There is still no satisfactory large-animal model of ischemic heart failure (IHF) with ideal survival rate and model time. The aim of this study is to explore a novel chronic IHF model in swine. 23 healthy Ba-Ma miniature pigs were included. Pigs in the experimental group underwent multiple strategic ligations on side branches of the left anterior descending (LAD) and circumflex coronary arteries. One week later, sequential intervention occlusion of the distal end of the LAD trunk was performed. In the experimental groups, LV end-diastolic (LVEDV) and end-systolic volume (LVESV) gradually increased starting at 4 weeks post operation. At 12 WPO, LVEDV increased from 45.0 ± 2.9 ml at baseline to 110.0 ± 9.8 ml and LVESV increased from 17.0 ± 1.4 ml at baseline to 42.0 ± 3.6 ml. Meanwhile, left ventricular ejection fraction significantly decreased from 73.8 ± 4.2 % at baseline to 31.0 ± 2.5%. According to histomorphometric assessment, viable cells were observed in infarction lesions, indicating the model has replicated the structural and functional features of chronic IHF.
Heart failure (HF) is the most important factor in the high mortality of cardiovascular disease. Epidemiological studies reveal an annual worldwide incidence of 0.3–1.0% (1, 2). Though ischemic myocardial injury induced by coronary artery disease is the most common cause of heart failure, the underlying mechanisms have not been fully elucidated and remain the subject of intense research in the cardiovascular field. There is still no ideal method of reversing or delaying the disease process of heart failure. Thus, a robust, reliable model of chronic ischemic heart failure (IHF) is essential for any effort to explore new prevention and treatment strategies. It is also beneficial for improving the drug and device development process and speeding their translation.
Creating and reproducing a model of chronic IHF with high efficiency and low mortality has proven arduous. Although various animal models of chronic heart failure have been reported (3-14), prolonged modeling periods and high mortality rates commonly limit their application. While one method to create an animal model of chronic ischemic heart failure is by coronary (3-7) or pulmonary (8) microembolizations, another option is to ligate the coronary arteries. The earliest studies in a large-animal model of chronic ischemic cardiomyopathy by ligating coronary arteries with simple suture techniques were described by Harris et al. in the 1940s (15,16). The authors ligated the left anterior descending (LAD) coronary artery in dogs. Moainie et al. modified this model by ligating the first and second branches of the LAD coronary artery, which reliably created a moderate-sized myocardial infarction (9). However, the limitation in that model was that animals developed a region of complete akinesia on the anterior and lateral wall of the left ventricle (LV), which led to compensatory hyperkinesis of the posterior regions of the heart.
Informed and inspired by the previous methods, we decided to perform multiple, strategic coronary artery ligations over the LV combined with sequential LAD balloon occlusion to create a robust, replicable, reproducible, low death-rate model of chronic ischemic heart failure in miniature Ba-Ma pigs.
The study was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, revised 2011) (17) and was submitted to, and approved by Animal Welfare and Ethics Committee in Fuwai Hospital, Peking Union Medical College, Beijing, China (permission number of 2012-1-15-BKJ). 23 Male Ba-Ma miniature pigs (26.2 ± 2.5 kg, 6 months old) were obtained from the Beijing Paike biotechnology company. All animals received humane care, had free access to autoclaved water, and were fed twice a day. Thirteen pigs were used to establish chronic ischemic heart failure models and underwent multiple coronary ligations and sequential balloon occlusion, while 10 pigs served as sham-operation controls. In the sham operation group, the experimental animals received thoracotomy without ligation, and the interventional balloon did not block after one week. Other operations were consistent with the model group.
General anesthesia was intramuscularly administered with pentobarbital sodium (5%, 2 - 3 mL/kg, intramuscularly). Animals were endotracheally intubated and mechanically ventilated with oxygen (2 L/min). Anesthesia was then maintained with 1–2% isoflurane. A left lateral thoracotomy was performed through the fourth intercostal space, followed by a pericardiotomy. Six to eight ligations were performed transmurally on three of four segments of the LV: anterior, lateral, and posterior, 2 cm apart. Side branches of the left anterior descending and circumflex arteries were occluded by suture ligations to create multiple patchy areas of myocardial infarction. A chest tube was placed before the thoracotomy was closed in layers. One week after the ligation, sequential LAD balloon occlusion (near the distal end of LAD, nearly 1/3 for 40 minutes) as described in previous study (18). After the operation, fentanyl (100 μg, IM) was administered as an analgesic. Animals received magnesium (2 mg intravenously), amiodarone (1.5 mg/kg intravenously), and lidocaine (3 mg/kg intravenously) before the infarction was induced and an infusion of amiodarone (0.01 mg/kg/min) and lidocaine (2 mg/min) for 60 min afterwards. Animals also received buprenorphine (5 μg/kg intramuscularly every 12 h for the first two postoperative days) for pain control and cefazolin (4 mg/kg intramuscularly every 12 h for the first two postoperative days) for antibiotic prophylaxis. At the end of the study, euthanasia was performed through intramuscularly administrating ketamine (35 mg/kg) and diazepam (1.5 mg/kg), and intravenously injecting potassium chloride (30 ml).
X-ray angiography was performed on pigs at baseline as well as immediately after surgery (both coronary ligation and balloon occlusion) using standard transfemoral Judkins techniques (19). Before X-ray angiography, 10 ml iopromide (Clarograf; Juste, Madrid, Spain) was administered via the right and left coronary arteries. Cardiac coronary angiograms were obtained by dynamic scanning of the pig heart at a speed of 3 frames per second with an X-ray machine (Philips).
Venous blood samples were collected and placed in ice-chilled tubes coated with EDTA at baseline and in weeks 1, 4, 8, and 12. Plasma was separated for 15 minutes by 3000 rpm centrifugation at 4°C and stored at −80°C. Levels of NT-proBNP and CRP were measured by enzyme-linked immunosorbent assay (ELISA, ALPCO, Salem, NH, USA).
Electrocardiography was performed at baseline, continuously during the intervention (pre-, intra-, and postoperatively), and at the end of the experiment, 12 weeks after coronary ligation. Electrocardiograms were recorded as previously described (14-17). Three standard bipolar limb lead (I, II, III) electrodes were placed on the cubital and stifle joints of the pig. For chest leads, V1 was located at the right edge of the sternum in the fourth intercostal space; while V2 was located at the left sternal border in the fourth intercostal space. V2 was also located at the midpoint of the connection of V3 and V4. V4 was located at the intersection of the left midclavicle line and fifth intercostal space. V5 was found at the left anterior axillary line and at the same level as V4. V6 was located at the left midaxillary line at the same level as V4. The paper speed was 25 mm/sec and sensitivity was 10 mm/mV.
Hemodynamic data were analyzed at baseline and 12 weeks after the operation. Mini-pigs were anesthetized as above, and the left femoral artery was used for catheterization. Two pigtail catheters were introduced into the LV and the ascending aorta and connected to mercury-calibrated water-filled transducers. A multilumen thermodilution catheter was inserted into the left jugular vein and positioned in the pulmonary artery to measure cardiac output as well as pulmonary artery and pulmonary capillary wedge pressures. A 25-mm balloon catheter was introduced into the inferior vena cava through the same vein to monitor LV volume and pressure by inflation and deflation during ventriculography. After a 15-min equilibration, a second ventriculogram was taken and synchronized with balloon deflation so that beat-by-beat increases in both LV pressure and volume were recorded simultaneously (13). The pressure-volume data produced from these alterations were used to construct the index of LV contractile function. Heart rate, mean aortic pressure, pulmonary capillary wedge pressure (PCWP), dp/dt max, and cardiac output were assessed.
Trans-thoracic 2-dimensional echocardiography was performed at baseline, and during weeks 1, 4, 8, and 12 using a 2.5 - MHz transducer coupled to Philips Sonos 7500 echocardiographic machine (Philips Medical Systems, Andover, MA). Standard 2-dimensional short and long parasternal views, as well as 4-chamber, 2-chamber, and 3-chamber apical views were obtained via standard procedure. LV end-diastolic volume (LVEDV), end-systolic volume (LVESV), and ejection fraction (EF) were measured three times using Simpson’s biplane method. The average was used for final data processing. Echocardiograms were interpreted by two cardiologists blinded to the animal groups.
Serial cardiac MRI was performed in all surviving animals 12 weeks after multiple coronary ligation and balloon occlusion to confirm the development of chronic heart failure. All surviving animals were studied in a 3.0 - T CMR system (Signa CV/i, GE Healthcare, Waukesha, WI, USA) in lateral decubitus position under general anesthesia and proper ventilation with an eight-element phased-array surface coil. Typical cardiac MRI study included cine steady-state free precession imaging. Peripheral pulse gating and breath holding were used as much as possible to minimize cardiac and respiratory motion, respectively. When breath holding was not successfully achieved, a free-breathing technique was applied using the multiple-excitations-per-cycle approach associated with reduced views per segment. Cine imaging was obtained in 8-14 matching short-axis (8 mm thick with 0 mm spacing) and three radial long-axis planes.
At week 12, animals were humanely euthanized under deep anesthesia and the relevant cardiac tissues were dissected and stored in 10% formalin for histological examination. Myocyte cross-sectional areas of paraffin-embedded tissue samples were cut at 5 μm thickness and stained with hematoxylin and eosin (H&E) or masson trichromic staining as previously described (21-23).
Electron microscopy was performed using a protocol previously described (24, 25). Tissue blocks (1 mm3) were fixed for 2 h with 2.5% glutaraldehyde in 100 mM sodium cacodylate (pH 7.4) and then post-fixed in 1% osmium tetroxide. After dehydration in increasing concentrations (30, 50, 70 95, and 100%) of ethanol (10 min for each step), infiltration proceeded overnight with 1:1 ethanol and LX112 Epon resin. Eighty nanometer sections were obtained using a Leica Ultracut E ultramicrotome, and contrasted with 2% uranyl acetate for 10 min and lead citrate for 5 min. Observations were performed on a JEOL 1400 TEM (JEOL, Tokyo, Japan) equipped with a side mount Gatan Orius SC1000 digital camera (Gatan, Munich, Germany).
The TUNEL assay was performed using a commercially available in situ apoptosis detection kit (No.11684795910, Roche Molecular Biochemicals, Mannheim, Germany) in accordance with the manufacturer’s protocol, as described previously (21). Samples were fixed with 4% paraformaldehyde solution for 30 min at room temperature. After a rinse with PBS, cells were treated with permeation solution (0.1% Triton X - 100 in 0.1% sodium citrate) for 2 min at 4°C. After washing with PBS, samples were incubated with TUNEL reagent for 30 min. The excitation wavelength was in the range of 450–500 nm and the detection wavelength was in the range of 515–565 nm.
Data are expressed as mean ± SEM. Both two-sided one-way ANOVA and two-sided Holm-Sidak post hoc t-test were performed using SPSS 16.0 statistical software. Proper and necessary data transformations (logarithmic or square root) were applied for non-normal distributed data prior to statistical analysis. P value < 0.05 was considered to be statistically significant.
Thirteen minipigs were used to establish models. The overall mortality rate was 7.4% (1/13 animals) (Figure 1A), reflecting the death of one pig in the experimental group during tracheal intubation. Two animals experienced brief ventricular fibrillation after ligation and were rescued to sinus rhythm after defibrillation. All other pigs (12/13) survived to study endpoint (3 months). All surviving animals developed clinical signs of heart failure as indicated by increased heart rate (72 ± 5 beats per minute (preoperatively) to 102 ± 4 beats per minute (12 weeks) (P < 0.05) and increased respiratory rate (25 ± 3 (preoperatively) to 42 ± 6 (12 weeks) (P < 0.05)) (all heart-failure pigs developed bilateral pulmonary rales).
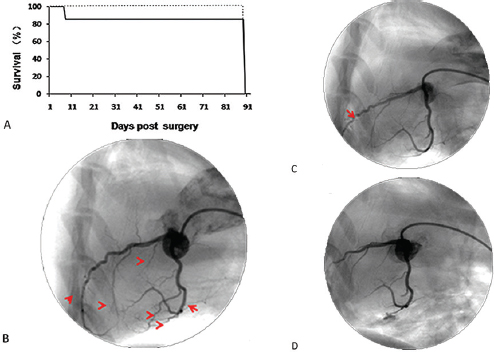 Figure 1
Figure 1Survival rate and coronary angiography of BA-MA mini-pigs at different time points post surgery. (A) The solid line is for the experimental group and the dotted line is for the sham group. Coronary angiography in the experimental pigs was performed before ligation (B), post ligation (C), and post secondary occlusion (D). Arrows in B indicate ligation sites; the arrow in C indicates occlusion site. Experimental group (n = 12); Sham group (n = 10).
Preoperative coronary angiograms showed that all coronary arteries in the experimental animals were normal (Figure 1B). After ligating second-grade vasculars (Figure 1 C) and subsequent LAD occlusion (Figure 1 D), coronary angiograms were performed to observe changes in coronary vascular blood compared with pre-operation. The blood flows in all target vessels were truncated.
Plasma NT - proBNP and CRP concentrations were measured in sera of pigs at baseline as well as 1, 2, 4, 8, and 12 weeks post-surgery. There was a peak (1210 ± 98 pg/L) in NT - proBNP levels at 8 weeks after occlusion. Plasma CRP level also gradually increased beginning 4 weeks post occlusion, and up to the peak value of 28.2 ± 2.2 mg/L at week 8 post occlusion, suggesting that ischemic injury was serious and that decompensation in systolic and diastolic function had occurred (Figure 2).
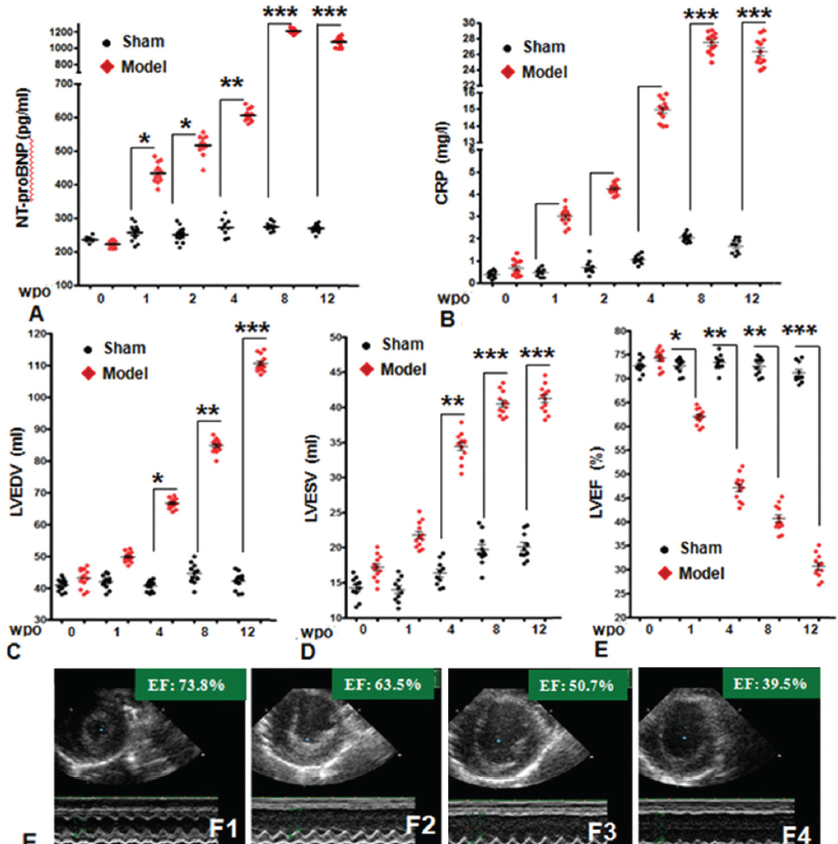 Figure 2
Figure 2The detection of plasma NT - proBNP & C - reactive protein levels and echocardiography assessment were performed at 0 (baseline) and 2, 4, 8, and 12 weeks post surgery. Red lines represent sham group (n = 10). According to echo, LVEDV (C) and LVESV (D) gradually increased beginning 4 WPO; meanwhile, LVEF (E) decreased significantly at 12 WPO in all experimental pigs. (F) The representative echo images of Pig 429 in experimental group at 0 WPO (F1), 4 WPO (F2), 8 WPO (F3) and 12 WPO (F4). LV = left ventricle/ventricular; EDV = end- diastolic volume; ESV = end - systolic volume; WPO = weeks post operation. n = 12 in experimental group; n = 10 in sham group. Data are represented as mean± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, versus baseline.
To detect structural and functional changes in Ba-Ma hearts after the experimental operation, 2D echocardiography was employed to detect abnormal movement of ischemic myocardium. The results showed large areas of abnormal movement in anterior and lateral walls 4 weeks post-surgery. In experimental pigs, LVEDV and LVESV gradually increased beginning 4 WPO. At 12 WPO, LVEDV and LVDSV were significantly increased compared to baseline values; LVEDV increased from a baseline 45 ± 2.9 ml to 110 ± 9.8 ml (P < 0.05) and LVESV increased from baseline 17 ± 1.4 ml to 42 ± 3.6 ml (P < 0.05). Meanwhile, the average EF in experimental animals significantly decreased from baseline 73.8 ± 4.2 % to 31 ± 2.5 (P < 0.05), indicating significant structural and functional remodeling in the left ventricle.
Twelve of thirteen minipigs survived the 12-week experimental period. Hemodynamic baseline data are presented in Table 1 and compared with postoperative data (12 WPO). Briefly, there was a significant difference between baseline and 12 WPO data between the control and experimental groups. At 12 WPO, the PCWP was significantly higher in the experimental group compared with controls (Control 5.8 ± 0.6 mm Hg vs. experimental 19.6 ± 1.2 mm Hg, P < 0.05). There was no significant difference in heart rate (HR) and mean aortic pressure (mAOP) between the groups. Additionally, dP/dt max decreased from 2380 ± 223 mm Hg/s at baseline to 982 ± 178 mm Hg/s (P <0.05). Cardiac output (CO) also decreased significantly compared with control group (Control 6.5 ± 0.6 L vs. experimental 4.1 ± 0.5 L).

To further confirm the data on structural and functional changes in LV obtained from echocardiography, MRI and histological assessment were employed. Cardiac MRI was performed in pig in sham group (Figure 3 A and B) and model group at 12 WPO (Figure 3 C and D). By this point all animals had developed heart failure, defined as an LV ejection fraction 35% and a 100% increase in LV volume at end-diastole. MRI images in short-axis views showing a significant increase in LV volume and a reduction in the thickness of the left ventricular free wall.
 Figure 3
Figure 3MRI and histological assessment of the myocardium was performed in experimental pigs in sham control group (A and B) and model group (C and D). H&E analysis (D1) and Masson trichrome staining (D2) of infarcted lesion from experimental pigs revealed left ventricle fibrosis. Transmission electron microscopy (D3) analysis revealed contraction bands and myocyte necrosis. TUNEL staining (D4) showed apoptotic myocytes in the ischemic area (40 x). The colored circles in Figure 3A and 3C represent the border of the myocardium. The part between the endocardium and the epicardium is the left ventricular wall. The red rectangle in Figure 3B and 3D represent the sampling sites. Images represent 3-6 experiments. E. Comparison of myocardiocyte viability between sham and experimental groups. n = 12 in experimental group; n = 10 in sham control group. Data are represented as mean± SEM.
Histological assessment was performed at 12 WPO. As shown in Figure 3 D, the ventricular anterior, lateral, and posterior walls in model pigs had obviously thinned, which is consistent with the results obtained from Echo and MRI. The infarcted lesions occupied 35 ± 4.8% of the overall LV volume. H&E (Figure 3 D1) and Masson trichrome staining (Figure 3 D2) revealed that chronic ischemia caused the cardiac pathological changes, including myocyte necrosis and tissue fibrosis within the ischemic central area of the left ventricle. Transmission electron microscopy (Figure 3 D3) and TUNEL staining (Figure 3 D4) further demonstrated the presence of contraction bands and dead myocytes. However, all of the above-mentioned techniques also indicated that the remaining regions of heart muscle contained intact nuclei and uniform cytoplasmic staining. The myocardial cell viability of the model group was significantly reduced, compared with the control group (Experimental 25 ± 4% vs. sham 95 ± 3%) (Figure 3 E).
Almost all research on new drugs or therapies for requires animal models. Here we successfully established a novel swine model of chronic ischemic heart failure induced by multiple coronary ligations and sequential balloon occlusion. Compared with previous studies, this large-animal model has a higher survival rate and faster progression to heart failure. Nearly all pigs (92.6%) survived the entire experimental process (12 weeks) and 100% of the surviving animals demonstrated heart failure at week 12, revealed by echocardiography and cardiac MRI.
The approach used in the present study differs from previous attempts (9, 15, 16). Experimental animal models of chronic ischemic heart failure have been established (2) using different approaches such as coronary microembolizations (3-7) and single coronary ligations (10). Although coronary microembolization models are well established and useful (3-7), their limitations include instability in embolism position and thrombus autolyzation. To create animal models with more-rapid heart dysfunction, another option is to perform ligation closer to the coronary artery trunk. Early in the 1940s, Harris et al. established chronic ischemic heart failure using suture ligation of the coronary arteries (15, 16). However, this significantly increased mortality despite prophylactic administration of antiarrhythmic agents, greatly hindering its popularization and application. Later, Smittho et al (26) successfully tested multiple ligations of coronary branches in sheep obtaining a limited mortality and significantly reduced ejection fraction (from 60 % to 28 %). In the present study, we further optimized the multipoint ligation method by performing transmural ligations of secondary vessels distributed throughout the LV to produce a robust degree of myocardial infarction and sequential occlusion of LAD. The adoption of a two-stage technique reduced the high incidence of ventricular fibrillation. These changes allowed us to improve the survival rate and reproducibility of chronic ischemic heart failure models in swine.
Assessment of the structural features of model hearts including electrocardiography, echocardiography, MRI, and histological analysis consistently indicated that all experimental animals suffered extensive myocardial infarction in anterior, lateral, and posterior walls of LV; the infarct occupied approximately a third of the entire LV; the volume of LV typically continued to increase; uniformity and reproducibility were acceptable.
EF is an important functional measurement to determine how well a heart is pumping and is also used to classify heart failure and guide treatment. In a healthy heart, the ejection fraction is 50 percent or higher, meaning that more than half of the blood that fills the ventricle is pumped out with each beat (27-29). The present study presents a clinically relevant model of ischemia-induced heart failure. Echocardiography and MRI data indicated heart EF < 50% in 75% of model pigs after 8 weeks and in all animals after 12 weeks post surgery. We observed clinical signs of chronic heart failure including tachycardia, tachypnea, and weight gain. An important feature of the present heart-failure model is the lack of LV function recovery, i.e., cardiac changes were irreversible.
Although heart function was significantly decreased, some viable myocardium was still detected in infarction lesions—another characteristic of human chronic ischemic cardiomyopathy, according to HE staining, masson staining, transmission electron microscopy, and TUNEL staining (30-32).
The present model also demonstrates several limitations. Ligations are performed via open chest surgery, which might produce postoperative adhesions. Because of a small number of animals involved, future research should involve more animals, in order to enrich the basic pathophysiological data and meet the requirements of different types of research.
We have developed a novel, robust, promising model of chronic ischemic heart failure with high survival rate and long treatment windows, using multiple ligations and sequential LAD occlusion. Our proposed model is highly effective and reproducible, providing the potential use in future experimental heart-failure research, such as cardiac assist-device implantation and heart transplant.
This work was supported by the Beijing Science and Technology Committee (grant number: Z161100005016014, Z101107052210004) and Beijing Key Laboratory of Preclinical Research and Evaluation of Cardiovascular Implant Materials (grant number: 2018-PT2-ZR04). The sponsor didn’t involve in study performance or paper preparation. All authors have read the journal’s authorship statement.
Abbeviations: IHF: Ischemic Heart Failure; LAD: Left Anterior Descending; LVEDV: Left Ventricular End-Diastolic Volume; LVESV: Left Ventricular End-systolic Volume; EF: Ejection Fraction; HR: Heart rate; mAOP: mean Aortic Pressure; CO: Cardiac Output; PCWP: Pulmonary Capillary Wedge Pressure
