Thyroid stimulating hormone (TSH) is the main regulator of thyroid cell proliferation and endocrine function. Here, we tested the effect of mir-22 in TSH induced proliferation and lipid metabolism of thyroid cells. TSH significantly down-regulated miR-22-3p, promoted proliferation and inhibited apoptosis of thyroid epithelial cells line, FRTL-5; effects that were opposed by overexpression of miR-22-3p. Overexpression of miR-22-3p significantly inhibited TSH- induced expression of lipid metabolic marker genes and increased the expression of lipid catabolism markers and IL6R and led to accumulation of intracellular lipid. IL6R overexpression also led to excessive proliferation, restrained apoptosis and promoted lipid accumulation. In conclusion, miR-22 regulates TSH-induced thyroid cell proliferation and lipid metabolism disorder by impacting IL6R.
Thyrotropin (TSH) is a hormone secreted by the anterior pituitary gland. Previous studies have shown that TSH is involved in abnormal proliferation, lipid metabolism and endocrine regulation of thyroid cells (1,2). Further studies on the mechanism of function show that TSH regulates the survival and proliferation of thyroid cells (3,4) by regulating the G protein / PI3K signaling pathway. In addition, TSH promotes the secretion of thyroid hormones by regulating lipid synthesis and catabolism (2,5).
MicroRNA (miRNA) is a non-coding RNA composed of 22 nucleotides. Recent studies have shown that miRNAs (mir) are involved in the proliferation of thyroid cells. Takeshi et al. found that thyroglobulin promotes the proliferation of thyroid cells by down-regulating the levels of mir-16, mir-24 and mir-195 (6). In addition, LeonV et al. showed that mir-23b and mir-29b inhibit the abnormal proliferation of thyroid cells induced by TSH (7). mir-22 is abnormally expressed in hyperthyroidism and thyroiditis, suggesting that mir-22 might be involved in the development of thyroid diseases (8,9). However, it is not clear whether mir-22 affects the abnormal proliferation of thyroid cells induced by TSH.
The pharmacological action of IL6 depends on the activation of IL6R (10,11). Studies have shown that IL6/IL6R signaling pathway play an important role in inflammatory response and apoptosis. In thyroid nodules, IL6/IL6R is abnormally expressed under the inducing effect of CD30L/CD30 (12 - 14). In addition, IL6/IL6R is also involved in the regulation of lipid metabolism, which induces the production of ROS, thus affecting the balance of liver lipid metabolism (15). Interestingly, mir-22 alleviates inflammatory damage by inhibiting IL6/IL6R signaling pathway (16,17). However, whether mir-22 affects the proliferation and lipid metabolism of thyroid cells through IL6/IL6R signaling pathway has not been reported.
JAK/STAT signaling pathway plays an important role in a variety of physiological and biological processes (18). IL6R is an important upstream regulator of JAK/STAT3 signaling pathway (19). The overexpression of IL6R promotes the phosphorylation of JAK tyrosine kinase and then activated STAT3 to p-STAT3. Hypoxia inducible factor-1 α (HIF-1 α) is an oxygen-sensitive transcription factor, which is related to the regulation of vascular endothelial growth factor signaling pathway, cell proliferation and apoptosis (20). Studies have shown that activated STAT3 (p-STAT3) could promote the expression of hypoxia inducible factor-1 α and ultimately promote tumorigenesis (21). Yang et al. reported that IL6 promoted the expression of vascular endothelial growth factor (VEGF) in bovine granulosa cells induced by follicle stimulating hormone (FSH) through JAK/STAT3/HIF1-α signaling pathway. (22) However, it is not clear whether JAK/STAT3/HIF1 α signaling pathway is involved in the abnormal proliferation and lipid metabolism disorder of thyroid cells.
Therefore, the purpose of this study was to investigate the role of mir-22 in TSH-induced thyroid cell proliferation and lipid metabolism disorder and its possible mechanism.
Fetal bovine serum (FBS) and DMEM / F12 medium were purchased from Gibco (Rockville, MD). The primary antibodies Ki67 (sc-23900, 1:1000, SCB , USA), PCNA (sc-25280, 1:1000, SCB, USA), Bcl-2 (sc-509, 1:1000, SCB, USA), Bax-6A7 (sc-23959, 1:1000, SCB, USA), GAPDH (sc-293335, 1:1000, SCB, USA), β-actin (sc-517582, 1:1000, SCB, USA), SREBP-1c (sc-13551, 1:1000, SCB, USA), ACS (sc-398559, 1:1000, SCB, USA), PPRAα (sc-398394, 1:1000, SCB, USA), CPT-1 (sc-515577, 1:1000, SCB, USA), IL6R (sc-374259, 1:1000, SCB, USA), JAK (sc-136225, 1:1000, SCB, USA), STAT3 (sc-293151, 1:1000, SCB, USA) and HIF-1α (sc-13515, 1:1000, SCB, USA) are purchased from Santa Cruz Biotechnology, and the second antibody is purchased from Dr. Deer Biotech (China, Wuhan). CCK8 kit, reverse transcription kit and fluorescence quantitative PCR kit are purchased from ThermoFisher Scientific (Waltham, USA). The TRIzol kit is purchased from Invitrogen (CA, USA). Endofectin lenti transfection kit is purchased from the United States GeneCopoeia company. Dual Luciferase reporter kit is purchased from Promega, USA.
Thyroid cell line FRTL-5 is purchased from the ATCC and incubated in DMEM/F12 medium supplement with 10% FBS at 37 ° C with 5% CO2. When the fusion rate reached 80%, the cells are treated with TSH for 24 h. To investigate whether hypoxia inducible factor-1 α (HIF-1α) is involved in the regulation of miR-22, the cells are incubated at 37 °C under 1% O2 and 5% CO2.
The hsa-mir-22-3p [the human microRNA-22 gene (hsa-miR-22) is processed from the 3 'end arm of the hsa-mir-21 precursor] mimic and NC mimics are designed and synthesized by Ruibo Biotechnology Co., Ltd. (Guangzhou, China). FRTL-5 cells induced by TSH are inoculated into 24-well plate. After more than 80% of the cells fused, mir-22-3p (processed at the 3 ' end) mimic or NC mimic are transfected into FRTL-5 cells. All the transfections are conducted according to the manufacturer's protocol of Lipofectamine 2000 transfection reagent (Invitrogen, Grand Island, NY, USA).
Recombinant lentiviruses packing IL6R vector (LV‐IL6R) and negative control vector (LV‐NC) are obtained from GeneChem (Shanghai, China). TSH-induced FRTL-5 cells are transfected with LV‐IL6R or LV‐NC with 5 μg/mL polybrene (GeneChem). After infection for 48 h, the transfection efficacy is determined by RT-qPCR. After 2 days of infection, the cells are screened with 2 μ g/mL puromycin for 2 weeks, and a stable overexpression cell line is established.
The total RNAs are extracted according to the manufacturer's protocol of TRIzol kit (Invitrogen, CA, USA). cDNA is synthesized by reverse transcription kit (Thermo Fisher Scientific, Waltham, USA) and detected by fluorescence quantitative PCR kit (Thermo Fisher Scientific, Waltham, USA). The data are quantitatively analyzed by 2-△△Ct method. The primers used in the experiments are designed and synthesized by Qiagen. The primers were as follow: GAPDH forward, 5'-AAGTATGACAACAGCCTCAAGA-3', and reverse, 5'-CACCACCTTCTTGATGTCATCA-3'. mir-22 forward, 5'-TTCGTAAGCTAGCAAGCCGC-3', and reverse, 5'-CCTAGAAATCGCAGCCGTT-3'.
The cells are inoculated in 96-well plate and cultured in DMEM/F12 medium supplemented with 10% FBS for 24 h. Cell proliferative ability is determined according to the manufacturer's protocol of CCK-8 (Cell counting kit-8, DOJINDO, Japan). The absorbance is measured at 450 nm with a microplate reader.
The total proteins are extracted from lysate and separated by SDS-PAGE. On this basis, the isolated protein is transferred to PVDF membrane and sealed with 5% skim milk powder at room temperature for 2 h. Proteins are then co-cultured with primary antibodies at 4 C overnight. After three times of washing with TBST, the corresponding secondary antibodies are added and incubated at room temperature for 1 h. Proteins are visualized using enhanced chemiluminescence reagents (Pierce, USA). Analysis is performed using ImageJ software (version 1.48, National Institutes of Health, Bethesda, MD, USA).
After trypsin digestion, the cells are collected and washed by PBS for 3 times. The cells are then fixed with 70% ethanol for 1 h and incubated with Annexin-V and PI at room temperature. Apoptosis is detected by flow cytometry.
The cells are inoculated and cultured in 24-well plate. After treatment with 10% formaldehyde for 10 min, the cells are washed with 60% isopropanol, stained with 1% oil red O for 40 min, and then treated with isopropanol. After treatment with hematoxylin complex, the morphology of the cells is observed under biomicroscopy.
Targetscan 7.0 is used to predict the binding sites of mir-22-3p to IL6R. IL6R wild type or mutant 3’UTR is amplified and inserted into pMIR-Report vector. IL6R wild type plasmid or IL6R mutant plasmid is transfected into cells, or co-transfected with mir-22-3p mimic. The fluorescence intensity is then measured according to the manufacturer's double luciferase reporting kit (Promega,USA).
The expression of STAT3 in FRTL-5 cells induced by TSH is detected by immunofluorescence. In short, FRTL-5 cells induced by TSH are cultured in the medium until the fusion rate reached 30 - 40%. After washing with PBS, the cells are fixed with 4% formaldehyde for 15 min, and then co-cultured with primary antibody at 4 °C (# 9139, 1 : 1600, Cell Signating Technology, USA). After washing 5 min for three times with PBS, the cells are incubated with Alexa Fluor ®647 second antibody (# 4410, 1 : 1000, CSTUSA, USA) for 2 hours and stained with Delay ®Gold AntiFade reagent (# 8961, CST, USA) at room temperature for 24 hours. The cells are then cleaned with PBS and examined by confocal microscopy (LSM 510 Meta,Zeiss,Oberkochen, Germany).
All the experimental data are analyzed by statistical software SPSS 10.0. The results are expressed as mean ± standard deviation. T or t’ test is used between the two groups. More groups are compared with ANOVA, with P < 0.05 indicating a statistically significant difference.
Researches have shown that miRNA expression in thyroid cells is closely related to the development of thyroid disease (23, 24). Therefore, we investigates the roles of mir-22-3p in thyroid cells. Firstly, RT-qPCR is applied to detect the expression level of mir-22-3p. As shown in Figure 1A, mir-22-3p level is obviously decreased in TSH-induced FRTL-5 cells, while with the transfection of mir-22-3p mimic, mir-22-3p level is dramatically increased compared with TSH + NC mimics group. (* p < 0.05, ##p < 0.01) Second, to explore the roles of mir-22-3p in progresses of FRTL-5 cells, CCK8, flow cytometry and western blot assays are employed. As shown in Figure 1B - Figure 1C,after transfection with mir-22-3p mimic, proliferation capacity is markedly inhibited, while apoptosis capacity is promoted in TSH-induced FRTL-5 cells. (* p < 0.05, #p < 0.05, ##p < 0.01) Western blot assay further confirms that the expression of Ki67 and PCNA are remarkedly decelerated in TSH-induced FRTL-5 cells transfected with mir-22-3p mimic. Besides, transfection with mir-22-3p mimic also elevated pro-apoptotic protein (Bax - 6A7) level, and reduced anti-apoptotic protein (Bcl-2) level in TSH-induced FRTL-5 cells. (Figure 1D,* p < 0.05, #p < 0.05) Taken together, the above results suggests that mir-22-3p suppresses proliferation and stimulates apoptosis in TSH-induced thyroid cells.
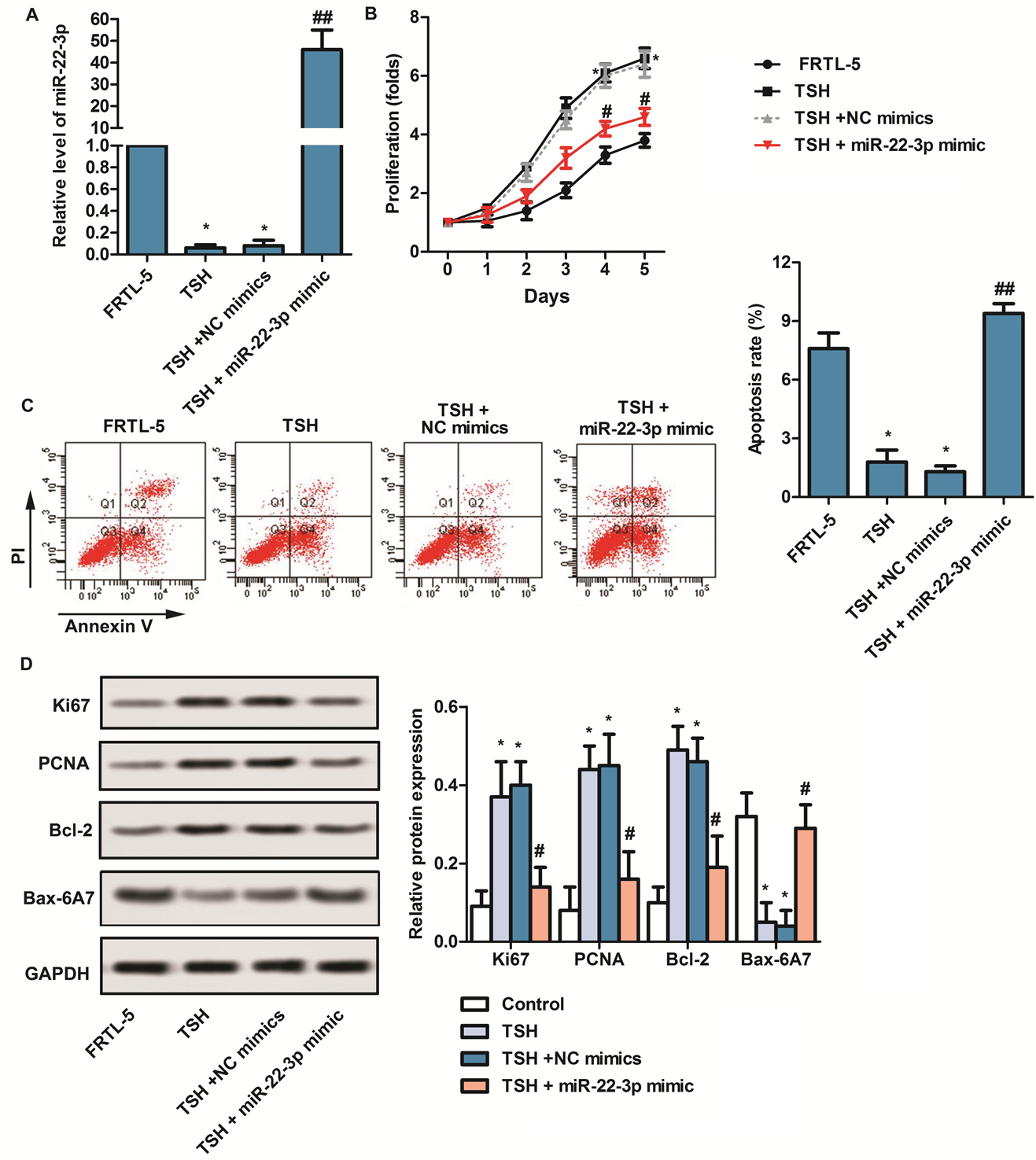 Figure 1.
Figure 1.
mir-22-3p suppressed proliferation and stimulated apoptosis in TSH-induced thyroid cells. Cells were divided into four groups: FRTL-5 group, TSH group (FRTL-5 cells were treated with TSH), TSH + NC mimics group (TSH-induced FRTL-5 cells were transfected with NC mimics) and TSH + miR-22-3p mimic (TSH-induced FRTL-5 cells were transfected with miR-22-3p mimics). (A) RT-qPCR was applied to detect the expression of miR-22-3p in each group. (B) CCK8 was applied to detect proliferation capacity of thyroid cells in each group. (C) The apoptosis was detected by flow cytometry in each group. (D) Western blot was used to detect the expression of Ki67, PCNA, Bcl-2 and Bax-6A7. FRTL-5 group was used as the control group. All results repeated three times. GAPDH was used as the internal reference. *p < 0.05 versus FRTL-5 group. #p < 0.05, ##p < 0.01 versus TSH + NC mimics group.
Researches have shown that mir-22-3p regulates the process of gluconeogenesis. But the direct evidence that mir-22-3p affects the anabolism of lipids has not been found (25). The results of oil red O staining shows that TSH promotes lipid accumulation, while upregulation of mir-22-3p significantly reduces lipid accumulation induced by TSH (Figure 2A). At the same time, TSH significantly promotes the expression of lipid synthesis-related markers (SREBP-1c and ACS), and inhibits the expression level of PPARα and CPT-1 (Figure 2B,*p < 0.05, ** p < 0.01). However, upregulation of mir-22-3p significantly attenuates the effects of TSH. (Figure 2B,*p < 0.05, ** p < 0.01, # p < 0.05, ## p < 0.01). It indicates that overexpression of mir-22-3p inhibits TSH-induced lipid accumulation.
 Figure 2.
Figure 2.
Upregulation of miR-22-3p by mimic inhibited lipid accumulation. Cells were divided into four groups: FRTL-5 group, TSH group (FRTL-5 cells were treated with TSH), TSH + NC mimics group (TSH-induced FRTL-5 cells were transfected with NC mimics) and TSH + miR-22-3p mimic (TSH-induced FRTL-5 cells were transfected with miR-22-3p mimic). (A) Oil red O staining was performed to detect lipid accumulation in each group. (B) Western blot was applied to detect the expression of SREBP-1c, ACS, PPARα and CPT-1 in each group. GAPDH was used as internal reference. *p < 0.05, **p < 0.01 versus FRTL-5 group. #p < 0.05, ##p < 0.01, versus TSH + NC mimics group.
Targeting relationship between mir-22-3p and IL6R is predicted by Targetscan7.0. (Figure 3A) Meanwhile, TSH promotes the expression of IL6R, while mir-22-3p significantly inhibits TSH-induced IL6R upregulation (Figure 3B,* p < 0.05, # p < 0.01). The luciferase reporter assay shows that mir-22-3p mimic significantly inhibits luciferase activity of wild-type plasmids compared to mutant plasmids (Figure 3C,** p < 0.05). These results further demonstrates that mir-22-3p targets IL6R and negatively regulates IL6R.
 Figure 3.
Figure 3.
miR-22-3p targeted IL6R and negatively regulated IL6R. Cells were divided into four groups: FRTL-5 group, TSH group (FRTL-5 cells were treated with TSH), TSH + NC mimics group (TSH-induced FRTL-5 cells were transfected with NC mimics) and TSH + miR-22-3p mimic (TSH-induced FRTL-5 cells were transfected with miR-22-3p mimic). (A) Targetscan7.0 were utilized to predicted the targeted relationship between miR-22-3p and IL6R. (B) Western blot was used to detect the expression of IL6R in each group. (C) The targeting relationship between miR-22-3 p and IL-6R was confirmed by luciferase reporter assay. GAPDH was used as internal reference. *p < 0.05 versus FRTL-5 group. #p < 0.05 versus TSH + NC mimics group. #p < 0.05 versus IL6R wt group.
To investigate the roles of IL6R in thyroid cells, we constructs the IL6R overexpression plasmid with lentivirus and overexpresses IL6R stably in FRTL-5 cells. (Figure 4A) Results showed that upregulation of IL6R expression significantly promotes the proliferation of thyroid cells, while mir-22-3p mimic has opposite effects (Figure 4B,* p < 0.05, # p < 0.05). Besides, overexpression of IL6R significantly promotes lipid accumulation, while upregulation of mir-22-3p by mir-22-3p mimic significantly inhibits the lipid accumulation induced by IL6R (Figure 4C). In addition, overexpression of IL6R significantly inhibits thyroid cell apoptosis (Figure 4D,** p < 0.01), whereas overexpression of mir-22-3p attenuates the inhibitory effect of IL-6R on apoptosis (Figure 4D,# p < 0.05). These results indicates that overexpression of IL6R attenuates the effects of mir-22-3p mimic on proliferation, lipid accumulation and apoptosis in TSH-induced thyroid cells.
 Figure 4.
Figure 4.
Overexpression of IL6R attenuated the effects of miR-22-3p mimic on proliferation, lipid accumulation and apoptosis in TSH-induced thyroid cells. (A) TSH-induced FRTL-5 cells were transfected with LV-NC or LV-IL6R. (**p < 0.01 versus TSH-induced FRTL-5 cells) (B-D) Cells were divided into four groups: NC mimics group (TSH-induced FRTL-5 cells were transfected with NC mimics), miR-22-3p mimic (TSH-induced FRTL-5 cells were transfected with miR-22-3p mimic), LV-IL6R group (TSH-induced FRTL-5 cells were transfected with LV-IL6) and mimic + IL6R group (TSH-induced FRTL-5 cells were co-transfected with miR-22-3p mimic and LV-IL6R ). (B) CCK8 was applied to detect proliferation capacity. (C) Oil red O staining was performed to detect lipid accumulation. (D) Flow cytometry was applied to detect apoptosis. *p < 0.05 versus NC mimics group. #p < 0.05 versus LV-IL6R group.
The present study further exhibits that upregulation of mir-22-3p significantly inhibits the protein level of IL6R, the decrease of IL6R further reduces the levels of p-JAK1, p-STAT3, HIF-1α and Bcl-2, and consequently regulates proliferation and apoptosis. (Figure 5A - Figure 5B,* p < 0.05, # p < 0.05). Immunofluorescence assay demonstrates that IL-6R accelerates STAT3 transfer to the nucleus, whereas overexpression of miR-22-3p has the opposite effect. (Figure 5C,* p < 0.05, # p < 0.05) These results suggests that mir-22-3p plays the roles by down-regulating the expression of IL6R to inhibit the nuclear translocation of STAT3 in TSH-induced thyroid cells. The above results show that the effect of miR-22-3p on TSH-induced thyroid cell proliferation and lipid metabolism disorder is associated with inhibition of IL6R and STAT3 nuclear translocation.
 Figure 5.
Figure 5.
mir-22-3p played the roles by regulating IL-6R/STAT3/HIF-1α pathway in TSH-induced thyroid cells. Cells were divided into four groups: NC mimics group (TSH-induced FRTL-5 cells were transfected with NC mimics), miR-22-3p mimic (TSH-induced FRTL-5 cells were transfected with miR-22-3p mimic), LV-IL6R group (TSH-induced FRTL-5 cells were transfected with LV-IL6) and mimic + IL6R group (TSH-induced FRTL-5 cells were co-transfected with miR-22-3p mimic and LV-IL6R). Cells were incubated under 1% O2 and 5% CO2 at 37 °C. (A) Western blot was applied to detect the expression of IL6R, p-JAK/JAK, p-STAT3/STAT3, HIF-1α and Bcl-2. (B) The bar graph represented relative protein expression of IL6R, p-JAK/JAK, p-STAT3/STAT3, HIF-1α and Bcl-2. (C) The expression of STAT3 in the nucleus was detected by immunofluorescence assay. β-actin was used as internal reference. *p < 0.05 versus NC mimics group. #p < 0.05 versus LV-IL6R group.
miRNA is abnormally expressed in hyperthyroidism (hyperthyroidism), thyroiditis (thyroiditis) and thyroid tumors. TSH plays an important role in regulating thyroid cell proliferation and maintaining endocrine balance. It has been reported that TSH promotes the proliferation of thyroid cells and inhibits the decomposition of lipids (5, 26). TSH also down-regulates the expression of mir-16 and mir-195. The upregulation of mir-16 and mir-195 slows down the cell cycle progression and inhibits DNA synthesis (4, 8, 27, 28). mir-22 regulates many of the body's life processes (29, 30). In recent years, the research on the role of mir-22 in tumor and its antitumor activity (31) has become a research hotspot. The expression of mir-22 is down regulated in multiple cancers. Upregulation of mir-22 expression inhibits the proliferation of cancer cells and induces apoptosis (32-34). Moreover, the expression of mir-22 is closely related to hyperthyroidism and Hashimoto's thyroiditis (8, 9). In this study, mir-22-3p is most severely down regulated in FRTL-5 cells with the treatment of TSH, and the decrease of mir-22-3p further promotes the proliferation of FRTL-5 cells and restrains apoptosis. However, transfection with mir-22-3p mimic markedly ameliorates cells progresses induced by TSH. These results suggests that mir-22 might play an important role in TSH-induced cell function.
Accumulated evidence shows that miRNAs are involved in the whole process of lipid synthesis. Silencing mir-133 promotes lipid synthesis by promoting the expression of SREBP1, resulting in lipid accumulation (35). Downregulation of mir-122 expression inhibits the synthesis of fatty acids by inhibiting the expression of FASN, ACC2 and SCD1. mir-370 affects the synthesis of lipids by regulating the expression of mir-122 (36, 37). The overexpression of miR-24 also lead to lipid accumulation (38). In this study, we investigates the effect of mir-22 on the expression of PPARα, CPT-1, SREBP-1c and ACS in lipid synthesis and decomposition-related proteins. PPARα and CRT-1 are mainly involved in the oxidation and transport of free fatty acids. SREBP-1c and ACS are mainly involved in the regulation of fatty acid synthesis (39). Overexpression of mir-22-3p significantly inhibits TSH-induced lipid accumulation, reduces the protein levels of SREBP-1c and ACS, and promotes the expression of PPARα and CRT-1. It shows that mir-22 regulates TSH-induced lipid metabolism disorders.
IL6R is closely related to the development of thyroid disease. Ruggeri RM et al. found that IL6 / IL6R signaling pathway is involved in the development of thyroiditis and is positively correlated with lymphocyte infiltration (40). At the same time, researches have shown that IL6R is closely related to obesity. Hsieh et al. reports that IL6R promotes the synthesis of triglycerides and inhibits high-density lipoprotein secretion (41). Consistent with the results, we found that upregulation of mir-22-3p inhibits TSH-induced lipid accumulation. Bioinformatics prediction and luciferase reporter assay shows that there is a regulatory relationship between mir-22-3p and IL6R. mir-22-3p inhibits the expression of IL6R in thyroid cells. Further studies have shown that upregulation of IL6R attenuates the inhibitory effect of mir-22-3p on TSH-induced abnormal proliferation and lipid accumulation of thyroid cells and inhibits cell apoptosis. It suggests that mir-22 regulates abnormal thyroid cell proliferation and lipid metabolic disorders by targeting IL6R expression.
IL6R plays an important role in promoting inflammation and regulating apoptosis, depending on the activation of downstream target genes. IL6R regulates the proliferation, migration, immune response and lipid synthesis of cancer cells by regulating the phosphorylation of STAT3 (42-44). Studies have shown that STAT3 promotes the expression of HIF-1α. Down-regulation of STAT3 expression inhibits tumor growth and angiogenesis by inhibiting the expression of HIF-1α and VEGF (45). Our investigation found that mir-22-3p mimic significantly inhibits the expression of STAT3, HIF-1α and Bcl-2 in the downstream target genes of IL6R. Upregulation of IL6R attenuates the inhibitory effect of mir-22-3p on STAT3, HIF-1α and Bcl-2 expression. It indicates that the effect of mir-22-3p on TSH-induced thyroid cell proliferation and lipid metabolism disorder is associated with inhibition of IL6R and STAT3 nuclear translocation.
In summary, mir-22-3p inhibits TSH-induced thyroid cell proliferation and lipid accumulation, promotes thyroid cell apoptosis via targeting IL6R. Further investigation reveals that mir-22-3p suppresses TSH-induced thyroid cell proliferation and lipid metabolism disorder by down-regulating the expression of IL6R to inhibit the nuclear translocation of STAT3. This study firstly investigates the effect of mir-22 on TSH-induced abnormal proliferation and lipid metabolism in thyroid cells, and may provide a new idea for the treatment of thyroid-related diseases.
Zaiyou Dai and Haihong Yan equally contributed to this work and should be considered co-first authors.
Abbreviations: TSH, Thyroid stimulating hormone; miRNA, microRNA; PCNA, Proliferation cell nuclear antigen; mRNA, Messenger RNA; IL, Interleukin; HIF-1, Hypoxia-inducible factor-1; SREBP-1, Sterol regulatory element binding protein-1; ACS, Acyl-CoA synthetase; PPARα, Peroxisome proliferation-activated receptor α; CPT-1, Carnitine palmitoyl transterase-1.
