miRNA-21 (miR-21) is overexpressed in various human cancers. Here, we show that miR-21 is overexpressed in human Non-Small Cell Lung Cancer (NSCLC) and that its up or down-regulation, respectively, increases or decreases cyclin D1 and cyclin E1 expression and coordinately promotes or inhibits proliferation of cancer cells. The perturbations of miR-21 also dramatically reduces or increases epithelial to mesenschymal transition (EMT). We show that regulation of proliferation and EMT are directed by PTEN/Akt/GSK3 beta signaling axis by regulating the expression of invasion markers including E-cadherin, vimentin, snail, slug and beta-catenin. Together, these findings show that miR-21 is a potential target for the development of treatment for NSCLC forms of human lung cancer.
Lung cancer quickly progresses, and has a low five year survival, causing death of 2 millions humans each year world-wide (1). Lung cancer is comprised of Small Cell Lung Cancer (SCLC) and Non-Small Cell Lung Cancer (NSCLC) with the vast majority of this class of tumors being adenocarcinomas (2). The current treatment options for lung cancer include surgery (for stage I and stage II tumors), chemotherapy, and radiotherapy. A new targeted treatment, which is based on the identification of mutations in the Epidermal Growth Factor Receptor (EGF) gene, can be used only in a small subset of patients. This necessitates identification of additional new targets for devising treatment for the remaining patients.
microRNAs (miRNA) are a class of small non-coding 18- to 25-nucleotide RNAs that regulate gene expression by binding to the 3'-untranslated region (3'-UTR) of target messenger RNAs (3). By suppressing a diverse set of genes, miRNAs play crucial roles in many cellular biological processes, including development, metabolism, survival, differentiation, proliferation, apoptosis and immune response (3-4). Oncogenic miRNAs (oncomir) are commonly expressed in many human cancers including carcinomas of the lung, breast, ovaries, colon, rectum, prostate, pancreas and thyroid (5). Many cancers specifically human lung cancer overexpress the miR-21, and such an overexpression is associated with a poor response to treatment and a poor survival rate (6, 7). In U87 MG malignant glioma cells, tamoxifen-induced cell death, is significantly amplified when combined with inhibition of miR-21 (8). Therefore, miR-21 is a promising target for the treatment of cancer (9).
In the present study, we investigated and show that miR-21 promotes human lung cancer cell proliferation, cell cycle progression, and EMT, through direct regulation of PTEN/Akt/GSK3β signaling cascades and its key downstream targets, including E-cadherin, vimentin, snail, slug and β-catenin. These findings show that miR-21 might be used as a target for the development of treatment for human NSCLC.
Human lung cell lines that were used included A549, ASTC-a-1, H1299, H322, and H441. A549 cells were cultured in F12K medium/Dulbecco’s Modified Eagle’s Medium (1:1) (Thermo Fisher Scientific, cat#11320033) containing 10% (v/v) fetal bovine serum (FBS, Sigma, cat# F2442) and 50 units/ml penicillin and 50 μg/ml streptomycin. Several primary human fetal lung fibroblast cells were used and included (IMR90). These cells and H1299, H322, and H441 cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific, cat#11875119) containing 10% FBS (v/v). ASTC-a-1 cells were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% FBS (v/v). All cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 and 95% air.
Tumors and adjacent control non-tumor tissues from 38 patients with the diagnosis of NSCLC (age range 36-68 years) included 20 cases of adenocarcinomas, 15 cases of squamous cell carcinoma, and 3 cases of adenosquamous carcinomas. Patients (n=18) without lymph node metastasis were included in N0 and those (n=20) with lymph node metastasis were included in N1 group. In addition, 12 patients were at stage I, 10 patients were at stage II, 8 patients were at stage III, and 8 patients were at stage IV. None of the patients received radiochemotherapy or other antineoplastic treatment. None of the patients had history of other types of tumors.
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. MiR-21 mRNA expression was analyzed by real time qPCR using primers shown in Table 1. qRT-PCR was carried out using SYBR-Premix Ex Taq™ (Takara) according to the manufacturer’s protocol using the ABI 7500 RT-PCR machine (Applied Biosystems, New York, USA). Relative mRNA expression was determined by the ΔΔ-Ct method.
| Gene | Forward 5′- 3′ | Reverse 5′- 3′ |
|---|---|---|
| MiR-21 | ACGTTGTGTAGCTTATCAGACTG | AATGGTTGTTCTCCACACTCTC |
| PTEN | ACCATAACCCACCACAGC | CAGTTCGTCCCTTTCCAG |
| GAPGH | GAAGGTGAAGGTCGGAGTCAACG | TGCCATGGGTGGAATCATATTGG |
Cells were transfected with miR-21 negative control (NC), miR-21 inhibitor or miR-21 mimics Invitrogen (Carlsbad, CA, USA). with Lipofectamine RNAiMAX (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instruction. After transfection, cells were cultured for indicated time points and the cell proliferation and protein expression were assessed.
A549 and ASTC-a-1 cells were seeded in the 96-well plate at the density of 1×103 or 5×103 cells per well, the day before transfection. Cell Counting Kit 8 (CCK8, Dojindo, Tokyo, Japan) was used for assessment of the cell viability at 0, 24, 48 and 72 h after transfection. Cell proliferation was assessed by absorbance at OD450 nm using an Infinite 200 plate reader (TECAN, Mönnedorf, Switzerland).
Western blot analysis was performed according to the standard procedures as described previously (10). Total protein was extracted from A549 and ASTC-a-1cells using lysis buffer (20 mM Tris, PH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 1% Triton X-100, 0.1% SDS and 100 mM phenylmethylsulfonyl fluoride). Extracted proteins were separated in SDS-polyacrylamide gels and transferred to PVDF membranes. Membranes were incubated with antibody against Cyclin E1 (Cell Signaling Technology, cat#20808), Cyclin D1 (Cell Signaling Technology, cat#2978), Akt (Cell Signaling Technology, cat#4691), p-Thr308-Akt (Cell Signaling Technology, cat#13038), p-Ser473-Akt (Cell Signaling Technology, cat#4060), total-GSK3β (Cell Signaling Technology, cat#12456), p-Ser9-GSK3β (Cell Signaling Technology, cat#5558), PTEN (Cell Signaling Technology, cat#9559), E-cadherin (Cell Signaling Technology, cat#3195), Vimentin (Cell Signaling Technology, cat#5741), p-β-catenin (Cell Signaling Technology, cat#9561), total-β-catenin (Cell Signaling Technology, cat#8480), Snail (Cell Signaling Technology, cat#3879), slug (Cell Signaling Technology, cat#9585) and Tubulin (abcam, cat#ab6046) antibodies at 4°C overnight. After incubation, the membranes were incubated with goat anti-mouse conjugated to HRP or goat anti-rabbit conjugated to HRP secondary Y antibodies (Santa Cruz Biotechnology, cat#sc-2004 and sc-2005). Data were analyzed using LI-COR Image Software (LI-COR, Biosciences, Lincoln, NE).
Immunofluorescence was performed according to the standard procedures as described previously (11). A549 and ASTC-a-1 cells were cultured on glass cover slips and incubated overnight to establish adherence. Cells were fixed with 3.7-4% paraformaldehyde for 15 min room temperature, followed by permeabilization with chilled methanol for 15 min at -20°C. Cells were incubated in blocking buffer (PBS containing 5% normal goat serum and 0.5% Triton X-100) for 1 h at room temperature followed by incubation with anti-ki67 antibody (Abcam, Cat#15580, diluted 1:200 in blocking buffer) at 4°C overnight. Cells were washed three times for 5 min in PBS, and then incubated for 1 h with Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (Thermo Fisher, Cat# A-11034, diluted 1:500 in blocking buffer; Invitrogen) at room temperature. Nuclei were labeled with DAPI (Sigma, Cat# D9542) for 30 min at room temperature. Images were captured with Nikon Eclipse E600 fluorescence microscope.
The putative binding sites of PTEN were predicted by http://cbcsrv.watson.ibm.com/rna22.html and the putative binding sites were located in the 3'-UTR of PTEN mRNA. To this end, the 3'-UTR of PTEN containing the putative miR-21 binding sites were cloned into the luciferase reporter gene of the pGL3-vector (Promega, Madison, WI, USA) referred to as pGL3-PTEN-3'-UTR-WT. We also generated three point mutations into the seed region of the miR-21 binding sites, and these are referred to as pGL3-PTEN-3'-UTR-MUT. For luciferase reporter assay, A549 cells were seeded in 48 well plates (1x103 cells per well). After 24 h, cells were co-transfected with pGL3-PTEN-3'-UTR-WT or pGL3-PTEN-3'-UTR-MUT, pRL-TK Renilla Luciferase Reporter Vector (Promega, Madison, WI, USA) in presence of miR-21 mimic or miR-21 inhibitor using Lipofectamine 2000 Reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA). After 48h, luciferase activities were quantified with dual-luciferase assay system (Promega, Madison, WI, USA). The firefly luciferase activity was normalized to renilla luciferase activity. All the experiments were performed in triplicates.
All values are represented as mean ± S.E.M. Statistical difference was analyzed by two tailed unpaired Student’s t-test using SPSS17.0 software. Difference with P<0.05 was considered as statistically significant.
miR-21 is ubiquitously expressed in many cancer types, including human lung cancer. We validated this by examining the miR-21 expression in lung cancer and surrounding control non-cancerous tissues. As shown in Figure 1A, miR-21 expression level was markedly increased in NSCLC tissues compared to the adjacent tissues. Further investigation showed that miR-21 expression was significantly higher in NSCLC patients who have lymph node metastasis as compared to those without metastasis (Figure 1B) as well as in patients at stages III/IV as compared to those in stages I/II (Figure 1C). These results show that miR-21 expression level varies and progressively increases in advanced stages of disease and presence of metastasis. miR-21 is also overexpressed in various lung cancer cell lines compared to normal human lung cells (IMR90) (Figure 1D). While, miR-21 mimics significantly increased expression of miR-21, the treatment of cells with miR-21 inhibitor significantly reduced expression of endogenous miR-21 in these cell lines (Figure 1E-J).
 Figure 1.
Figure 1.
miR-21 is overexpressed in human lung cancer tissues and cells.A. miR-21 expression level was detected by qRT-PCR in lung cancer tissues and normal tissues. *P<0.05 vs. normal tissues. B. miR-21 expression level was detected by qRT-PCR in lung cancer tissues from patients with (N1) or without (N0) lymphatic metastasis. *P<0.05 vs. N0 group. C. miR-21 expression level was detected by qRT-PCR in lung cancer tissues from patients at the different clinical stages. *P<0.05 vs. stage I/II. D. miR-21 expression level was determined by qRT-PCR in different human lung cancer cell types and fetal lung fibroblast cells (IMR90). n=3,*P<0.05 vs.IMR90 cells. E-G. ASTC-a-1, H1299 and A549 cells were transfected with or without miR-21 inhibitor and the miR-21 expression level was determined by qRT-PCR. n=3, *P<0.05 vs. MOCK and NC group. H-I. ASTC-a-1, H1299 and A549 cells were transfected with or without miR-21 mimics and the miR-21 expression level was determined by qRT-PCR. n=3, *P<0.05 vs. MOCK and NC group.
Treatment of lung cancer cells, ASTC-a-1 and A549 cells with miR-21 inhibitor led to the inhibition of cell proliferation as assessed by cell count, labeling of cells with the proliferation marker, Ki67, and changes in cell cycle markers (cyclin D1 and E1) at different time intervals (Figure 2A-G).
 Figure 2.
Figure 2.
Down-regulation of miR-21 reduces prevents lung cancer cell proliferation and cell cycle progression. A and B. ASTC-a-1 and A549 cells were transfected with or without miR-21 inhibitor and the cell viability was measured at the indicated time points. n=5,*P<0.05 vs. MOCK and NC group. C and D. ASTC-a-1 and A549 cells were transfected with or without miR-21 inhibitor and ki-67 staining was performed at 48h after transfection (C), Bar=100μm. Quantitative analysis of ki-67 positive cells (D). 1000 cells were counted for each group (n=3, *P < 0.05 vs. MOCK and NC group). E. Western blot analysis of cyclin D1 and cyclin E1 expression level in ASTC-a-1 and A549 cells which were transfected with or without miR-21 inhibitor. F and G. Quantitative analysis of cyclin D1 and cyclin E1 expression level ASTC-a-1 and A549 cells. Data represent mean ± SEM (n=3, *P < 0.05 vs. MOCK and NC group).
Next, we carried gain-of-function studies by treatment of ASTC-a-1 and A549 cells with miR-21 mimics. These studies showed increased cell proliferation as assessed by cell count, labeling of cells with the proliferation marker, Ki67, and changes in cell cycle markers (cyclin D1 and E1) at different time intervals (Figure 3A-G). These loss-of-function studies are consistent with the idea that miR-21 regulates the proliferation of lung cancer cells.
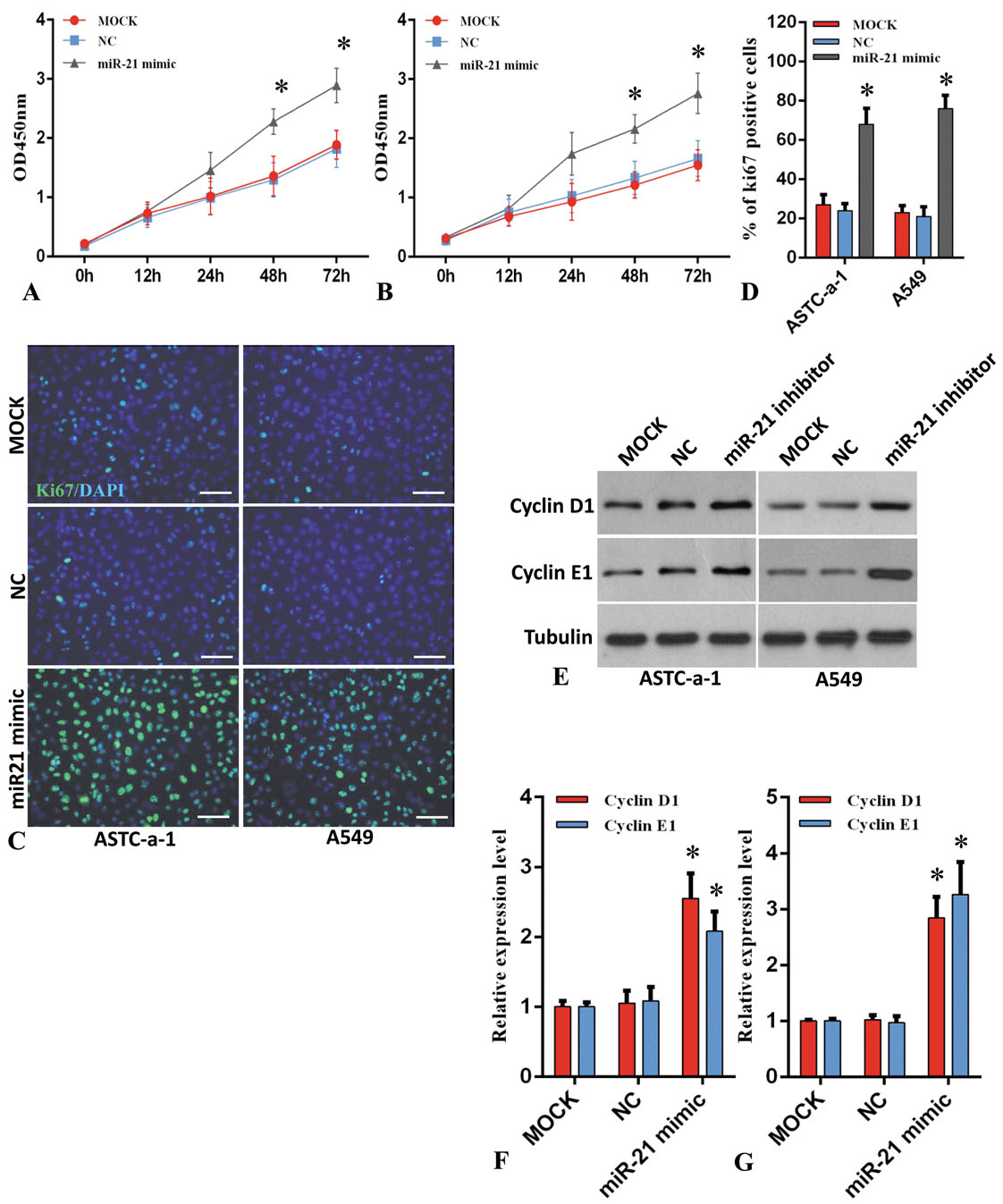 Figure 3.
Figure 3.
Up-regulation of miR-21 reduces prevents lung cancer cell proliferation and cell cycle progression. A and B. ASTC-a-1 and A549 cells were transfected with or without miR-21 mimics and the cell viability was measured at the indicated time points. n=5, *P<0.05 vs. MOCK and NC group. C and D. ASTC-a-1 and A549 cells were transfected with or without miR-21 mimics and ki-67 staining was performed at 48h after transfection (C), Bar=100μm. Quantitative analysis of ki-67 positive cells (D). 1000 cells were counted for each group (n=3, *P < 0.05 vs. MOCK and NC group). E. Western blot analysis of cyclin D1 and cyclin E1 expression level in ASTC-a-1 and A549 cells which were transfected with or without miR-21 mimics. F and G. Quantitative analysis of cyclin D1 and cyclin E1 expression level ASTC-a-1 and A549 cells. Data represent mean ± SEM (n=3, *P < 0.05 vs. MOCK and NC group).
Together, the loss or gain-of-function studies are consistent with the idea that miR-21 regulates the proliferation of lung cancer cells.
To decipher the molecular mechanisms that underlie the impact of miR-21 in proliferaiton through PTEN/Akt/GSK3β signaling cascade, we first predicted the putative miR-21 binding sites on PTEN (Figure 4A). Based on this prediction, the luciferase reporter assay was then performed using a construct that harbored the miR-21 binding sites in 3'-UTR of PTEN mRNA. As shown in Figure 4B and 4F, miR-21 inhibitor significantly increased the relative luciferase activity of the PTEN construct (Figure 4B, 4F). Moreover, the mutant reporter plasmid abolished miR-21 inhibitor-induced increase in luciferase activity (Figure 4B). We also performed the same experiment with miR-21 mimic in A549 cells. miR-21 mimic significantly reduced the relative luciferase activity when cells were co-transfected with the WT reporter plasmid as compared to the negative control. The mutant reporter plasmid also diminished miR-21 mimic-induced decrease in luciferase activity (Figure 4F).
 Figure 4.
Figure 4.
miR-21 promotes lung cancer cell replication via regulation of PTEN/Akt/GSK3β signaling axis. A. The predicted miR-21 binding sites within the 3'-UTR of PTEN mRNA. The arrows indicate the mutations. B. The pGL3-PTEN-3'-UTR-WT or pGL3-PTEN-3'-UTR-MUT was co-transfected with pRL-TK renilla luciferase reporter vector into A549 cells in the presence of miR-21 inhibitor. After 48h, luciferase activity was measured. Data represent mean ± SEM (n=3, *P < 0.05 vs. NC group). C. A549 cells were transfected with or without miR-21 inhibitor and PTEN mRNA expression level was detected by qRT-PCR (n=3, n.s means no significance). D. A549 cells were transfected with or without miR-21 inhibitor and indicated proteins expression were analyzed by Western blotting. E. The protein expression level of PTEN, p-Akt-Thr308, p-Akt-Ser473 and p-GSK3β-Ser9 was quantified. Data represent mean ± SEM (n=3, *P < 0.05 vs. MOCK and NC group). F. The pGL3-PTEN-3'-UTR-WT or pGL3-PTEN-3'-UTR-MUT was co-transfected with pRL-TK renilla luciferase reporter vector into A549 cells in the presence of miR-21 miR-21 mimics. After 48h, luciferase activity was measured. Data represent mean ± SEM (n=3, *P < 0.05 vs. NC group). G. A549 cells were transfected with or without miR-21 mimics and PTEN mRNA expression level was detected by qRT-PCR (n=3, n.s means no significance). H and I. A549 cells were transfected with or without miR-21 mimics and indicated proteins expression were analyzed by Western blotting (H). The protein expression level of PTEN, p-Akt-Thr308, p-Akt-Ser473 and p-GSK3β-Ser9 was quantified (I). Data represent mean ± SEM (n=3, *P < 0.05 vs. MOCK and NC group).
We then examined the PTEN mRNA expression level in the presence or absence of miR-21 inhibitor or mimics. As shown in Figure 4C and 4G, PTEN mRNA was unchanged when cells were transfected with miR-21 inhibitor or mimics compared to MOCK and NC group, indicating that miR-21 regulated PTEN expression level not through transcriptional level.
The down-regulation or up-regulation of miR-21 respectively increased or decreased PTEN protein (Figure 4D and 4H). These results suggest that miR-21 controls PTEN expression at the post-transcriptional level, but not transcriptional level.
PTEN is a pivotal effector of PI3K/Akt/GSK3β signaling pathway and plays a crucial role in cell proliferation and cell cycle progression in various human cancers (12). Then, by Western blotting in presence or absence of miR-21 inhibitor or mimics, we determined whether the changes in PTEN protein modifies its down-stream targets, Akt and GSK3β, and their activation as evidenced by phosphorylation at AktThr308, AktSer473, GSK3βSer9. The phosphorylation of Akt was markedly reduced when cells were transfected with miR-21 inhibitor compared to MOCK and NC group (Figure 4D, 4H). In contrast, the phosphorylation of Akt and GSK3β was dramatically enhanced when cells were transfected with miR-21 mimics compared to MOCK and NC group ( (Figure 4E, 4I). These findings suggested that effects of miR-21 by PTEN controls the down-stream phosphorylation and subsequent activation of Akt and GSK3β.
Accumulating evidence show that Akt/GSK3β signaling pathway and their key downstream targets are involved in EMT which occurs in many human cancers and plays an important role in cancer cell invasion and tumor metastasis (13-15). To decipher whether the EMT regulation might be regulated by miR-21, we examined the markers of this state including expression of E-cadherin, vimentin, snail and slug and related key factor β-catenin. To do this, cells were treated with were miR-21 inhibitor or mimics. E-cadherin expression level was distinctly increased when cells were transfected with miR-21 inhibitor compared to MOCK and NC group (Figure 5A-B). Conversely, vimentin, snail and slug expression levels were significantly reduced. Moreover, phosphorylation of β-catenin at Ser33/37 and Ther41 which is regulated by GSK3β and involved in cancer cell invasion and proliferation, was also significantly reduced. In contradistinction to these results, the E-cadherin expression level was distinctly reduced while vimentin, snail, slug and phosphorylation of β-catenin at Ser33/37 and Ther41 was increased when cells were transfected with miR-21 mimics as compared to MOCK and NC group (Figure 5C-D).
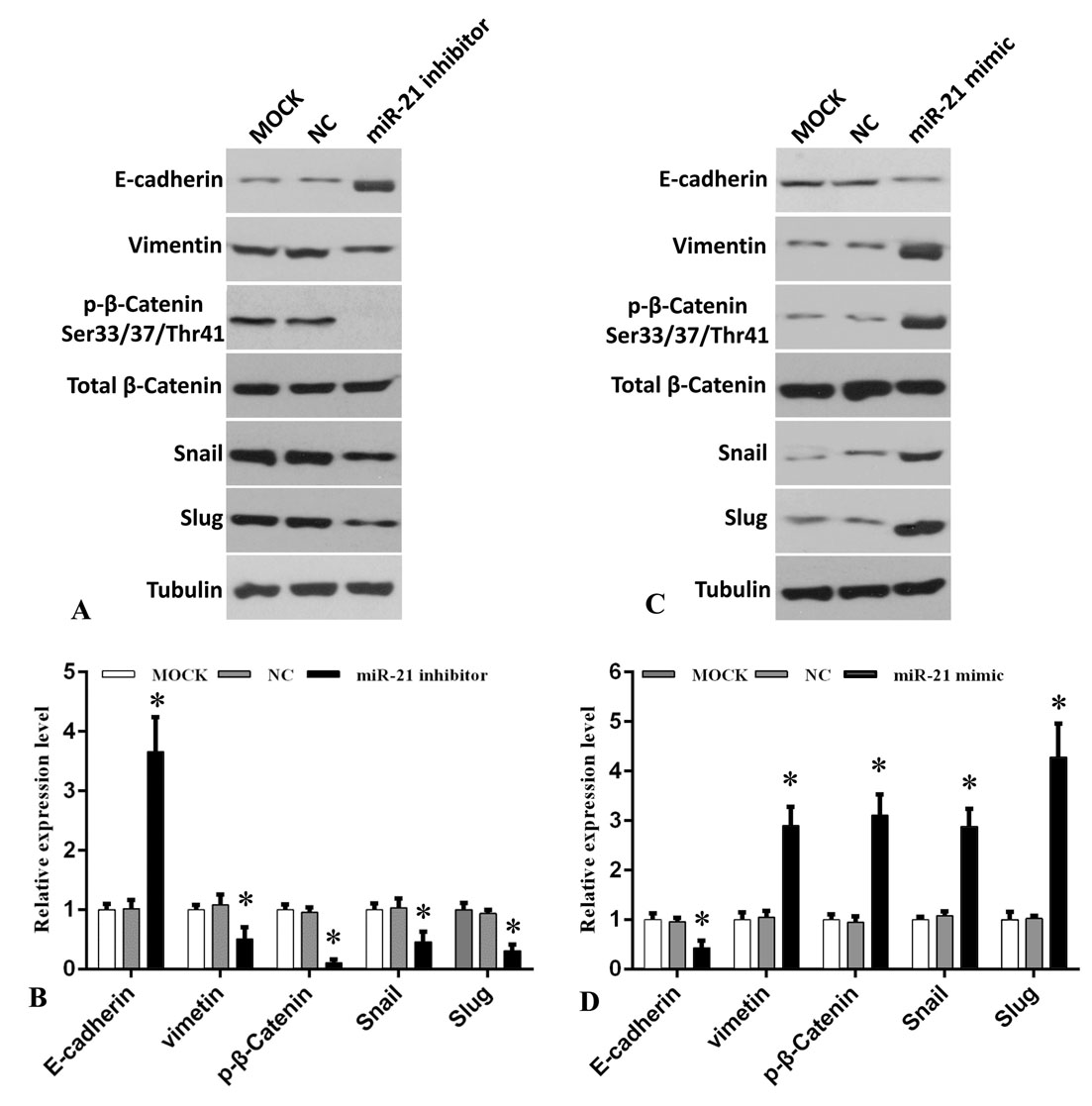 Figure 5.
Figure 5.
miR-21 promotes lung cancer cell EMT and invasion via regulation of Akt/GSK3β downstream targets. A. A549 cells were transfected with or without miR-21 inhibitor and indicated proteins expression were analyzed by Western blotting. B. Quantitative analysis of E-cadherin, vimentin, p-β-catenin-Ser33/37/Thr41, Snail and Slug. Data represent mean ± SEM (n=3, *P < 0.05 vs. MOCK and NC group). C. A549 cells were transfected with or without miR-21 mimics and indicated proteins expression were analyzed by Western blotting. D. Quantitative analysis of E-cadherin, vimentin, p-β-catenin-Ser33/37/Thr41, Snail and Slug. Data represent mean ± SEM (n=3, *P < 0.05 vs. MOCK and NC group). Figure 6. The proposed model of miR-21 regulates lung cancer cell proliferation and EMT via PTEN/Akt/GSK3β signaling axis.
Together, the loss or gain-of-function studies are consistent with the idea that miR-21 regulates the EMT in lung cancer cells, in principle, through Akt/GSK3β downstream targets including regulation of E-cadherin and phosphorylation of β-catenin at Ser33/37 and Ther41which, in turn, regulates the EMT proteins including down-ergulation of E-cadherin as well as increased expression of vimentin, snail and slug.
Some miRNAs serve as an oncogene while others behave as a tumor suppressor depending on the context. For example, miR-126, miR-145, miR-144 and miR-34a are identified as tumor suppressors (16, 17), while others such as miR-21, miR-17-92, miR-221, miR-222 and miR-31 are designated as oncogenes (oncomir) (18, 19). miR-21 is the first oncogenic miRNA that is shown to be commonly expressed in many human cancers including carcinomas of the lung, breast, ovaries, colon, rectum, prostate, pancreas and thyroid (5). Many cancers, and, more specifically human lung cancers, overexpress miR-21 and such an overexpression is associated with a poor response to treatment and a poor survival rate (6, 7). These miRNA regulate many of the biological properties of cancer including their proliferation, apoptosis, migration, metastasis, invasion, and development of drug-resistance. To further decipher the oncogenic role of miR-21, we examined the expression of miR-21 in lung cancers. We show that miR-21 is overexpressed in human Non-Small Cell Lung Cancer (NSCLC) with the pathologic classification of adenocarcinomas, squamous cells carcinomas as well as mixture of these tumors. The up or down-regulation, respectively, increases or decreases cyclin D1 and cyclin E1 expression and coordinately promotes or inhibits proliferation of cancer cells by targeting PTEN/Akt/GSK3β signaling axis. The perturbations of miR-21 also dramatically reduces or increases epithelial to mesenschymal transition (EMT), by regulation of PTEN, directly at the post-transcriptional level and through Akt/GSK3 beta signaling axis, regulate the expression of invasion markers including E-cadherin, vimentin, snail, slug and beta-catenin.
Previous studies have shown that elevation of miR-21 expression has been identified in several types of human cancers (20, 21). In addition, it has been shown that miR-21 promotes cancer cell proliferation, colony formation, migration and invasion. miR-21 also represses apoptosis by targeting multiple targets, such as by regulating PTEN, a tumor suppressor and key modulator of cell survival and cell cycle progression (22, 23). A recent study showed that miR-21 is overexpressed in lung cancer patients and this expression is associated with a poor prognosis (24). Furthermore, a previous study demonstrates that NSCLC patients with distal metastasis have a higher miR-21 expression than those without distal metastasis (25). Based on such strong correlation, it is suggested that overexpression of miR-21 may be involved in the invasion and metastasis in lung cancer (25). We validated these findings by showing that the miR-21 indeed is overexpressed in NSCLC. We also showed that such an expression was linked to the stage of the disease as well as presence or absence of metastasis.
PI3K/Akt signaling axis plays an important role in the development and progression of human tumors. PI3K family are activated by a variety of tyrosine receptors and exert catalytic phosphatidylinositol kinase activity leading to the recruitment of a large number of second messenger 3, 4, 5-triphosphate phosphatidylinositol (PIP3) to the cell surface and their activation. Activated Akt, in turn, activates or inhibits downstream substrates such as GSK3β, NF-κB, Bad, caspase-9, etc., and regulates the phosphorylation of numerous targets that regulate cell proliferation, differentiation, migration, metastasis and apoptosis (26-27).
The PI3K/Akt signaling axis negatively reguates PTEN which is mutated at a high frequency in many types of human cancer, including NSCLC (28). Down-regulation of PTEN expression promotes proliferation and invasion and in several cancers (29). Conversely, up-regulation of PTEN suppresses tumor cell growth and promotes tumor cell apoptosis. Under normal conditions, tumor suppression by PTEN relies on negative regulation of the PI3K/Akt signaling axis (30).
There is an inverse relation between miR-21 and PTEN expression in colorectal cancer (35). PTEN is a direct target of miR-21 and their balance regulate proliferation, apoptosis, and invasion in various cancers including NSCLC (31-34). We validated the existence of this inverse relation between the miR-21 and PTEN expression in NSCLC. Moreover, down-regulation or up-regulation of miR-21, respectively, increases or reduces PTEN protein level but not PTEN mRNA level compared to control cells. This miR-21 signaling through Akt/GSK3β, leads to phosphorylation of cyclin E1, cyclin D1, β-catenin, and snail. Previous studies showed that cyclin D1 expression and its subcelluar localization is closely controlled by GSK3β and by PTEN/Akt signaling (36-37). We also showed that miR-21 promotes EMT in lung cancer cells through regulation of invasion markers, E-cadherin, vimentin, β-catenin, and nail and slug, which are all the key downstream targets of Akt/GSK3β signaling. These results are consistent with the role of miR-21 as an oncogene in NSCLC.
In summary, we demonstrated that miR-21 govern lung cancer cell proliferation, cell cycle progression and ETM through regulation of PTEN/Akt/GSK3β signaling cascades (Figure 6). We also provided hard evidence for down-regulating miR-21 as a new therapeutic strategy for human lung cancer. Given, that the miR-21 regulates proliferation and apoptosis in NSCLC and that its expression leads to a proor progrnosis, the findings reported here show that miR-21 might be used as a target for the development of treatment for human NSCLC.
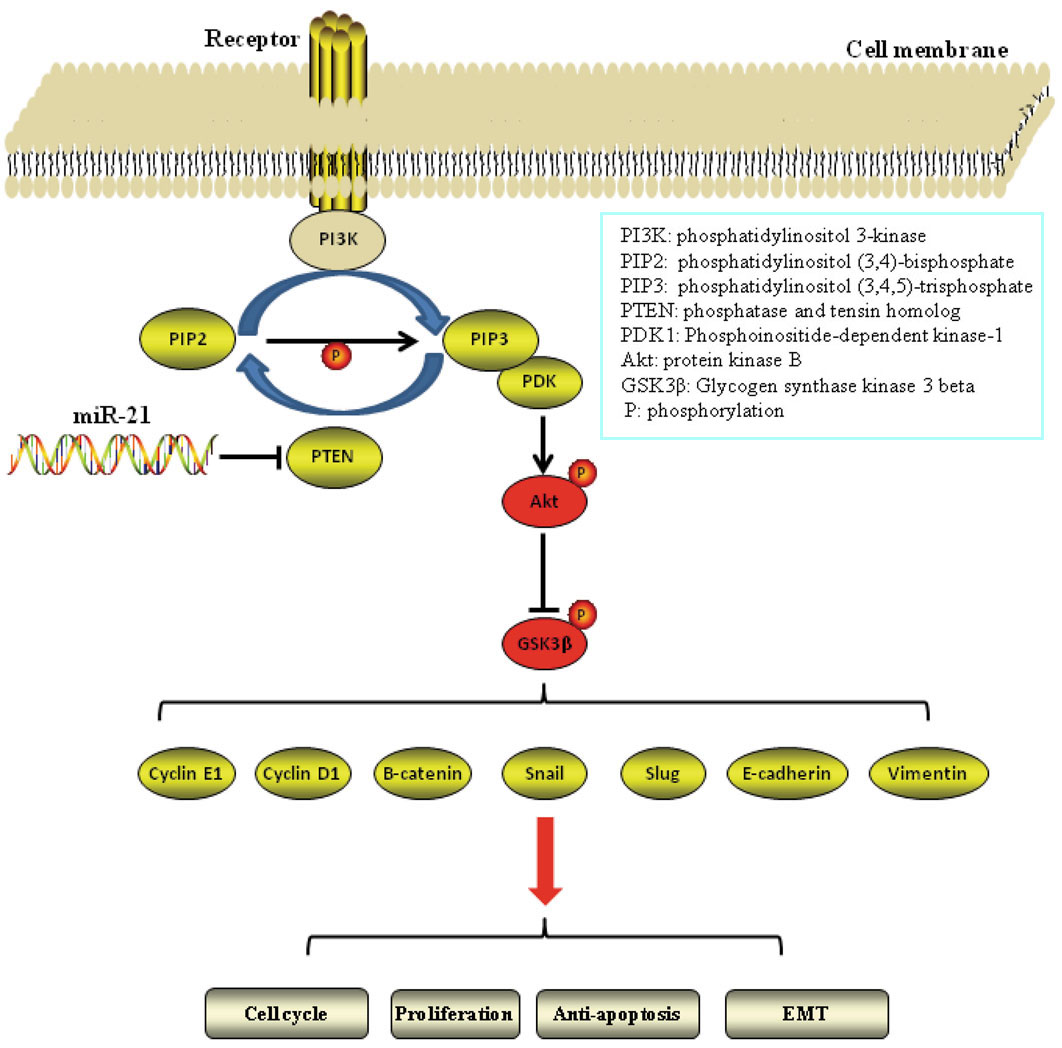 Figure 6.
Figure 6.
The proposed model of miR-21 regulates lung cancer cell proliferation and EMT via PTEN/Akt/GSK3β signaling axis.
Lihua Dai and Fenghua Chen contributed equally to this paper. This study is partially supported by National Natural Science foundation of China (Grant number 81470261) and by Health Industry Clinical Research Project of Shanghai Municipal Commission of Health and Family Planning (Grant number 201840222).
Abbreviations: EMT, epithelial-mesenchymal transition; IMR90, Primary human fetal lung fibroblast cells; miRNA, MicroRNA; NSCLC, non-small cell lung cancer; PIP3, 4, 5-triphosphate phosphatidylinositol; PDK1, phosphoinositide dependent kinase; SCLC, small cell lung cancer; 3'-UTR 3'-untranslated region
