Oxaliplatin is one of the most common chemotherapy drugs for colorectal cancer (CRC), but its application is greatly limited owing to the drug resistance. Nobiletin is a natural flavonoid isolated from citrus peel and has many biological functions, including anti-inflammatory, antitumor and neuroprotective activities. However, little is known about the effect of nobiletin on the anti-tumor activities of other chemotherapy drugs. In this study, we examined the effect of nobiletin on the efficacy of oxaliplatin in treatment of CRC by using two CRC cell lines. In vitro experiments indicated that nobiletin enhanced the inhibitory effect of oxaliplatin on the proliferation of CRC cells. Meanwhile, nobiletin promoted oxaliplatin-induced apoptosis of CRC cells, as demonstrated by the increased expression of pro-apoptotic proteins (Bax and cleaved-caspse3) and the down-regulation of anti-apoptotic protein Bcl-2. Mechanically, nobiletin sensitized CRC to oxaliplatin chemotherapy by down-regulating the PI3K/Akt/mTOR pathway. Taken together, our study has demonstrated that nobiletin could enhance the sensitivity of CRC to oxaliplatin chemotherapy, and provided a molecular basis for nobiletin’s potential applications in the chemosensitization of CRC.
Colorectal cancer (CRC) is one of the most common malignancies worldwide. The diagnostic rate of CRC is ranked third in males and second in females, and the 5-year survival rate is relatively low compared with other types of cancer(1). Although surgery, new chemotherapeutic agents and targeted therapies have significantly reduced the mortality of CRC in the past 20 years, short-term tumor recurrence and drug resistance has greatly inhibited their further clinical applications(2, 3). Oxaliplatin, one of the most effective drugs for CRC therapy, is a platinum-based chemotherapeutic agent that blocks DNA replication, leading to cell cycle arrest and cell death(4-7). It was reported that oxaliplatin significantly enhanced the survival rate of CRC patients(8-10). However, the decreased platinum influx, improved base excision repair, and increased detoxification, which lead to platinum resistance has largely debilitated the efficacy of oxaliplatin(4, 6). Thus, it is urgently needed to develop novel and effective therapeutic interventions to overcome oxaliplatin resistance.
Nobiletin is a polymethylated flavonoid extracted from the citrus peel of Citrus reticulata Blanco. Nobiletin has been reported to possess various biological effects, such as anti-inflammatory, anti-cancer and neuroprotective properties(11, 12). Nobiletin exerts its anti-cancer activity in various cancers by inhibiting cell proliferation and angiogenesis and possibly leading to cell cycle arrest or apoptosis(13, 14). However, the therapeutic effects of combinational nobiletin and chemotherapeutics on cancer have not been reported.
The PI3K/AKT/mTOR pathway plays a critical role in oncogenic process by regulating the proliferation, survival and metabolism of cancer cells(15-17). In addition, recent studies have demonstrated that inhibition of PI3K/AKT/mTOR signaling sensitized cancer cells to chemotherapeutics(18-20). Therefore, PI3K/AKT/mTOR signaling could be a potential target to search new chemosensitizers.
In this study, we examined the anti-tumor activity of nobiletin combined with oxaliplatin in CRC cells and explored its underlying mechanism. The results showed that nobiletin effectively sensitized CRC cells to oxaliplatin treatment by inhibiting proliferation and promoting apoptosis. Further study demonstrated that the chemosensitive effect of nobiletin was mediated by PI3K/AKT/mTOR signaling pathway.
HT29 and SW480 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM complemented with FBS 10% (vol/vol; Life Technologies, Grand Island, USA) at 37°C in a 5% CO2 incubator.
HT29 and SW480 cells were cultured on a 96-well plate and were treated with various doses of nobiletin and/or oxaliplatin. After 24 h of incubation, cell viability was measured by CCK-8 kit according to the manufacturer’s instruction (Beyotime Biotechnology, Shanghai, China).
HT29 and SW480 cells were treated with oxaliplatin and different concentrations of nobiletin for 24 h. After washing with ice-cold PBS, cells were digested, centrifuged and stained with Annexin V-FITC and PI for 15min at room temperature. Cell apoptosis was then analyzed with a LSR-II flow cytometer.
Cells were lysed by RIPA Lysis Buffer (Shengxing Biotech, Nanjing, China). Equivalent amounts of protein sample were separated by 10% SDS-PAGE and transferred to a PVDF membrane (Millipore, Billerica, USA). After blocking with 5% non-fat dry milk, the membrane was then incubated with respectively primary antibodies and HRP-conjugated secondary antibodies (Abcam, Cambridge, MA). The blots were visualized by ECL and detected using ImageQuant LAS 4000 (Pittsburg, PA, USA).
Data were analyzed by GraphPad Prism software and the results were expressed as mean ± standard deviation (SD). The statistical significance of the studies was analyzed using one way ANOVA. The difference was considered significant at P < 0.0.5.
To gain insight into the anti-cancer activity of nobiletin, HT29 and SW480 cells were treated with various doses of nobiletin. As shown in Figure 1A and B, nobiletin significantly decreased the viability of CRC cells in a dose-dependent manner. In addition, the proliferation of CRC cells was markedly decreased after oxaliplatin treatment, and the inhibitory effect was further enhanced when combined with nobiletin (Figure 1C and D). These results suggest that nobiletin and oxaliplatin may act synergistically to enhance cytotoxicity in CRC cell lines.
 Figure 1
Figure 1Nobiletin decreases the viability of oxaliplatin-treated CRC cells. (A and B) HT29 (A) and SW480 (B) cells were treated with various doses of nobiletin (0-40 μM) for 24 h. Cell viabilities were measured by CCK-8 kit. (C and D) HT29 (C) and SW480 (D) cells were treated with oxaliplatin (5 μM) and/or 20-40 μM of nobiletin for 24 h. Cell viability were measured by CCK-8 kit. All the experiments were repeated at least three times. *P < 0.0.5 versus untreated control. #P < 0.0.5 versus oxaliplatin treated alone.
To further investigate the effect of nobiletin on oxaliplatin-induced cell apoptosis, CRC cells were treated with oxaliplatin and nobiletin, and cell apoptosis was measured by flow cytometry. The results showed that nobiletin further enhanced the pro-apoptotic effect of oxaliplatin on CRC cells (Figure 2B). In addition, oxaliplatin combined with nobiletin significantly increased the expression of pro-apoptotic proteins (Bax and cleaved-caspase-3) and decreased the expression of anti-apoptotic protein (Bcl-2) in CRC cells when compared with oxaliplatin treated alone (Figure 2C-F). These results indicate that nobiletin promotes oxaliplatin-induced apoptosis of CRC cells.
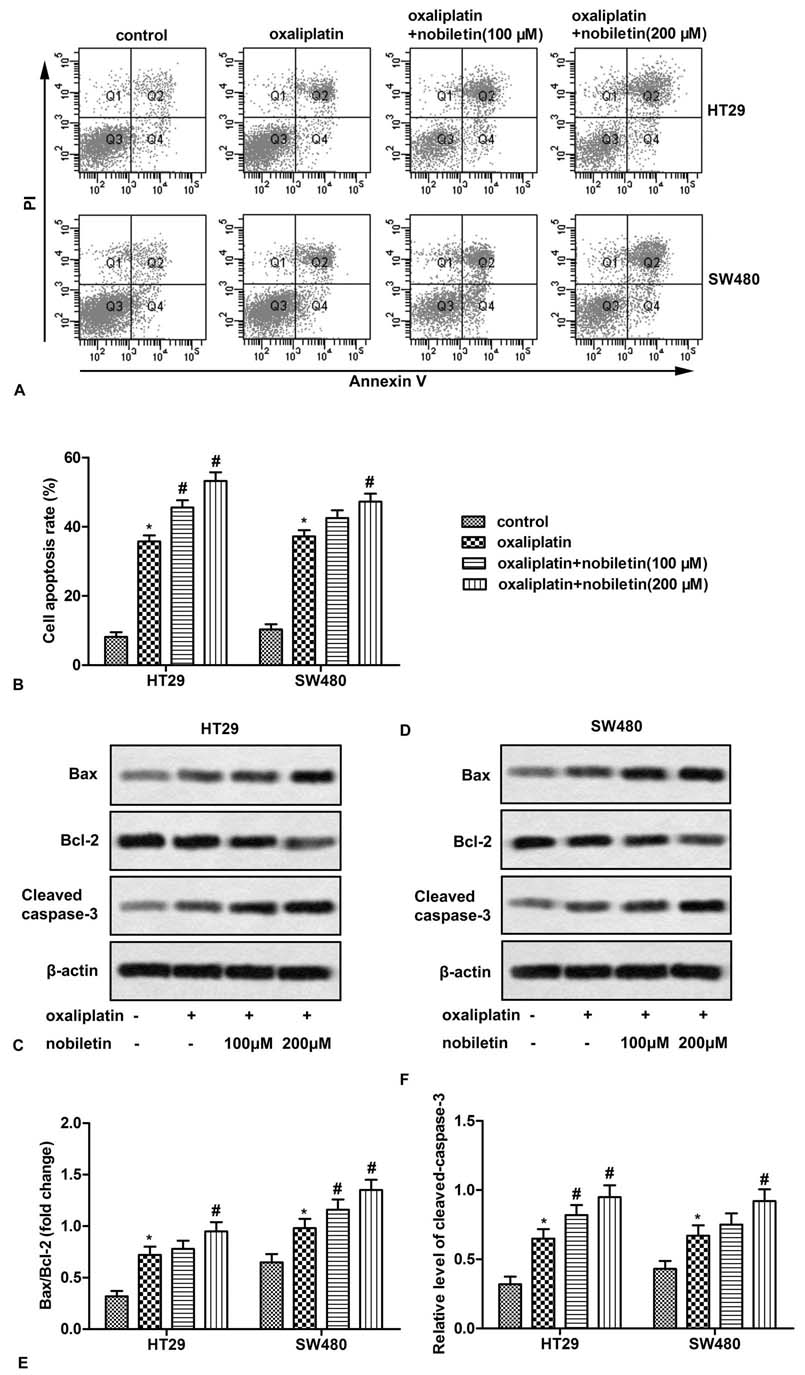 Figure 2
Figure 2Nobiletin promotes oxaliplatin-induced CRC cell apoptosis. HT29 and SW480 cells were treated with oxaliplatin (5 μM) and/or 20-40 μM of nobiletin for 24 h. (A) Cell apoptosis was analyzed by Annexin V flow cytometry. (B) Apoptotic cell quantification. (C and D) The expression of Bax, Bcl-2 and cleaved-caspase3 was measured by Western blot. (E and F) Quantification of Figure 2C and D. All the experiments were repeated at least three times. *P < 0.0.5 versus untreated control. #P < 0.0.5 versus oxaliplatin treated alone.
The PI3K/AKT/mTOR signalling pathways is an important driver of CRC growth and progression. To further explore the mechanism underlying the chemosensitive effect of nobiletin, we examined the effect of nobiletin on PI3K/Akt/mTOR signaling. HT29 and SW480 cells were treated with oxaliplatin in presence or absence of nobiletin for 24 h, the phosphorylated Akt and mTOR were determined by Western blotting. The results showed that oxaliplatin increased the phosphorylation of Akt and mTOR, and this effect was augmented in the presence of nobiletin (Figure 3).
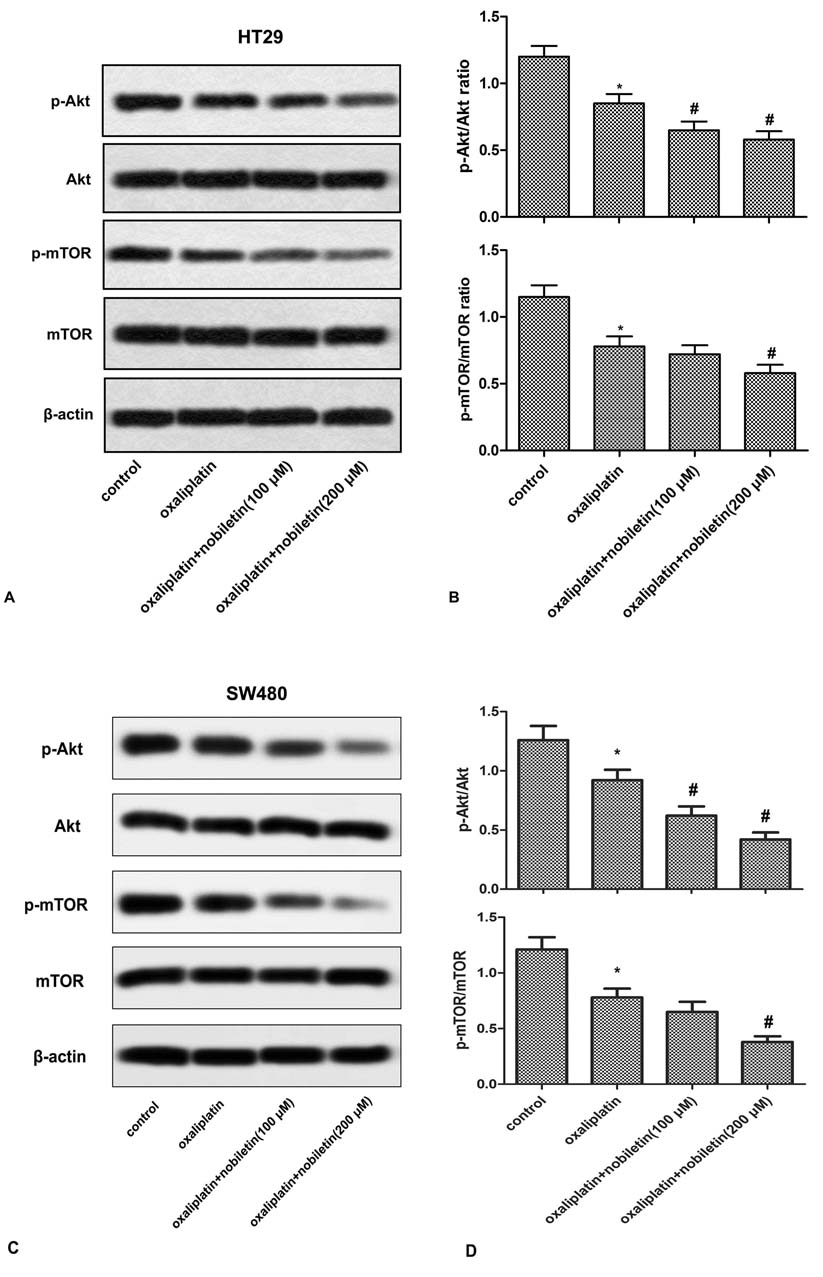 Figure 3
Figure 3Nobiletin promotes oxaliplatin-mediated down-regulation of PI3K/Akt/mTOR. HT29 and SW480 cells were treated with oxaliplatin (5 μM) and/or 20-40 μM of nobiletin for 24 h. (A and C ) The phosphorylation of Akt and mTOR was detected by Western blot. (B and D) Quantification of Figure 3A and C. All the experiments were repeated at least three times. *P < 0.0.5 versus untreated control. #P <0.0.5 versus oxaliplatin treated alone.
To determine whether nobiletin inhibition of PI3K/AKT/mTOR contributes to its chemosensitization, IGF-1 was used to reactivate the PI3K signaling. HT29 and SW480 cells were treated with nobiletin and oxaliplatin in presence or absence of IGF-1 for 24 h. As shown in Figure 4A and 5A, IGF-1abolished the effect of nobiletin on cell viability in oxaliplatin-treated cells. Similarly, IGF-1 also reversed the inhibitory effect of nobiletin on cell apoptosis and apoptosis-related proteins in oxaliplatin-treated cells (Figure 4B-E, Figure 5B-E). Taken together, IGF-1 effectively inhibited the chemosensitization effect of nobiletin, indicating that nobiletin sensitizes CRC cells to oxaliplatin via down-regulating PI3K/Akt/mTOR pathway.
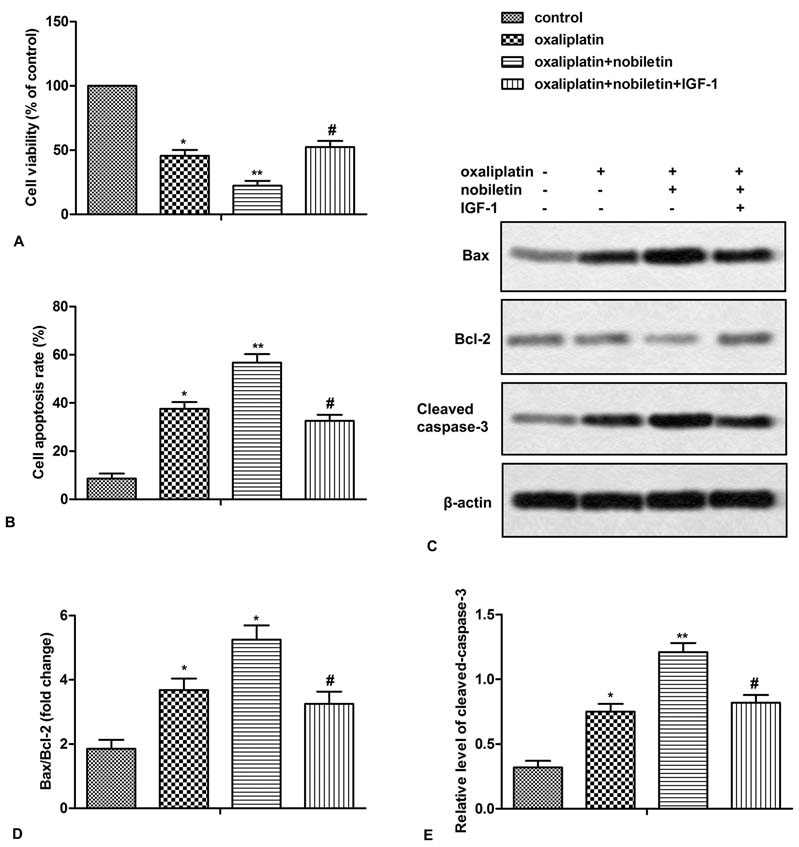 Figure 4
Figure 4Nobiletin sensitizes CRC cells to oxaliplatin via down-regulating PI3K/Akt/mTOR pathway. HT29 cells, pretreated with or without nobiletin (40 μM) and/or IGF-1 (10 ng/ml), were incubated with oxaliplatin (5 μM) for 24 h. (A) Cell viability was measured by CCK-8 kit. (B) Cell apoptosis was analyzed by flow cytometry. (C) The expression of Bax, Bcl-2 and cleaved-caspase3 was measured by Western blot. (D and E) Quantification of Figure 4C. All the experiments were repeated at least three times. *P < 0.0.5, **P < 0.0.1 versus untreated control. #P < 0.0.5 versus oxaliplatin plus nobiletin.
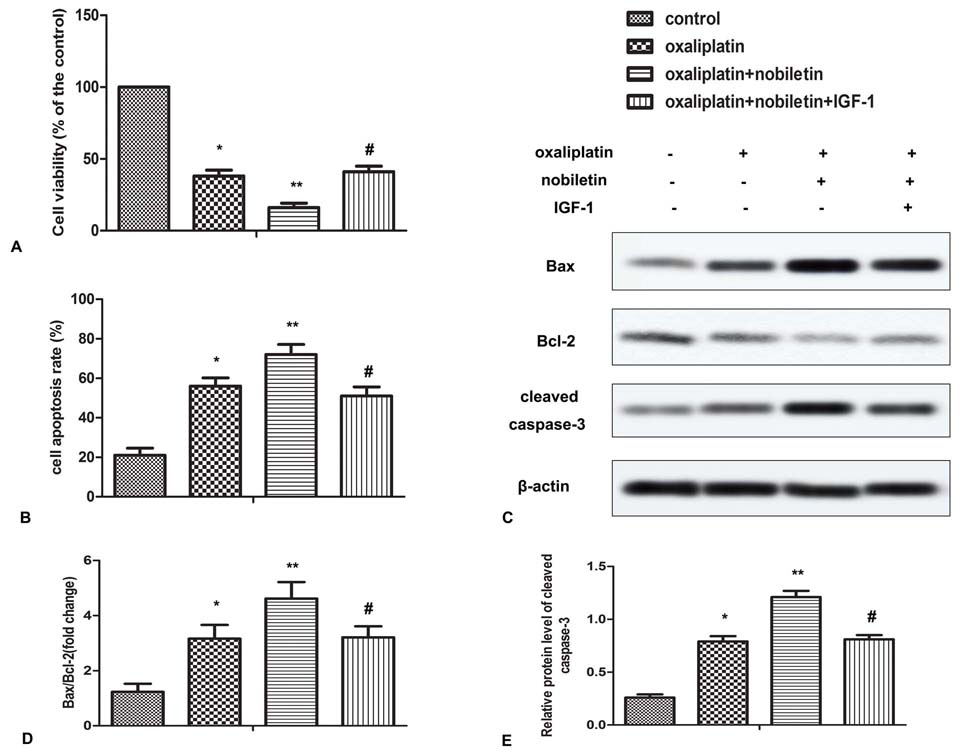 Figure 5
Figure 5Nobiletin sensitizes CRC cells to oxaliplatin via down-regulating PI3K/Akt/mTOR pathway. SW480 cells, pretreated with or without nobiletin (40 μM) and/or IGF-1 (10 ng/ml), were incubated with oxaliplatin (5 μM) for 24 h. (A) Cell viability was measured by CCK-8 kit. (B) Cell apoptosis was analyzed by flow cytometry. (C) The expression of Bax, Bcl-2 and cleaved-caspase3 was measured by Western blot. (D and E) Quantification of Figure 5C. All the experiments were repeated at least three times. *P < 0.0.5, **P < 0.0.1 versus untreated control. #P < 0.0.5 versus oxaliplatin plus nobiletin.
Chemotherapy is a mainstream anti-cancer therapy that effectively suppresses the growth of malignant tumors(21). It was reported that chemotherapeutic drugs are delivered to tumor by microcirculation. However, the maldistribution of drugs may lead to some specific changes of tumors in the microenvironment(22), including formation of hypoxic areas, elevation of interstitial fluid pressure and oligotrophy. These changes decrease the chemosensitivity of tumors and come about drug resistance(23). Thus, improving the drug resistance of cancer cells has become a research focus. Peng et al. have demonstrated that Astragaloside IV sensitizes non–small cell lung cancer cells to gefitinib via regulating of SIRT6(24). Ganji et al. have reported that HSP90 inhibition down-regulates thymidylate synthase and sensitizes colorectal cancer cell lines to 5FU(25). In this study, our results demonstrated that combined treatment with nobiletin could increase CRC cells response to oxaliplatin inhibition through PI3K/Akt/mTOR pathway.
Nobiletin has been shown to have anti-tumor activities in various types of cancer, such as gastric cancer, lung cancer and breast cancer(26-28), although its molecular mechanism remains unclear. In addition, recent studies showed that nobiletin sensitized cancer cells to chemotherapy. For example, nobiletin induces apoptosis and enhances the effects of the anticancer drug 5-fluorouracil in human gastric cancer cells(29). Co-treatment of nobiletin and atorvastatin displays a strong synergy in inhibiting colon carcinogenesis(30). Our results showed that nobiletin treated alone significantly decreased the proliferation of CRC cells at the dose of 100 and 200 μ?, which was consist with previous studies. Combined treatment with nobiletin increased the cytotoxicity of oxaliplatin on CRC cells, suggesting that nobiletin was capable of potentiating the anti-tumor activity of oxaliplatin.
The PI3K/Akt/mTOR signaling pathway is involved in various aspects of tumor progression, including cell proliferation, differentiation, survival, apoptosis, and metastasis(31, 32), PI3K promotes tumor growth by activating mTOR. Akt inhibits cell apoptosis by up-regulating the phosphorylation of Bax and inactivating Bad(33). It was reported that mutations in the individual component of PI3K/Akt/mTOR signaling pathway account for as much as 30% of all known human cancers(34). Thus, many researchers have turned their focus on PI3K/Akt/mTOR pathway for treating cancer or enhancing chemotherapy sensitivity of cancer cells. For example, Guizhi Fuling Wan, a traditional Chinese herbal formula, sensitizes cisplatin-resistant Human ovarian cancer cells through inhibition of the PI3K/AKT/mTOR Pathway(35). Oxymatrine synergistically enhances the anti-tumor activity of oxaliplatin in colon carcinoma through PI3K/AKT/mTOR pathway(36) miRNA-7 increases cisplatin sensitivity of gastric cancer cells through suppressing mTOR(37). More importantly, PI3K/Akt signaling is also involved in the inhibitory effect of nobiletin on invasion and migration of cancer cells(11, 12). In the present study, we found oxaliplatin inhibited the phosphorylation of Akt and mTOR, which was augmented in the presence of nobiletin. Gain-of-function study showed that nobiletin enhanced the anti-tumor effect of oxaliplatin through PI3K/Akt/mTOR pathway.
In conclusion, we found that nobiletin enhanced the anti-proliferative and apoptotic effect of oxaliplatin in CRC cells by modulating the PI3K/Akt/mTOR pathway. Since PI3K/Akt/mTOR signaling is involved in the pathogenesis of many types of cancer, our findings not only elaborate a novel mechanism of nobiletin’s chemosensitive effect, but also provide new insights into the combination of nobiletin and chemotherapeutic drugs in treatment CRC and other cancers.
We sincerely appreciate the technical support from Beijing Luhe Hospital, Capital Medical University.
Abbreviations: CRC, Colorectal cancer; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2 Associated X protein; PI3K/Akt/mTOR, Phosphatidyl inositol 3-kinase/Akt/ mammalian target of rapamycin; CCK-8, Cell Counting Kit 8; PI, Propidium iodide; IGF-1, Insulin-like growth factors-1.
