millimeter at follow up between patients with high and low plasma s(P)RR baseline concentration .
Further investigating prognostic capabilities of plasma s(P)RR for prediction of long-term mortality, rehospitalization rate due to heart failure worsening and stroke in CHF patients, we conducted Kaplan-Meier analysis. Within different tertiles of baseline s(P)RR plasma concentration in CHF cohort (inter-tertile range=0.92 Units/ml) we found no significant differences in respect to mortality, rehospitalization due to heart failure worsening and stroke rate at mean follow up of forty-eight months (Figures 7-9).
 Figure 7
Figure 7Kaplan-Meier curves showing differences in mortality rate at mean follow-up of five years between chronic heart failure patients with low, medium and high baseline s(P)RR plasma concentration.
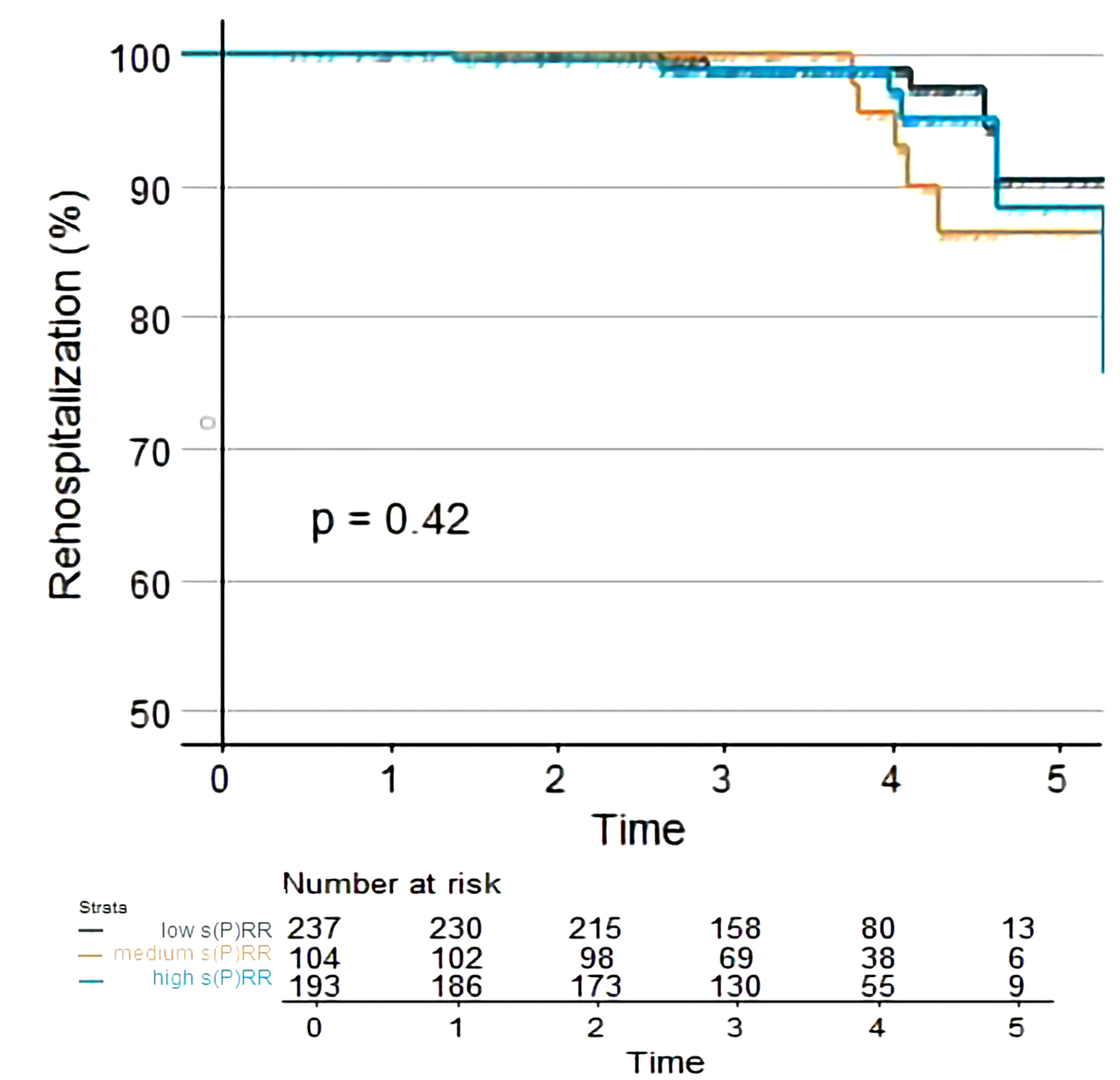 Figure 8
Figure 8Kaplan-Meier showing differences in rehospitalization rate due to worsening of heart failure at mean follow-up of five years between chronic heart failure patients with low, medium and high baseline s(P)RR plasma concentration.
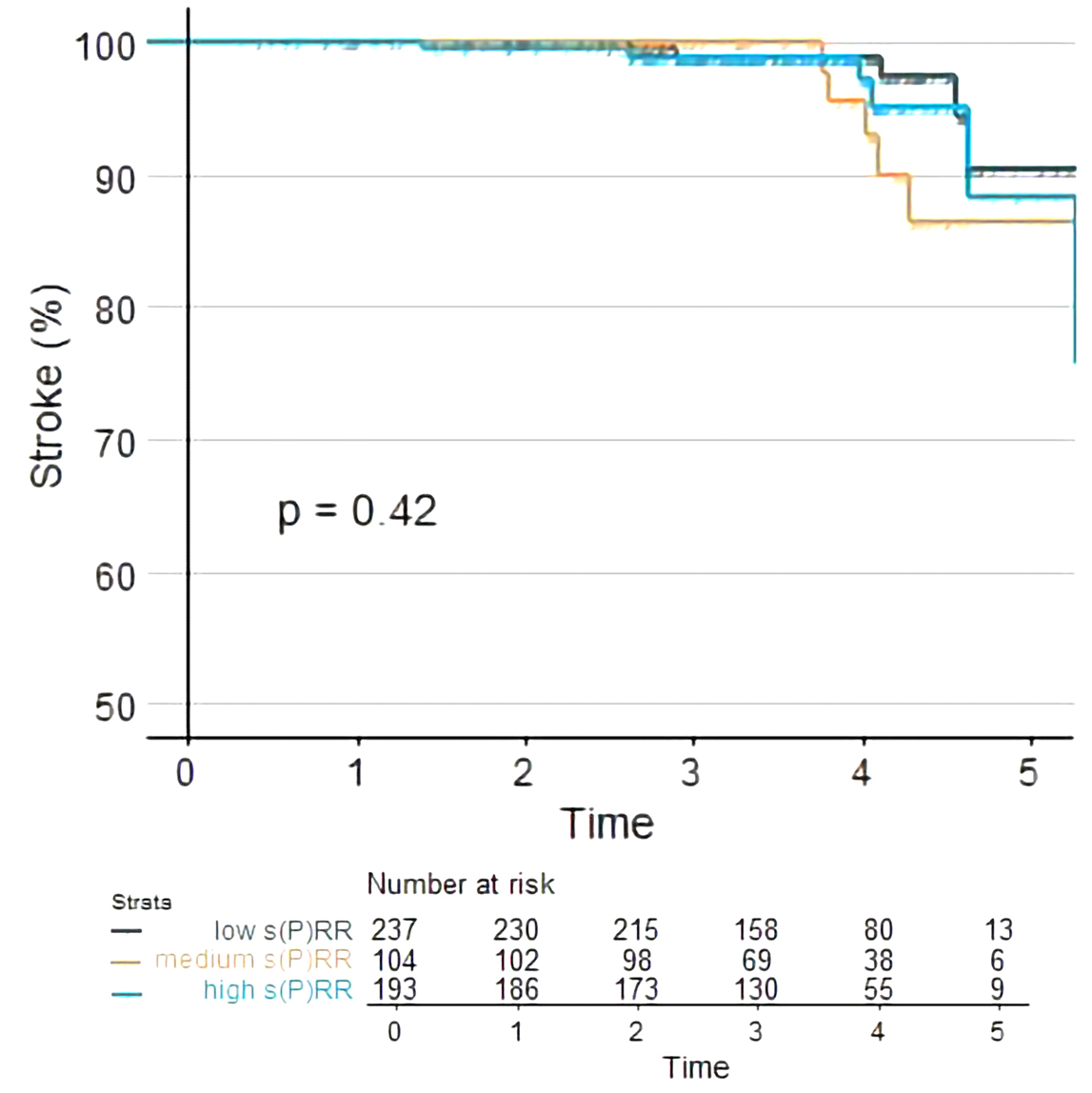 Figure 9
Figure 9Kaplan-Meier curves showing differences in stroke rate at mean follow-up of five years between chronic heart failure patients with low, medium and high baseline s(P)RR plasma concentration.
Our results showed that soluble (pro)renin receptor is elevated in plasma of elderly CHF patients. Furthermore, s(P)RR levels exhibited negative correlation with the left ventricular ejection fraction and positive correlation with the left ventricular end-diastolic and end-systolic diameter, showing its association with degree of functional and structural left ventricular remodeling in CHF patients. Interestingly, evaluating influence of guideline-conform medical therapy of chronic heart failure on s(P)RR plasma distribution pattern, we established that only treatment with diuretics led to a significantly higher s(P)RR blood levels in CHF patients.
The clinical relevance of the human (P)RR became in the past years a topic of intensive research, since numerous clinical studies suggested involvement of the (P)RR in development of plethora of cardiovascular diseases (hypertension, diabetes mellitus, preeclampsia etc.)(13, 14). Considering that some of these clinical entities play a crucial role in the development of heart failure, several research groups found important to investigate whether (P)RR and its plasma product s(P)RR are involved in cardiac damage in course of chronic heart failure.
Gong et al. were first to report elevated s(P)RR plasma levels in the heart failure patients with reduced left ventricular ejection fraction (HFrEF) compared to healthy controls, where the left ventricular mass index and glomerular filtration rate were independent predictors of plasma s(P)RR level in HFrEF patients (15). Extrapolating these findings to the elderly chronic heart failure patients, population know to be more frequently encountered in daily clinical setting, our results underline involvement of s(P)RR in evolution of chronic heart failure.
Additionally, evaluating s(P)RR plasma distribution pattern in a large heart failure patient cohort, we observed no differences in plasma s(P)RR concentration between patients with HFrEF and HFpEF. Knowing that previous studies found no linear relationship between the degree of activation of tissue RAS and renin/prorenin plasma activity level (16), it is reasonable to assume, that the tissue renin-angiotensin system and (P)RR as its most important effector, plays equally important pathophysiological role in both major clinical subentities of chronic heart failure.
The finding that prorenin can induce cardiac remodeling with loss of cardiac function through angiotensin-independent pathways, raised the question, whether these effects are strictly (P)RR mediated (16, 17). The (P)RR gene transcription is up-regulated in hearts of rats with congestive heart failure (18, 19). In animal model of ischemic cardiomyopathy with left ventricular remodeling increased myocardial (P)RR expression was reported. In line with these findings, in human patient population with dilated cardiomyopathy the cardiac mRNA for (P)RR and (P)RR protein were up-regulated compared to healthy controls (5.3 and 8.8 fold respectively)(20).
An important observation of our study is that in clinical setting of the chronic heart failure, irrespective to its etiology, progressive deterioration of left ventricular structural and functional performance (left ventricular ejection fraction reduction, progressive left ventricular and left atrial dilatation) significantly correlates with higher levels of s(P)RR in plasma. It is worth mentioning that previous studies also found link between (P)RR over-expression and atrial remodeling leading to higher incidence of atrial fibrillation (21) Acknowledging strong association of left ventricular remodeling with ventricular rhythm disturbances, we believe that our results should foster further clinical studies, aiming more in depth evaluation of s(P)RR as prognosticator of malignant ventricular arrhythmias in CHF patients.
RAS is a well-established therapeutic target in the medical treatment of heart failure (22). Previous studies about the effects of RAS inhibitors (ACE-inhibitors, ARBs and MRAs) on tissue concentration and activation level of renin, prorenin and (P)RR implied complexity of these relationships. Research evidence concerning effect of RAS inhibitors on (P)RR metabolism is limited. One of few studies found that aliskiren therapy leads to higher renin levels in cortical renal tissue and at the same time induces lower expression level of (P)RR (23).
Our results offer further insights in the effects of inhibitors of the RAS on s(P)RR metabolism. In both study subgroups, HFrEF and HFpEF, up to 86% of patients were treated with ACE inhibitor or ARBs at study enrollment and we did not find differences in s(P)RR concentration between treated and not treated patients. Interestingly, only treatment with diuretics in our cohort induced statistically significant higher plasma amount of s(P)RR in comparison to diuretic naive CHF counterparts. It is known that diuretic therapy leads to higher plasma renin activity (24). The diuretic induced higher plasma rennin activity with consecutive (P)RR activation and s(P)RR plasma release, is one of possible explanations of our findings.
The B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (Nt-pro-BNP) are crucial for diagnosis of heart failure (25). In spite of extensive research on biomarkers in heart failure (e.g. BNP, Nt-pro-BNP, ST2, galectin, copeptin, adrenomedulin), there is no definitive circulatory biomarker, with accurate predefined concentration cut-off range, recommended for prognostication of CHF in clinical practice (1). In our study baseline plasma s(P)RR concentration in elderly CHF group was insufficient to identify those patients at risk for death, stroke and rehospitalization due to heart failure at forty-eight months follow-up. To the best of our knowledge, this is the first study to examine prognostic potential of s(P)RR in large cohort of CHF patients. Considering our findings and appreciating complexity of pathophysiological cascades involved in heart failure development, serial s(P)RR sampling and evaluation of temporal plasma distribution patterns of s(P)RR in course of heart failure evolution, in combinations with other biomarkers, clinical features of CHF and non-invasive diagnostic tools, could reveal real potential of s(P)RR in diagnosis and treatment of heart failure patients.
Certain limitations of our study need to be addressed. First, we used cross-sectional, retrospective approach to evaluate distribution pattern of s(P)RR in the collective of a chronic heart failure patients. Secondly, although CHF and the control group have been clinically well characterized and matched concerning majority of evaluated parameters, there are significant age differences between CHF patients from CIBIS cohort and control group. Thirdly, the plasma concentration of the s(P)RR used for measurements remained at a very low range, which makes an identification of deviations in the s(P)RR concentration challenging. Lastly, we did not measure plasma levels of RAS components in addition to s(P)RR, which deprives us from valuable insights in cross-talk between circulatory and tissue renin-angiotensin system, question we plan to further delineate in following studies.
Plasma s(P)RR is elevated in elderly patients with heart failure, showing no differences in plasma distribution pattern between patients with HFrEF and HFpEF. Although we observed significant correlation of plasma s(P)RR with degree of left ventricular remodeling in heart failure patients, s(P)RR was not able to identify elderly CHF patients at risk for mortality, stroke and rehospitalization at follow up from forty-eight months.
Danilo Obradovic and Goran Loncar share first authorship. Ralf Dechend and Hans-Dirk Duengen equally contributed as last authors of paper.
CHF
chronic heart failure
soluble (pro)rennin receptor
renin-angiotensin system, tissue
renin-angiotensin system
left ventricular ejection
heart failure with reduced ejection fraction
heart failure with preserved ejection fraction
angiotensin-converting enzyme inhibitor
angiotensin II receptor blocker
mineralocorticoid-receptor antagonists
receiver operating characteristics curve analysis.
