Studies have shown that CD4+ CD25+ regulatory T cells (Tregs) could inhibit cytokine-induced killer (CIK) cells against tumor cells, but minimal data have been reported on the underlying mechanisms. The purpose of this study was to investigate the potential suppressive mechanisms of cord blood Tregs on CIK cells in vivo and in vitro. The in vitro study demonstrated that Tregs were normally proliferated and had potent suppressive characteristics. CD4+ CD25+ LAP+ cells were highly expressed as part of activated Tregs, which limited CIK cell-mediated cytotoxicity and reduced the expression of the NKG2D receptor. Interestingly, the inhibitory ability of Tregs could be mimicked by soluble TGF-β1 and neutralizing TGF-β1 antibody could abrogate the inhibitory function of Tregs on CIK cells. In vivo results showed that adoptively transferred CIK cells could delay the tumor growth in nude mice. Moreover, depletion of CD4+ CD25+ Tregs in preculture or blockade of TGF-beta 1 strikingly enhanced CIK cells cytotoxicity. These data indicate that Tregs inhibit CIK cells cytotoxicity mainly by down regulating the expression of NKG2D receptor in a TGF-β dependent manner.
Cytokine-induced killer (CIK) cells, which have a potent role in the non-restricted killing of tumor cells, exist as a heterogeneous cell population expressing both T cell and natural killer (NK) cell markers (1, 2). Additionally, CIK cells are readily expanded from peripheral blood or cord blood mononuclear cells with the addition of different cytokines (3, 4). The CD3+ CD56+ cell subset, the main effector cells, have the most potent cytotoxic activity against target cells by interaction with NKG2D receptor and MHC-related ligands (5, 6).
CIK cells have shown significant antitumor activity, and can efficiently lyse a wide range target of tumor cells (7). However, some inhibitory factors limit the function of CIK cells, and CD4+ CD25+ regulatory T cells (Tregs) may be one of the most important inhibitory factors. Tregs dramatically decrease the CIK cell cytotoxicity (8). The production of CD4+ CD25+ Tregs could be increased via incubation with an anti-CD3 antibody, interferon-γ (IFN-γ), and interleukin (IL)-2 obtained from peripheral and cord blood. However, CD4+ CD25+ Tregs in cord blood express a higher level of TGF-β, which is closely related to highly effective Tregs suppressor functions (9). To improve the antitumor activity of CIK cells and overcome this unwanted suppressive factor, some studies have optimized the culture protocol and have included certain additional cytokines or chemotherapeutic drugs in the CIK cell culture medium (10-14). Furthermore, genetic engineering and changing the culture environment can enhance the antitumor activity of CIK cells (15, 16).
Although Tregs can inhibit the cytotoxic activity of CIK cells, the suppressive mechanism of Tregs toward CIK cells is not well clarified, especially that of cord blood Tregs. Tregs are potent immunosuppressive cells that suppress effector cells (17, 18). The growth of many tumors is correlated with Tregs enriched in tumor sites and peripheral circulation (19, 20). LAP+ Tregs. that express latency-associated peptides (LAPs) on the surface of CD4+ CD25+ T cells, are overexpressed in tumor patients and have immunosuppressive effects via a TGF-β mediated mechanism (21, 22). Therefore, it is believed that TGF-β may be responsible for the inhibitory function of Tregs. Further, TGF-β down regulates surface expression of the NK-activating receptor (NKG2D) that contributes to the cytotoxic activity of NK cells to lyse target tumor cells (23-26).
Tregs-mediated inhibition of NK cells is only linked with the down regulation of NKG2D receptors by TGF-β1 (27). To date, no study has described the effect of TGF-β on Tregs and NKG2D mediated cytotoxicity on CIK cells. Therefore, this study aimed at interpreting how the cord blood-derived Tregs block CIK cell functions in a TGF-β dependent manner.
BALB/c nude mice (aged 6-8 weeks) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd (Beijing, Chinese) and were maintained in a specific pathogen-free environment. The gastric cancer cell line SGC-7901 was cultured for inoculation of BALB/c nude mice, and the influence of Tregs on the antitumor activity of CIK cells in vivo was analyzed. All procedures of animal experiments conducted were in compliance with institutional guidelines.
CD4+ CD25+ T cells were isolated from cord blood mononuclear cells (CBMCs) via magnetic cell separation (MACS; Miltenyi Biotech, Germany). CD4+ CD25+ T cells were negatively and positively enriched using a two-step isolation kit according to the manufacturer’s instructions. The purity of CD4+ CD25+ T cells was determined at 95%–98% by fluorescence-activated cell sorting (FACS) analysis. Tregs were used in subsequent experiments in parallel with cord blood-derived CIK cells to study their suppressive function.
Cord blood was collected from full-term healthy newborn infants at delivery (38–40 weeks), and CBMCs were isolated by centrifugation on Ficoll–Hypaque gradient. CIK cells (conventional CIK cells) and CIK-Tregdel cells (depleted CD4+ CD25+ T cells by MACS isolation system) from CBMCs were cultured. The culture medium used was DMEM (Hyclone) supplemented with 10% fetal calf serum (Gibco) and 1000 U/mL recombinant human IFN-γ (PeproTech) added on day 0 and 50 ng/mL anti-human CD3 monoclonal antibody (eBioscience), 500 U/mL recombinant human IL-2 (eBioscience), and 100 U/mL recombinant human IL-1α (PeproTech) added on day 1. Culturing was performed for 21 days.
Cells were analyzed using FACS Calibur (BD Biosciences, USA). All antibodies were purchased from eBioscience except for anti-human LAP (TGF-β1), which was purchased from Biolegend. All cells were stained with directly labeled antibodies in a three-color staining analysis. CIK and CIK-Tregdel cells were characterized with anti-CD3- FITC (HIT3a clone), and anti-CD56-PerCP-eFluor710 (CMSSB clone) respectively. Activating receptors on the surface of CIK and CIK-Tregdel cells were labeled with anti-NKG2D-PE (BAT221 clone), anti-NKp30-PE (1D11 clone), anti-NKp46-PE (AF29-4D12 clone), anti-NKp44-PE (KS38 clone), and anti-TRAIL-PE (RIK-2 clone). Tregs were stained with anti-CD4-PerCP-Cyanine5.5 (RPA-4 clone), anti-CD25Alexa Fluor488 (BC96 clone) or anti-CD25 PE (BC96 clone), anti-CD127-PE (eBioRDR5 clone) and anti-human LAP-FITC (TGF-β1) (TW4-2F8 clone). All samples were stained with appropriate isotype controls (eBioscience).
Proliferation was assessed using Cell Counting Kit-8 (CCK-8) (Dojindo, Kumamoto, Japan), CIK-Tregdel cells were seeded in 96-well plates or co-cultured with Tregs and/or TGF-β1 (R&D Systems) and/or anti-TGF-β1 blocking antibodies (R&D Systems) at the indicated number or concentration. After incubating for 24 h, the cells were incubated with 10 μl CCK-8 again for 1.5 h at 37°C. At the end of the incubation period, absorbance was measured at an optical density of 450 nm, and this analysis was carried out in triplicates for each group.
Human myeloid leukemia cell line K562 and gastric cancer cell line SGC-7901 were used for cytotoxicity testing. K562 and SGC-7901 cells were washed and resuspended at 2×106 cell/ml PBS and labeled with a final concentration of 200 μM of green fluorescent dye CFSE (eBioscience) for 10 min at room temperature in the dark in cold complete DMEM medium which stopped reactions. Labeled target cells were cultured with CIK or CIK-Tregdel cells for 4, 8 and 24 h at a ratio of 1:5 or CIK-Tregdel cells were precultured with Tregs for 4 h at the indicated ratio (CIK/Tregs). Immediately before analysis, 7-amino actinomycin (7-AAD) (eBioscience) as suggested by the manufacture was added to each sample (1×106 cell/ml), 7-AAD-positive cells were analyzed using a flow cytometer. The cytotoxicity of CIK and CIK-Tregdel cells was determined by calculating the percentage of 7-AAD positive cells in the total labeled target cells. To further test Tregs-mediated inhibition on CIK cells in a TGF-β1-dependent manner, before incubation with labeled SGC-7901 target cells, CIK-Tregdel cells were precultured with Tregs and/or reagents (1 ng/ml TGF-β1, 10 ng/ml anti-TGF-β1 blocking antibody, and an isotype control antibody) for 4 h. After incubation for 24 h, 7-AAD-positive cell in labeled target cells were enumerated by flow cytometry.
BABL/c nude mice injected with SGC-7901 cells were used as animal models. When the tumor diameter reached 5 mm, the mice were treated by transferring 200 μl CIK-Tregdel cells (5×107/ml) alone or 2×106 Tregs and/or reagents (anti-TGF-β1 blocking antibody), An equivalent volume of PBS was used as control, which was injected every three days, for a total of six cycles. The volume of the tumor was measured every 3 days and was calculated using the following formula: volume (mm3) = (L (mm) × W (mm)2)/2. The survival periods of mice were recorded for the in three treatment groups and PBS control group.
Statistical analysis was performed with SPSS 20.0 software package. Data were expressed as mean ± SD. A two-tailed Student’s test was used to analyze the differences between the groups. The level of statistical significance was set at p < 0.05.
The procedures were approved by the ethical committee of the Taishan Medical University Affiliated Taishan Hospital and informed consent was obtained from the parents of all newborn infants. All studies were conducted in accordance with the Declaration of Helsinki (1964) and all possible steps were taken to avoid animal suffering at each stage of the experiment.
Cord blood CD4+ CD25+ T cells have previously been known to be a discrete group and include a high population of CD25+brih T cells compared with its adult peripheral blood counterparts (27). Our experiments showed that cord blood CD4+ CD25+ T cells were a relatively discrete population and comprise a high number of CD4+ CD25+ brih T cells with few CD25dim cells (Figure 1A). Therefore, cord blood CD4+ CD25+ T cells are more readily purified by MACS. After cord blood mononuclear cells were negatively and positively selected by MACS, the purity of CD4+ CD25+ T cells was > 95% (data not shown). To better characterize Tregs, we detected the expression of CD127 on CD4+ CD25+ T cells. Purified cord blood CD25+ cells contained a higher percentage of CD127low (Figure 1B). Via a combination of CD25 and CD127 marker expression, we confirmed that isolated cord blood CD4+ CD25+ T cells largely comprised natural Tregs.
 Figure 1.
Figure 1.
Cord blood CD4+ CD25+ T cells are natural Tregs. (a) Cord blood derived mononuclear cells were analysed by flow cytometry using three-color staining with anti-CD4-PerCP-Cyanine5.5, anti-CD25Alexa Fluor488 on day 0. (b) purified CD4+ CD25+ T cells was stained with anti-CD127-PE. The expression of CD127low was also analysed by flow cytometry. Results are representative at least nine different cord blood donors.
To assess the influence of Tregs on CIK cells, CIK and CIK-Tregdel cells were generated from CBMCs. After Tregs were depleted, the expansion of total cells and relative percentage of CD3+ CD56+ cells in CIK-Tregdel cells increased compared with that of conventional CIK cells. Starting from 5×106 CBMCs, CBMCs depleted CD4+ CD25+ T cells in preculture, and the final expansion levels in CIK and CIK-Tregdel cells on day 21 were 223.23×106 and 321.64×106, respectively. The growth curve showed that depletion of Tregs could improve the proliferation of CIK cells (Figure 2A). More importantly, CD3+ CD56+ double positive cells gradually increasing culture duration in CIK and CIK-Tregdel cells, the percentage of CD3+ CD56+ in CIK-Tregdel cells was higher than that in CIK cells culture on days 7,14 and 21 (Figure 2B). In terms of marker expression of CD3+ CD56+ cells, there were marked differences between CIK (images shown in the upper panels) and CIK-Tregdel cells (images shown in the lower panels) as observed using immunophenotyping (Figure 2C).
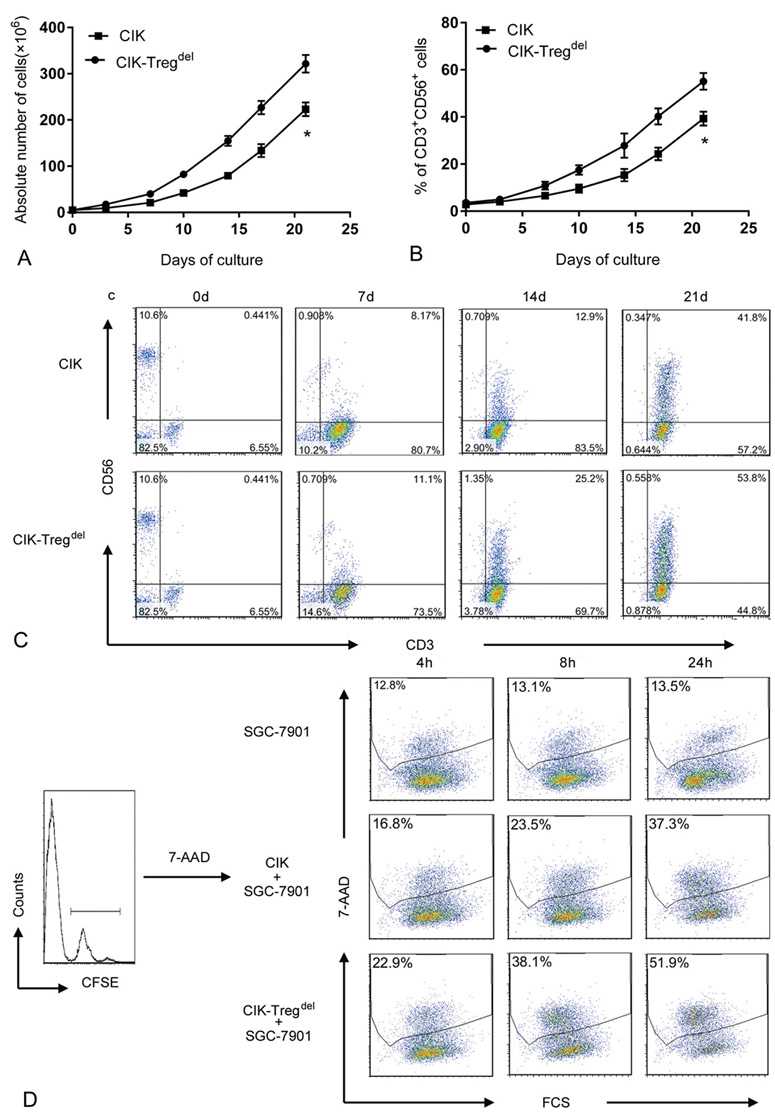 Figure 2.
Figure 2.
Comparison multiplication and cytotoxiciy on CIK and CIK-Tregdel cells. (a) CIK and CIK-Tregdel cells were generated in vitro, total cells were counted at different time points. Data are the mean absolute number of cells ± standard deviation from nine independent experiments performed using nine different cord blood, compared to CIK group, *p<0.05. (b) The percentage of CD3+ CD56+ double positive cells were investigated on both CIK and CIK-Tregdel cells at different time points, compared to CIK group, *p<0.05. (c) The marker expression of CD3+ CD56+ cells in CIK and CIK-Tregdel cells was shown throughout the whole process of culture in one cord blood sample by FACS analysis. (d) CFSE-labeled SGC-7901 cells used as target cells were co-cultured with CIK and CIK-Tregdel cells at 1:5 ratio for 4, 8, 24 h, respectively, CFSE-labeled SGC-7901 cell was used as the control. 7-AAD-positive cells was analysed by flow cytometry, the percentage of 7-AAD+ cells conducted on CFSE-labeled SGC-7901cells were utilized to determine inhibition effect of Tregs on CIK cells. A representative experiment of at least eleven independent experiments.
We also compared the suppressive ability of Tregs on the cytotoxicity of CIK and CIK- Tregdel cells using 7-AAD method. The data showed that Tregs exerted an inhibitory effect on the cytotoxicity of CIK cells, and the ability of CIK-Tregdel cells to kill SGC-7901 target cells was more enhanced than CIK cells at the corresponding ratio and same time points, as shown by representative staining (Figure 2D). This implied that depletion of CD4+ CD25+ T cells at the beginning of culture can boost cytotoxic activity of CIK cells.
To specifically verify the suppressive effect of Tregs on CIK cells, we analyzed the influence of Tregs on CIK cells against both tumor lines. CIK-Tregdel cells were co-cultured with Tregs at a variable Tregs/CIK-Tregdel cell ratio on day 14, and Tregs strongly decreased the proliferation and cytotoxicity of CIK-Tregdel cells in a dose-dependent manner. However, the suppressive action of Tregs was evident between ratios 1:1 and 1:10, Interestingly, lowering the Tregs/CIK-Tregdel cells ratio to 1:20 did not inhibit proliferation or cytotoxicity of CIK cells (Figure 3A and Figure 3B).
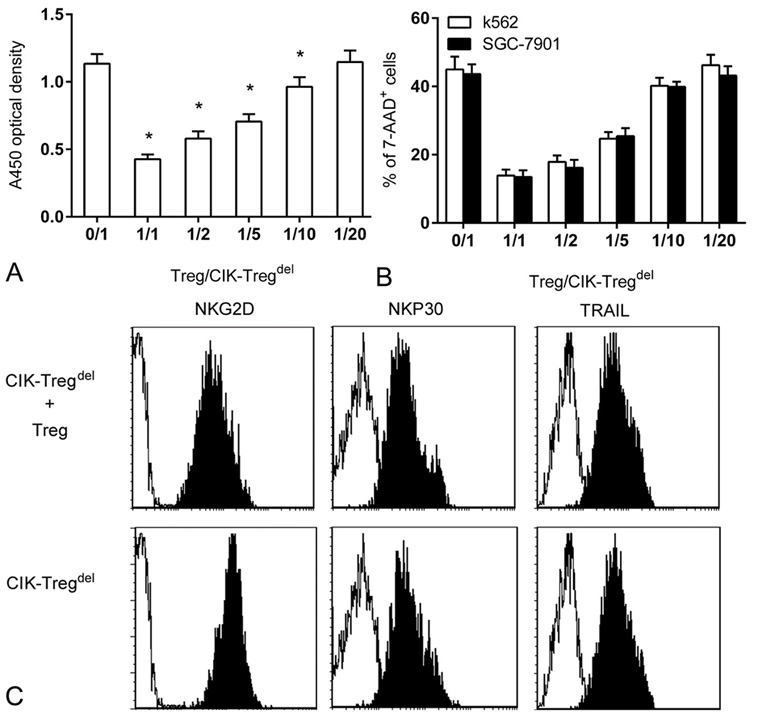 Figure 3.
Figure 3.
Suppressive function of Tregs on CIK cells. (a) A volume of 1×105/well CIK-Tregdel cells was cultured with Tregs at a variable Treg/CIK-Tregdel cells ratio (as indicated), CIK-Tregdel cells alone were taken as the control. After cells co-cultured for 24 h, proliferation was measured by CCK-8 assay, compared to 0/1 group, *p<0.05. (b) CIK-Tregdel cells were cultured alone or with Tregs for 4 h at indicated ratio before incubating with CFSE-labeled SGC-7901 and K562 cells for 24 h, effect /target ratio was 5:1. CFSE-labeled SGC-7901 cells and K562 cells alone were regarded as a control. The percentage of 7-AAD-positive cells was analysed. (c) CIK-Tregdel cells alone or co-cultured with Tregs for 24 h at 5:1 were stained with anti-CD3, anti-CD56 and anti-NKG2D or anti-NKp30 or anti-TRAIL. The CD3+ CD56+ cells were gated to analyse the expression of NKG2D, NKP30, TRAIL by flow cytometric (black area). Negative control was the isotype antibody (white area).Values are expressed as the mean of triplicate cultures and the results represent means values and standard deviations of seven independent experiments.
Next, we investigated whether Tregs inhibited CIK cells cytotoxicity by down regulation of activating receptors, including natural cytotoxicity receptors (NCRs), NKG2D and TNF-related apoptosis inducing ligand (TRAIL). The results indicated that CIK-Tregdel cells highly expressed NKG2D receptors with slightly low levels of NKP30 and TRAIL expression, as determined by mean fluorescence intensity, whereas the surface densities of NKP44 and NKP46 were only marginal (data not shown). Moreover, addition of Tregs sharply down regulated the expression of NKG2D receptors but did not interfere with the surface density of NKP30 and TRAIL (Figure 3C). Similarly, surface expression of other natural cytotoxicity receptors (NCRs) receptors was not significantly affected by additional Tregs.
Taken together, these data indicate that the inhibitory function of Tregs on CIK cells might be mediated by down-regulation of the NKG2D receptor.
Latency-associated peptide (LAP) is no covalently linked to TGF-β1 on Tregs, and it is an excellent marker to identify TGF-β-dependent Tregs, it has greater suppressive effects exerted in a TGF-β-dependent manner in vitro and in vivo (21, 28). LAP+ CD4+ Tregs represent an activated Tregs subgroup (29).We examined the surface marker expression of LAP on freshly isolated cord blood Tregs and activated Tregs, The results revealed that Tregs had larger proliferative responses and acquired potent suppressive function in CIK cell culture, and the expression of LAP+ was more pronounced on activated Tregs than freshly isolated Tregs and reached a peak between days 7 and 14. Subsequently, a slow progressive decline in LAP+ was detected in culture (Figure 4A).
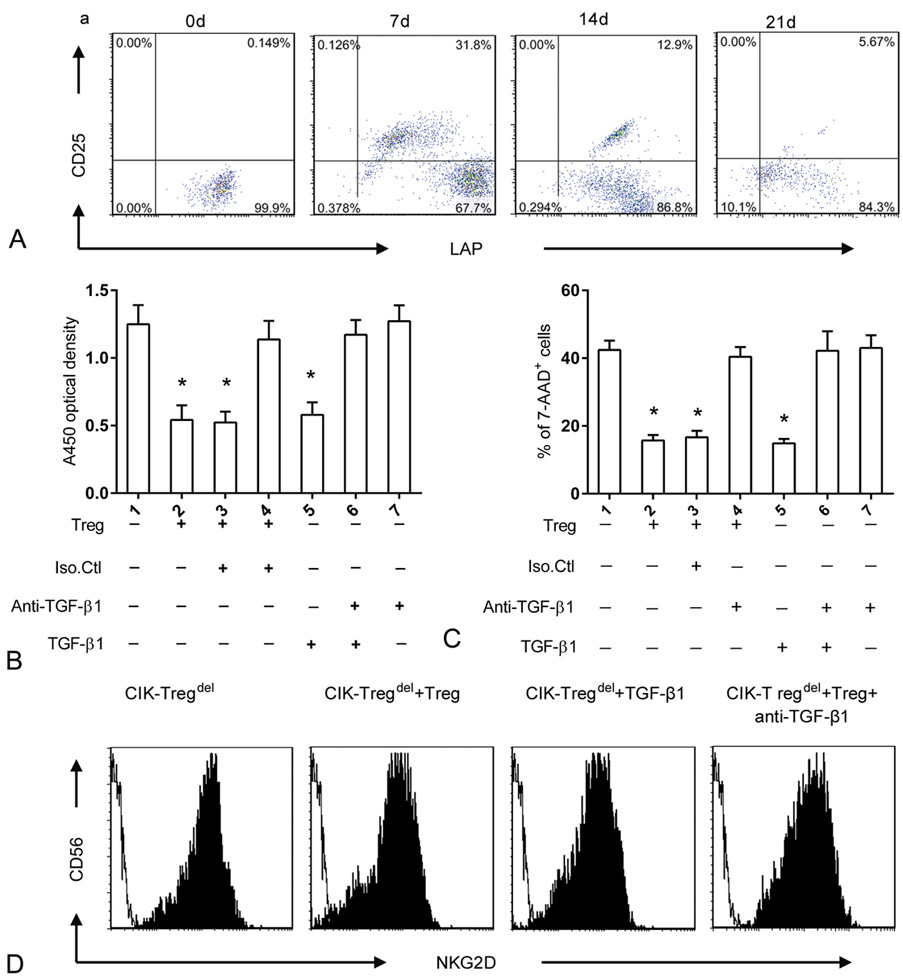 Figure 4.
Figure 4.
Role of TGF-β1 in Tregs–mediated CIK cells inhibition in vitro. (a) The expression of LAP+ cells in the gated CD4+ CD25+ subpopulation was shown at different time points of culture, compared to group 1, *p<0.05. (b) A volume of 1×105/well CIK-Tregdel cells were cultured alone or with Tregs (CIK-Tregdel : Treg ratio; 5:1) or TGF-β1 (1 ng/ml) and/or anti-TGF-β1 blocking antibody (10 ng/ml). CCK-8 based proliferation assay was performed after cells co-cultured for 24 h, compared to group 1, *p<0.05. (c) CIK-Tregdel cells were cultured alone or with Tregs or soluble TGF-β1 (1 ng/ml) and/or anti-TGF-β1 blocking antibody (10 ng/ml) for 4 h before incubation with CFSE-labeled SGC-7901 tumor targets. The Treg /CIK-Tregdel /target ratio was 1:5:1 for 24h. CFSE-labeled SGC-7901 cell alone was taken as the control. The percentage of 7-AAD positive cells was analysed, the results represent means values and standard deviations of seven independent experiments. (d) CIK-Tregdel cells were cultured alone or with Tregs at 5:1 ratio or 1 ng/ml TGF-β1 or with Tregs in the presence of 10 ng/ml anti-TGF-β1 blocking antibody for 24 h. Cells were monitored by flow cytometry.
To examine the functional role of TGF-β1 in Tregs-mediated suppression, we analyzed the influence of Tregs on CIK cells in the presence of soluble TGF-β1 or anti-TGF-β1 blocking antibodies. We observed that soluble TGF-β1 acquired a similar suppressive effect as as Tregs and could effectively inhibit proliferation and cytotoxicity of CIK cells, whereas neutralization of the TGF-β1 antibody could counteract the inhibitory ability of Tregs (Figure 4B and Figure 4C).
Down-regulation of phenotypic marker of NKG2D by Tregs is related to the level of TGF-β (26). In our study, it appeared that Tregs down regulated NKG2D receptor expression on CIK cells. Thus, we hypothesized that TGF-β1 is a contributing factor in down-regulation of the NKG2D receptor by Tregs. To confirm this, CIK-Tregdel cells were added to Tregs or TGF-β1 reagent. Soluble TGF-β1 displayed equivalent suppressive level as Tregs and markedly down regulated the surface NKG2D expression. Conversely, addition of neutralizing anti-TGF-β1 antibody could restore NKG2D receptor expression (Fig.4D).
These results indicate that Tregs inhibited CIK cell cytotoxicity mainly via TGF-β1 mediated down regulation of the NKG2D receptor expression that was mainly responsible for activation of CIK cells cytotoxicity against tumor cells.
Our study indicated that Tregs dramatically inhibit cytotoxicity of CIK cells, which were mediated by TGF-β1 in vitro, we investigated whether such phenomena also existed in vivo. BABL/C nude mice were inoculated with SGC-7901 gastric cancer cells. CIK-Tregdel cells alone or with Tregs or reagents anti-TGF-β1 blocking antibody were adoptively transferred into nude mice when the tumor diameter reached 5 mm. As shown by tumor growth curve (Figure 5A), the growth of tumor significantly slowed in three treated groups as compared with the controls, which were injected PBS in nude mouse model. Addition of Tregs could more risen the tumor volume than that of CIK-Tregdel cells alone group or with Tregs in the presence of anti-TGF-β1 blocking antibody group. However, there were no obviously difference in the tumor growth both CIK-Tregdel cells alone group and adding anti-TGF-β1 blocking antibody group. And similar phenomena were observed in survival periods of the mice. The mice of three treatment groups had obviously longer survival periods than PBS control group, the addition of Tregs to CIK-Tregdel cells significantly shortened survival periods of the mice compared with mice received CIK-Tregdel cells treatment alone or in the presence of anti-TGF-β1 blocking antibody (Figure 5B). Collectively, these results demonstrated that Tregs inhibited cytotoxic activity of CIK cells in vivo, which mediated primarily depended on TGF-β1.
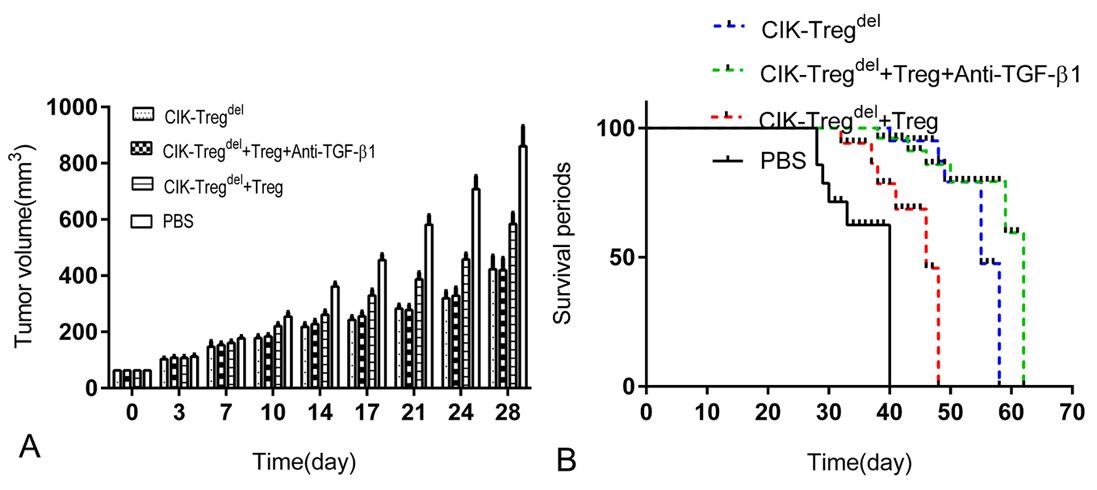 Figure 5.
Figure 5.
Role of TGF-β1 in Tregs–mediated CIK cells inhibition in vivo. (a) BABL/C nude mice were inoculated in dorsal subcutaneous with SGC-7901 gastric cancer cells. When the tumor diameter reached 5 mm, the mice were treated with different combinations of cells (CIK-Tregdel cells alone or with Tregs or with Tregs adding anti-TGF-β1 blocking antibody), PBS was taken as the control, The volume of tumor was measured every 3 days after the first cells transfer. (b) The average survival periods of mice were exhibited in CIK-Tregdel cells alone or with Tregs or with Tregs in present of anti-TGF-β1 blocking antibody or PBS control groups respectively. the survival periods in four groups were compared in the animal model. Six mice were used for a group and results represent four groups independent experiments.
CIK cells have been shown to be prime candidate for strong cytotoxic activity against a variety of target cells. However, Tregs gain suppressive function in CIK cell culture system and inhibit CIK-induced cytotoxicity to some extent. In this study, we focus on a strategy for effective improving the cytotoxic activity of CIK cells, and exploring the molecular mechanism whereby cord blood Tregs inhibiting CIK cells expanded from cord blood mononuclear cells.
Despite the fact that cord blood Tregs and the purest of adult-derived Tregs exhibit similar phenotype and function, cord blood Tregs own higher level of CD25+ high and lack CD25dim memory cells than adult peripheral blood that exist a large and wide spectrum CD25dim (28). Thus, it was easily purified. At present, the expression of CD127low on CD4+ CD25+ T cells has been described to be unambiguous surface marker distinguish between Tregs and the activated effector T cells (29). Freshly sorted cord blood CD4+ CD25+ T cells expressed CD127low so high that it was regarded as an actual natural Tregs, rather than the activated effector T cells. The freshly isolated cord blood Tregs are phenotypic immaturity and absent of inhibitory function (30). However, Tregs show abundant expansion, and obtain vigorous suppressive capacity by certain cytokines or antigen encounter (31). Particularly, depletion of Tregs can increase proliferation of CD3+ CD56+ cells and improve their cytotoxic potential (8). Our assays also appeared that not only the amounts of cord blood Tregs but also suppressive function ware concomitantly increased following CIK cell culture. CIK cells depleted Tregs preculture ameliorated cytotoxic activity. Applied Tregs to CIK-Tregdel cells could inhibit proliferation and cytotoxic potential of CIK cells, and the suppressive intensity was markedly associated with its frequencies. In other words, the higher Tregs/CIK-Tregdel cells ratio, the greater suppressive capacity of Tregs was found.
TGF-β is widely reported involved in Tregs-mediated CIK cytotoxity suppression, however the functional role is controversial (32). Some researches showed that suppressive function of CD4+ CD25+ Tregs was not necessarily to TGF-β (33). And another research groups proposed that CD4+ CD25+ Tregs might not completely reflect the Tregs capacity. Tregs included multiple subsets, among which the functions of each population were different. Studies revealed that CD4+ CD25+LAP+ Tregs possessed manifest potent suppressive ability compared to others (22, 34). Moreover, LAP+ Tregs principally represent the subset of activated Tregs (35).
LAP serve as a reliable marker to discriminate TGF-β+CD4+ CD25+ Tregs from other Tregs subsets. Our studies observed that freshly isolated cord blood CD4+ CD25+ Tregs was a discrete group that highly expressing CD127low but hardly expressing LAP+. However, after cytokines stimulation (during the preparation of CIK cells) in vitro, Tregs showed efficient proliferation with higher LAP+ expression on their surface, including CD4+ CD25+ LAP+ Tregs, CD4+ CD25+ LAP- Tregs and so on, which inhibited CIK cytotoxicity possibly via different mechanisms. Applied Tregs or soluble TGF-β1 could decline CIK cytotoxicity. Moreover, TGF-β1-blocking antibody could reverse the inhibitory capacity of Tregs on the proliferation as well as cytotoxicity of CIK cells. Previous studies also underscored that absence of TGF-β on cord blood Tregs failed to suppress effector cell functions (30). Similar results were also found in vivo. we established gastric cancer xenograft mouse model, the mice received CIK cells had significantly slower tumor growth compared with the untreated control, however additional Tregs accelerated tumor growth. Conversely, the inhibitory capacity of Tregs could be blunted by TGF-β blocking antibody. Based on our current study, we concluded that augmented LAP+ Tregs may partly contribute to the attenuated CIK cytotoxity via TGF-β.
CIK cells share several surface markers with NK cell, such as high levels of CD56 and NKG2D, and low level of NKp30, whereas NKp44, NKp46 and inhibitory receptors (KIR, NKG2A) do not express on the CIK cells (36). Antitumor activity of CIK cells is exerted by activating a variety of receptors, among which NKG2D has been considered to be a specific triggering receptor that result in cytotoxicity by interaction with signal transducing adapter protein DAP10 and IL-2 (37-40). NKP30 receptors are also involved in recognition of tumor target cells (1). Along with NCR and NKG2D, TRAIL-mediated caspase-3 activation pathway that provoke caspase-dependent target cell apoptosis by the interactions of TRAIL ligand and its receptors on target cells have been confirmed (41-43). Our experiment displayed that cord blood CIK cells expressed high NKG2D and TRAIL, low NKp30 and almost no NKp46 and NKp44, which are as published by Durrieu et al (26). Although multiple activating receptors are capable of endowing recognition and killing tumor cells, Tregs play suppressive functions only by down-regulated expression of NKG2D. Interestingly, soluble TGF-β engagement significantly descended expression of NKG2D receptor, whereas neutralizing TGF-β antibodies could attenuate Tregs–mediated inhibitory function on NKG2D. All these results indicated that TGF-β might facilitate Tregs suppressive function via affecting NKG2D receptor on CIK cells.
In conclusion, cord blood derived Tregs induced by the certain cytokines in CIK cell culture system exhibited potent suppressive characteristic to antitumor activity of CIK cells both in vitro and in vivo experiments. TGF-β was an important factor in Tregs-mediated suppressive function on CIK cells. The underlying mechanism may be that TGF-β participated into suppression function of Tregs by down-regulating the expression of NKG2D. These data illustrate that removing of Tregs or blockading the function of TGF-β in cord blood derived CIK may be a feasible approach to improve antitumor effect of CIK cells.
This work was supported by grants from the Shandong Province Natural Science Foundation of China (No. ZR2013HL054). The authors declare no conflicts of interest.
Abbreviations: Tregs: regulatory T cells; CIK: Cytokine-induced killer cells; NK: natural killer cells; IFN-γ: interferon-γ; IL-2: interleukin-2; LAP: latency-associated peptide; CBMCs: cord blood mononuclear cells; MACS: magnetic cell separation; 7-AAD: 7-amino actinomycin method; NCRs: natural cytotoxicity receptors; TRAIL: TNF-related apoptosis inducing ligand
