Pulmonary arterial hypertension (PAH) is a syndrome caused by restricted blood flow in the pulmonary circulation, which results in a poor patient prognosis. The serotonin (5-HT), TRPC1 (Transient receptor potencial channel 1), TRPC6 (Transient receptor potencial channel 6), calcineurin A, and NFATc3 (an isoform of nuclear factor of activated T-cells family) are involved in cell proliferation and hypertrophy and the crosstalk between these molecules may play an essential role in the pathogenesis of pulmonary arterial hypertension. We hypothesized that 5-HT promotes PAH by affecting TRPC channels. We investigated the effects of sarpogrelate, a 5-HT2A receptor antagonist, on pulmonary arterial pressure, cardiac remodeling, pulmonary artery remodeling, and TRPC1, TRPC6, calcineurin A, and NFATc3 expression in pulmonary arteries from rats with PAH. The results showed that sarpogrelate reduced pulmonary arterial pressure, cardiac remodeling, pulmonary artery remodeling, and expression of TRPC1, TRPC6, calcineurin A, and NFATc3 in pulmonary arteries. In conclusion, Sarpogrelate reduced the severity of PAH in rat model and decreased the expression of TRPC1, TRPC6, calcineurin A, and NFATc3 in pulmonary arteries.
Pulmonary arterial hypertension (PAH) is a syndrome caused by the blockage of blood flow in the pulmonary circulation, resulting in increased pulmonary vascular resistance, and eventually leading to right heart failure (1,2). Despite the use of modern treatments, the prognosis for PAH is poor, with an approximate mortality rate of 15% within 1 year (3) and about 50% within 3 years (4). The causes of PAH are complex and are due to many factors. It is now well accepted that an imbalance between vasoconstriction and vasorelaxation plays a significant role in PAH. Changes in the substances that regulate vasoconstriction and vasorelaxation, such as NO and endothelin, have been found in PAH and may be related to the development of PAH (2,4).
Serotonin (5-HT) is an important neurotransmitter and vasoactive substance involved in the regulation of cardiovascular physiology, such as vasoconstriction and vascular remodeling (2). 5-HT enters the cells by binding to 5-HT receptors and then to 5-HT transporters. There is a variety of 5-HT receptor subtypes, among which 5-HT2A receptors are closely related to the cardiovascular system. Increased levels of plasma 5-HT, as well as increases in pulmonary artery 5-HT receptors and 5-HT transporter expression often accompany PAH (5-8). However, the role of 5-HT in the pathogenesis and progression of PAH remains unclear.
TRPC channels are involved in the regulation of intracellular calcium homeostasis, and the opening of these channels leads to a continued increase in intracellular calcium levels. Calcium is an essential second messenger within the cell that causes cell proliferation and vasoconstriction (9). Studies of pulmonary arteries in patients with idiopathic PAH and animal models of PAH have shown that TRPC channel expression is increased (10) and that 5-HT increases intracellular calcium as well (11). However, whether 5-HT and TRPC channels interact during the development or pathogenesis of PAH has not yet been elucidated.
Therefore, we tested the hypothesis that 5-HT promotes PAH by affecting TRPC channels. A rat model of PAH was established using monocrotaline, and the effects of the 5-HT2A receptor antagonist, sarpogrelate was tested on these rats, and TRPC expression and its downstream signaling pathways were examined.
Four-week-old male Sprague-Dawley rats, weighing between 150 g and 200 g, were purchased from the Experimental Animal Center of Medicine School of Xi‘an Jiaotong University.
A BL-420E biometric signal acquisition system (Chengdu Thai Union Company), HX-200 animal respirator (Chengdu Thai Union Company), high-speed low-temperature centrifuge (Germany Heraeus Company), iQ5 fluorescence quantitative PCR detection system (Bio-Rad), and gel Imaging Analysis System ChemiDocTM (Bio-Rad) were used in the present investigation.
Monocrotaline (Sigma), sarpogrelate (Mitsubishi), TRPC1 antibody (Alomone, ACC-010), TRPC6 antibody (Alomone, ACC-017), Calcineurin A antibody (Santa Cruz, sc-9070), NFATc3 antibody (Santa Cruz, sc-8321), GAPDH monoclonal antibody (Epitomics, 2251-1), and HRP-labeled goat anti-rabbit polyclonal antibody (Abcam, ab6721) were used in the present investigation.
Rats were randomly divided into 3 groups: a control group, a monocrotaline-induced PAH model group (MCT group) and a 5-HT2A receptor antagonist sarpogrelate intervention group (sarpogrelate group). For the control group an intraperitoneal injection of saline was performed along with intragastric administration of saline once daily. In the MCT group, intraperitoneal injection of monocrotaline (50 mg/kg) (12) along with intragastric administration of saline once daily was performed. While in the sarpogrelate group, an intraperitoneal injection of monocrotaline along with intragastric administration of sarpogrelate (100 mg/kg) was provided once daily. Pulmonary arterial pressure measurements and samples were taken after 4 and 8 weeks of the interventions.
Rats were anesthetized via intraperitoneal injection of 10% chloral hydrate. Rats were then placed in a supine position, trachectomized, and intubated. The intra-tracheal tube was connected to a small animal ventilator (tidal volume 4–6 mL, respiratory rate 60 breaths/min, and inspiratory-to-expiratory ratio 4:5). One end of a catheter was then placed into the pulmonary artery and the other end connected to a tension transducer. The pressure signal was delivered to a BL-420E biological signal acquisition system. The mean pulmonary arterial pressure was calculated using the BL-420E system.
The rats were then euthanized by a chloral hydrate overdose. After removal of the heart and lung tissues, the pulmonary artery was dissected, and the lung tissues were frozen in liquid nitrogen. The right ventricle and the left ventricle with septum were harvested and weighed separately. Peripheral sections of the lung tissue were cut for routine HE staining.
Trizol was used to extract the total RNA from the tissues and reverse transcription polymerase chain reaction (RT-PCR) was used to detect TRPC1, TRPC6, calcineurin A, and NFATc3 expression in the pulmonary arteries.
About 50 mg of pulmonary artery tissue was used for protein extraction, and the proteins were preserved at −80°C. The proteins were separated using SDS polyacrylamide gel electrophoresis and subsequently transferred to a nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% skim milk powder and shaken slowly for 1 h. The membrane was then incubated overnight with a primary antibody (against TRPC1, TRPC6, calcineurin A, NFATc3, or GAPDH). After washing with Tris-buffered saline Tween (TBST), a secondary antibody was added, and the membrane was incubated at room temperature for 1 h. The membrane was washed again with TBST and chemiluminescence was used for imaging in a gel imaging system. Quantity One software was then used for image analysis.
Results are expressed as a mean ± standard deviation. SPSS 13.0. software was used for statistical analysis, and an analysis of variance was used to compare the groups. P <0.0.5 was considered statistically significant. Graphs were prepared using GraphPad Prism 5.
The rat model of PAH was established by intraperitoneal injection of monocrotaline, and the pulmonary arterial pressure was measured to confirm successful modeling. As shown in Figure 1, the pulmonary arterial pressure in the PAH group was significantly higher at 4 and 8 weeks when compared with the control group (P<0.0.5). The sarpogrelate group had a lower pulmonary arterial pressure at 4 and 8 weeks compared with the PAH group (P <0.0.5). The pulmonary arterial pressure in the control group at 8 weeks was higher than that at 4 weeks (P<0.0.5). This change was also observed in the PAH group and may be associated with aging. The pulmonary arterial pressure was not significantly different between the 4 week and 8 week sarpogrelate groups.
 Figure 1.
Figure 1.Effects of sarpogrelate on the pulmonary arterial pressure in rats with pulmonary arterial hypertension (PAH). Sarpogrelate decreased pulmonary arterial pressure at 4 and 8 weeks compared with the PAH group. pulmonary arterial pressure of control group and MCT group increased at 8 weeks compared with at 4 weeks. Control group; MCT group: PAH group; sarpogrelate group: intervention group. * P<0.0.5, vs Control group at 4 weeks; # P <0.0.5, vs. MCT group at 4 weeks; ** P<0.0.5, vs Control group at 8 weeks; ## P <0.0.5, vs. MCT group at 8 weeks; a P <0.0.5, vs. 4-week, n = 6.
Cardiac remodeling was observed in the transverse section of the heart. The right cardiac index was calculated by dividing the right ventricular weight by the sum of the left ventricular and septum weights. Figure 2 shows the cross-section of the heart. The cross-section of the heart was morphologically normal and right ventricle was not enlarged at 4 or 8 weeks in the control group. The right ventricular cavity was enlarged, the left ventricular septum shifted left, the right ventricular free wall thickened, and the left ventricular cavity was relatively small at 4 and 8 weeks in the PAH group. After the sarpogrelate intervention, the cross-section of the heart had a reduced right ventricular cavity, the right ventricular wall was not thickened, and the ventricular septum was not shifted at 4 or 8 weeks. Then the right cardiac index was calculated as RV / (LV + S), namely, the right ventricular weight / (left ventricular weight + septum weight). Figure 3 shows the right cardiac index calculation results: The PAH group had a significantly increased right cardiac index at 4 and 8 weeks compared with the control group (P<0.0.5). There was no significant difference in the right cardiac index at 4 weeks in the sarpogrelate compared with PAH group (P> 0.0.5). However the right cardiac index was significantly lower at 8 weeks in the sarpogrelate group compared with the PAH group (P <0.0.5).
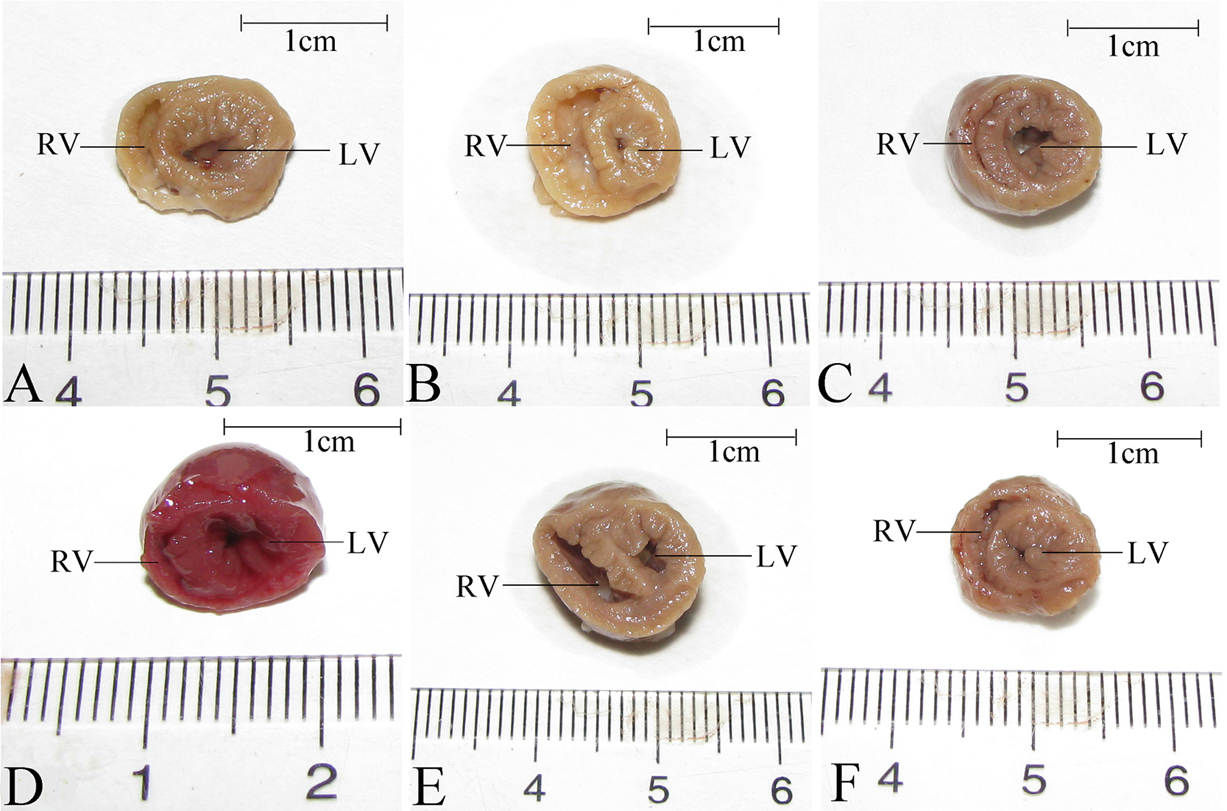 Figure 2.
Figure 2.Effects of sarpogrelate on cardiac remodeling in PAH rats. Sarpogrelate intervention reduced right ventricular remodeling at 4 and 8 weeks. A, B, and C are cardiac cross sections of control, MCT, and sarpogrelate groups, respectively at 4 weeks; D, E, and F are cardiac cross sections of control, MCT, and sarpogrelate groups, respectively at 8 weeks. Control group; MCT group: PAH group; sarpogrelate group: intervention group. RV: right ventricle; LV: left ventricle, n = 6.
 Figure 3.
Figure 3.Effects of sarpogrelate on the right cardiac index in PAH rats. Sarpogrelate reduced the right cardiac index in rats with PAH at 8 weeks. The right cardiac index was calculated as RV / (LV + S), namely, the right ventricular weight / (left ventricular weight + septum weight). Control group; MCT group: PAH group; sarpogrelate group: intervention group; RV: right ventricle; LV: left ventricle; S: septum. *P<0.0.5, vs control group at 4 weeks; **P<0.0.5, vs control group at 8 weeks; ## P <0.0.5, vs. MCT group at 8 weeks; n = 6.
Sections of the peripheral lungs of the rats were subjected to HE staining to observe pulmonary artery remodeling. Figure 4 shows a cross-section of the pulmonary artery of peripheral lung tissues after HE-staining. Figure 5 shows assessment of pulmonary artery wall thickness, which was calculated by multiplying the pulmonary media thickness by 2 and dividing that number by the outer diameter. The results showed that the pulmonary artery wall of the PAH group was significantly thicker at 4 and 8 weeks when compared with the control group (P <0.0.5). Pulmonary artery wall thickness was significantly lower after 4 and 8 weeks in the sarpogrelate compared with the PAH group, (P <0.0.5). Pulmonary artery wall thickness in the PAH group was significantly higher at 8 weeks than at 4 weeks (P <0.0.5). However, there was no significant difference in pulmonary artery wall thickness between the 4th and 8th week in the control or sarpogrelate groups.
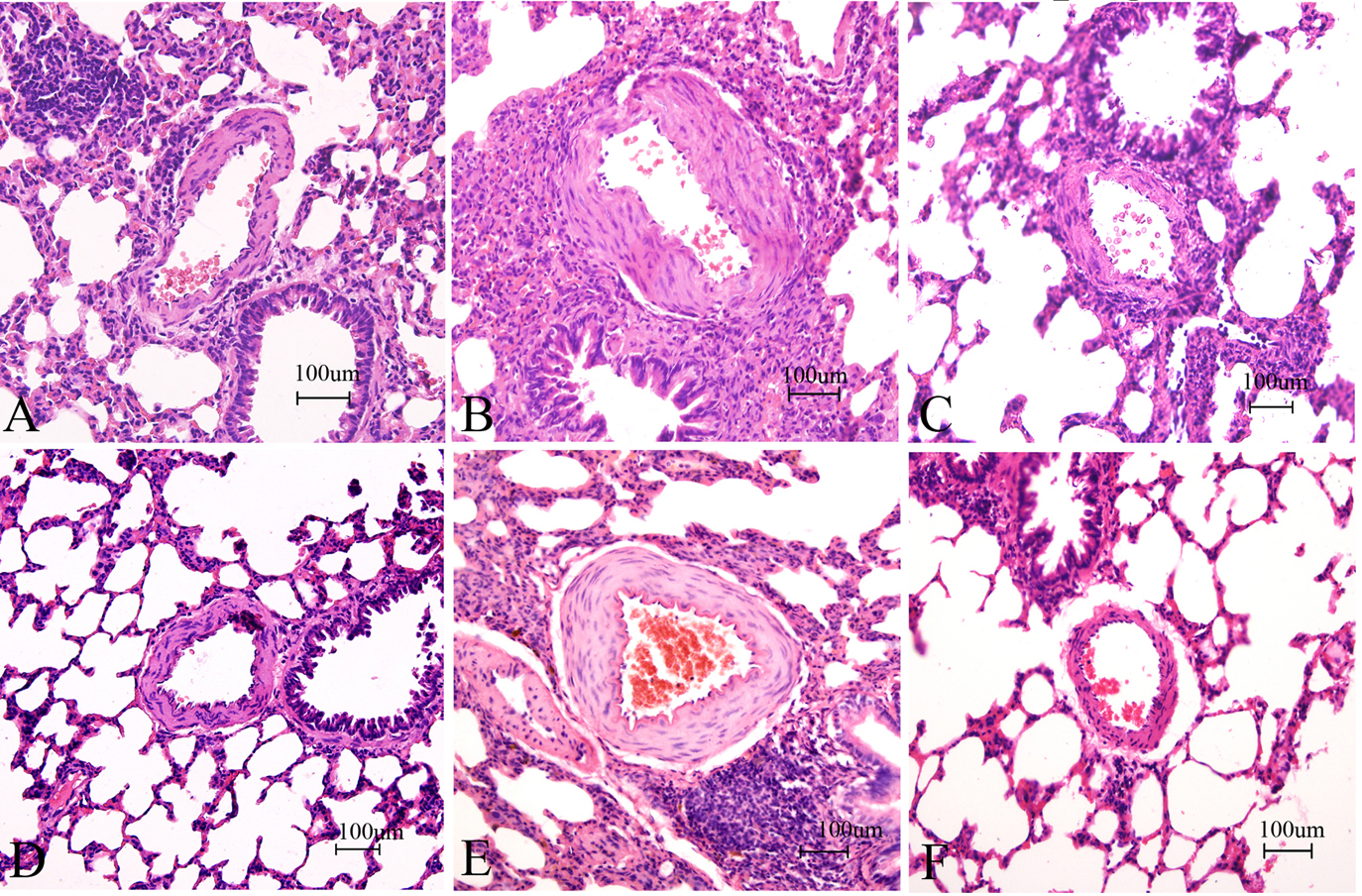 Figure 4.
Figure 4.Effects of sarpogrelate on pulmonary artery remodeling in PAH rats (HE Staining of Lung Tissues, 20×). Sarpogrelate reduced the extent of pulmonary artery wall thickening at 4 and 8 weeks. A, B, and C are the control, MCT and sarpogrelate groups, respectively, HE-stained at 4 weeks. D, E, and F are the control, MCT, and sarpogrelate groups, respectively, HE-stained at 8 weeks. Control group; MCT group: PAH group; Sarpogrelate group: intervention group, n = 6.
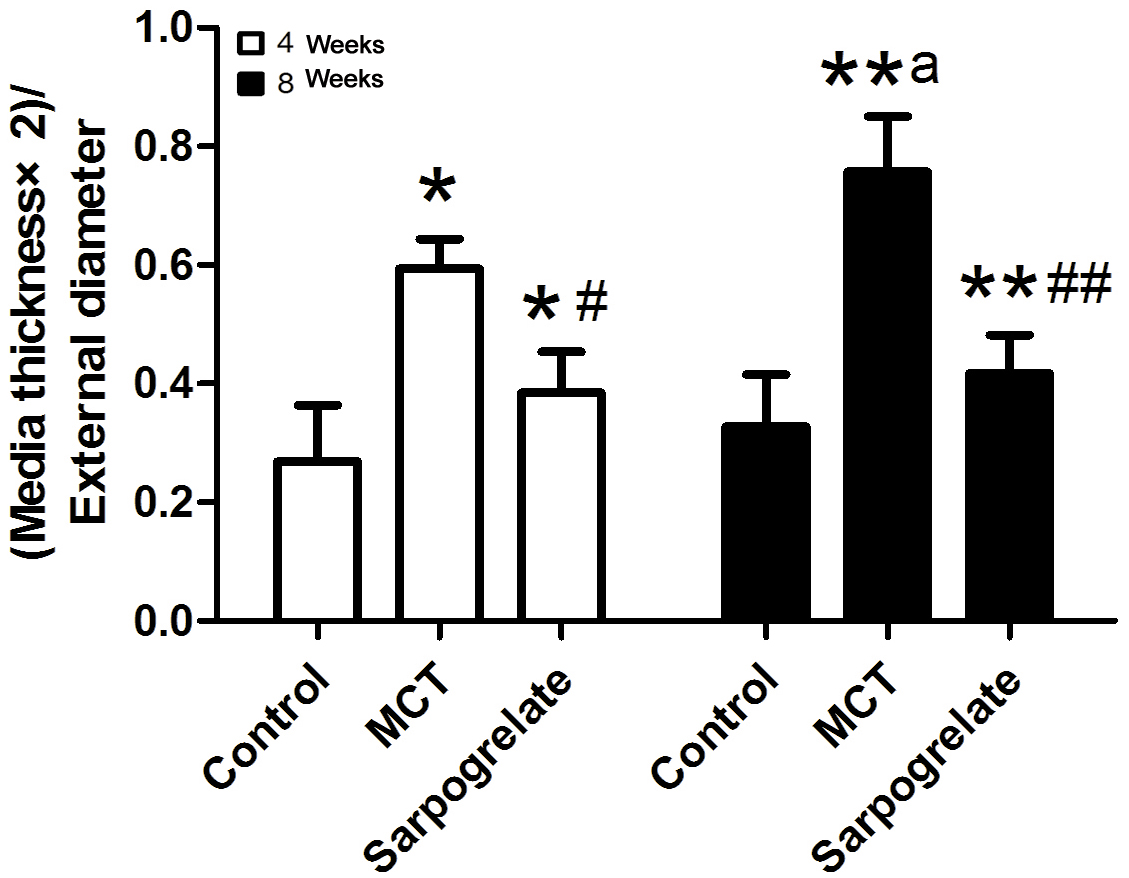 Figure 5.
Figure 5.Effects of sarpogrelate on pulmonary artery remodeling in PAH rats. Sarpogrelate reduced pulmonary artery remodeling in rats with PAH. Pulmonary artery wall thickness in the PAH group was significantly higher at 8 weeks than at 4 weeks. The degree of pulmonary artery remodeling was evaluated as (Media thickness × 2) / External diameter. Control group; MCT group: PAH group; sarpogrelate group: intervention group. * P <0.0.5, vs. Control group at 4 weeks; # P <0.0.5, vs. MCT group at 4 weeks; **P<0.0.5, vs control group at 8 weeks; ## P <0.0.5, vs. MCT group at 8 weeks; a P <0.0.5, vs. 4-week; n = 6.
TRPC channels regulate intracellular calcium levels, which are associated with cell proliferation. Calcium ions bind to calcineurin subunits for calcineurin activation and then promote NFAT phosphorylation and NFAT translocation to the nucleus. Within the nucleus, NFAT binds to DNA motifs, thus promoting transcription and cell proliferation. We then investigated the effects of sarpogrelate on the mRNA expression of TRPC1, TRPC6, calcineurin A, and NFATc3 in pulmonary arteries of rats with PAH. After 4 weeks of the sarpogrelate intervention, rat pulmonary artery mRNA expression was analyzed as shown in Figure 6. The mRNA expression of TRPC1, TRPC6, calcineurin A, and NFATc3 was higher in the PAH compared to the control group (P <0.0.5). TRPC1, TRPC6, calcineurin A, and NFATc3 mRNA expression levels were lower in the sarpogrelate group when compared to the PAH group (P <0.0.5). Similarly, protein expressions in rat pulmonary arteries were analyzed after 4 weeks of the sarpogrelate intervention. As shown in Figures 7, the protein expressions of TRPC1, TRPC6, calcineurin A, and NFATc3 were significantly higher in PAH when compared to the control group (P <0.0.5). The expressions of TRPC1, TRPC6, calcineurin A, and NFATc3 was significantly lower after sarpogrelate treatments compared to the PAH group (P <0.0.5). These results suggest that sarpogrelate, a 5-HT2A receptor antagonist, attenuated pulmonary artery modulation of TRPC1, TRPC6, calcineurin A, and NFATc3 in PAH rats.
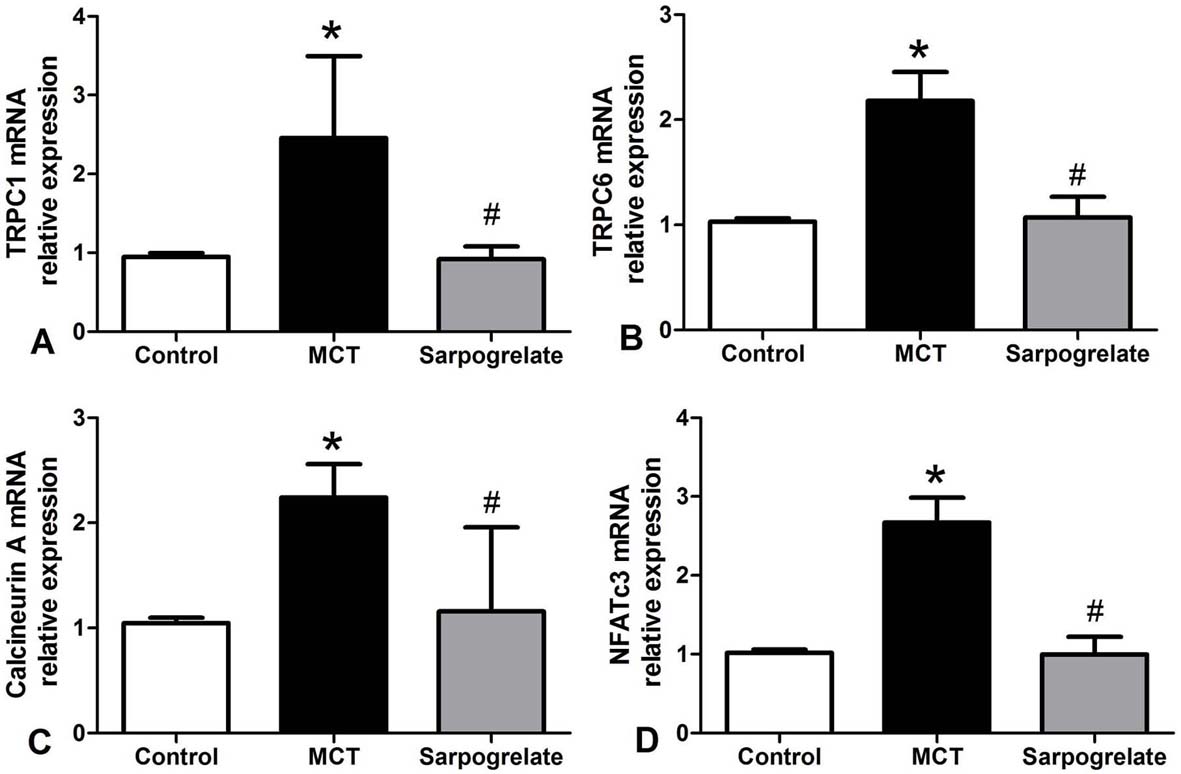 Figure 6.
Figure 6.Effects of sarpogrelate on pulmonary artery mRNA expressions in PAH rats. Sarpogrelate reduced TRPC1 (A), TRPC6 (B), calcineurin A (C), and NFATc3 (D) mRNA expressions in pulmonary arteries of rats with PAH. Control group; MCT group: PAH group; Sarpogrelate group: intervention group. * P <0.0.5, vs. Control group; # P <0.0.5, vs. MCT group, n = 6.
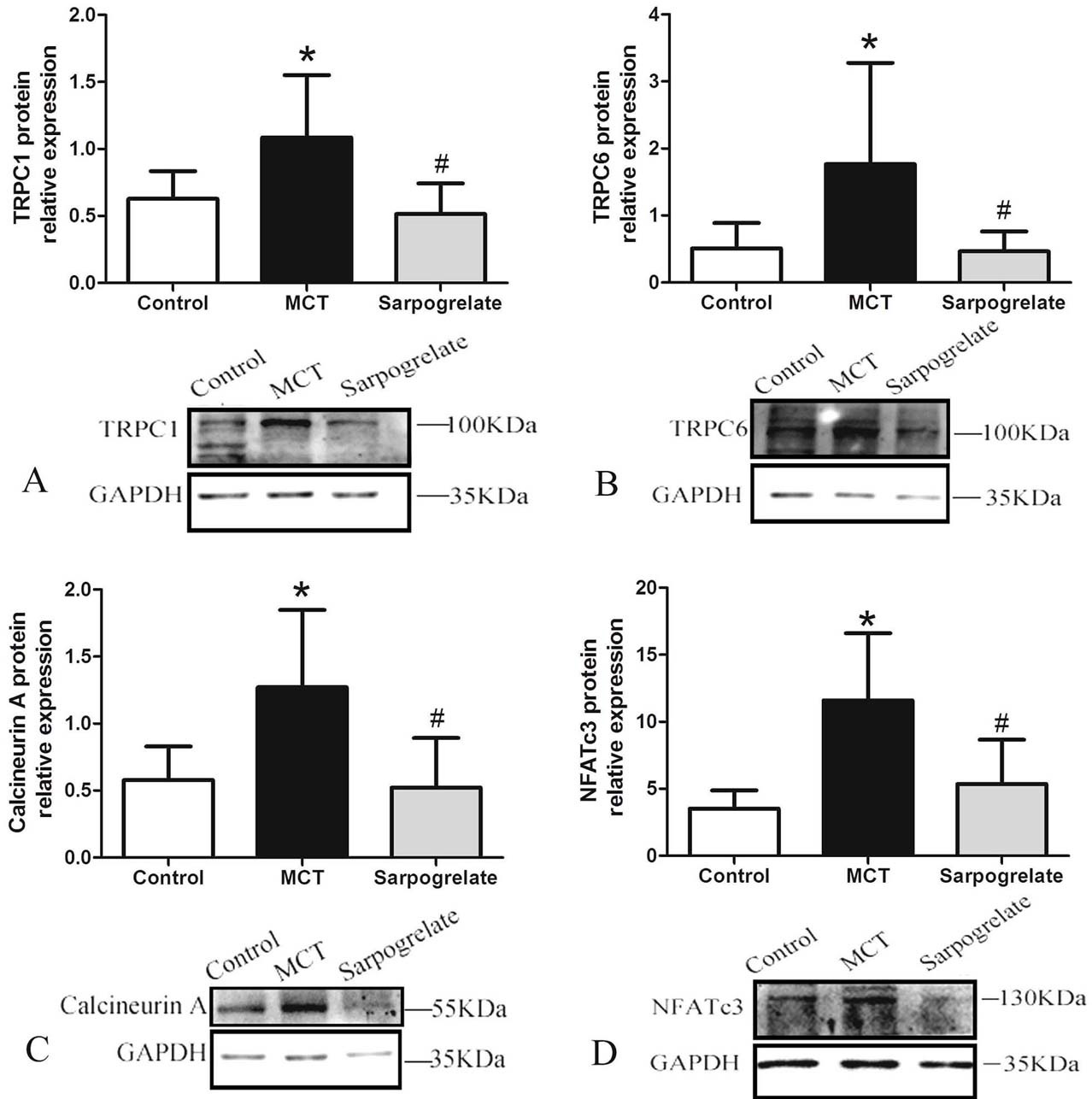 Figure 7.
Figure 7.Effects of Sarpogrelate on pulmonary arterial protein expressions in rats with PAH. Sarpogrelate reduced TRPC1 (A), TRPC6 (B), calcineurin A (C), and NFATc3 (D) protein expressions in rats with PAH. Control group; MCT group: PAH model group; Sarpogrelate group: intervention group. * P <0.0.5, vs. Control group; # P <0.0.5, vs. MCT group, n = 6.
The median survival for PAH is 2.8. years, and the 1-, 3-, and 5- year survival rates are 68%, 48%, and 34%, respectively (13). The causes of PAH include contraction of the pulmonary arterioles, vascular remodeling, and thrombosis (13-15). 5-HT is an important neurotransmitter and vasoactive substance, which has been studied extensively by neurophysiologists. In the 1960s, an association between 5-HT and PAH was recognized. At that time, the appetite suppressant, aminorex, was found to promote PAH. These appetite suppressants are 5-HT receptor agonists, which increase 5-HT levels in the local tissues and the plasma (16). Since then, many studies have shown that patients with idiopathic PAH have increased levels of 5-HT (5,17), while some other studies have found that patients with PAH did not have high 5-HT levels (18). These disparities may be related to different detection methods used in these studies. 5-HT plays a role in promoting vasoconstriction and promoting proliferation by binding with 5-HT receptors and 5-HT transporters. There are different subtypes of 5-HT receptors, 5-HT1 to 5-HT7, and 3 subtypes in the 5-HT2 receptor family: 5-HT2A, 5-HT2B, and 5-HT2C, which have 46–50% structural homology (6). 5-HT transporters are membrane proteins that translocate 5-HT into cells since 5-HT cannot permeate the lipid membrane. The 5-HT receptors and 5-HT transporters are abnormal in PAH. For example, the pulmonary artery 5-HT2B receptor expression has been found to be elevated, along with vascular remodeling and right ventricular hypertrophy in PAH rats (19). Immunohistochemistry has confirmed that 5-HT2A/B receptors are in the vascular smooth muscle layers of the human lung, and PCR has demonstrated increased expression of 5-HT2A/B receptors in pulmonary arterial smooth muscle cells in PAH patients (7). Therefore, 5-HT and 5-HT receptors may be effective therapeutic targets for the treatment of PAH. The inhibition of 5-HT or 5-HT receptors may reduce the degree of PAH. In the current study, sarpogrelate, a 5-HT2A receptor antagonist, decreased pulmonary arterial pressure and reduced pulmonary artery remodeling as well as right ventricular hypertrophy in rats. Also, sarpogrelate continuously reduced severity of PAH at 4 weeks, and up to 8 weeks. These findings indicate that sarpogrelate can reduce the degree of PAH in rats and further suggest that blocking the 5-HT pathway may be one strategy for treating PAH.
This study also investigated the effects of sarpogrelate on the expression of TRPC1 and TRPC6 channels and the expression of calcineurin A and NFATc3 in PAH rats. Sarpogrelate reduced TRPC1, TRPC6, calcineurin A, and NFATc3 levels in the pulmonary arteries. The TRPC channel belongs to the TRP channel family, and there are 7 TRPC subtypes (TRPC1–7). The TRPC channels that are expressed in rat, mouse, and human pulmonary artery and pulmonary arterial smooth muscle cells mainly include two types: TRPC1 and TRPC6. TRPC3 and TRPC4 channels are sometimes found; however, TRPC5 and TRPC7 are not. Activation of TRPC channels can cause a persistent increase in intracellular calcium levels that can activate the downstream calcineurin/NFAT signaling pathway, causing dephosphorylated NFAT to enter the nucleus (10). The NFAT then binds to the gene motifs that promote proliferation and inhibit apoptosis. NFAT can also up-regulate TRPC channel expression after it binds to the NFAT binding site on the TRPC gene sequence (20,21). Studies have also shown that 5-HT can promote intracellular calcium levels (11), while the effect of 5-HT on TRPC channel and downstream calcineurin/NFAT signaling pathway had been studied less. Previous studies have found that both TRPC channels and calcineurin/NFAT are associated with PAH and pulmonary arterial smooth muscle cell proliferation. Yu et al. reported the elevated expression of TRPC3 and TRPC6 in the lung and pulmonary arterial smooth muscle cells of patients with idiopathic PAH (10). De Frutos et al. showed that hypoxia increased the activity of NFAT in the mouse pulmonary artery and that hypoxia resulted in an increased NFAT in the cell nucleus of pulmonary arteries. Inhibition of calcineurin also reduces hypoxia-induced right ventricular hypertrophy (22). Collectively, it is speculated that 5-HT may play a role in the pathogenesis of PAH by affecting the TRPC channel, calcineurin A, and NFATc3 signaling pathway. Inhibition of this pathway may interrupt this positive feedback loop and relieve PAH, but further study is needed to confirm this hypothesis.
In conclusion, sarpogrelate, a 5-HT2A receptor antagonist, reduced the severity of PAH in rats and decreased TRPC1, TRPC6, calcineurin A, and NFATc3 levels in the pulmonary arteries of PAH rats. 5-HT, TRPC channels and calcineurin A/NFATc3 signaling pathways may be potential new therapeutic targets for PAH.
This research was funded by Scientific and Technological Project for Social Development of Shaanxi (NO. 2016SF-326) and Fundamental Research Funds of Xi‘an Jiaotong University (NO. Xjj2014079).
