Academic Editor: Gianluca Caruso
Background: The main aim of the research was to study short-term changes in the concentrations of elements in two widely distributed plant species, couch grass and nettle and in the rhizosphere soil of the plants. Methods: The sampling of plants and soil was carried out on three dates: 3, 10, and 25 May 2021. On each day of sampling, the plants and soil were collected three times: at 9:00, 14:00, and 19:00. The ICP-OES and ICP-MS analytical techniques were used for determination of elements in the plant and soil samples. The Raman spectroscopy was applied to study variations in the organic compounds. Results: The concentrations of both macro-nutrients and trace elements in plants varied greatly over daytime on all dates of sampling. The differences between concentrations of many elements in the plants collected at different times during a day were statistically significant. There were also statistically significant differences between concentrations of some elements (Na, Mg, P, K, Fe, Ba) in the plants collected on different dates. The relative intensity of diffuse luminescence of the rhizosphere soil of couch grass and nettle was different during daytime and also differed between the soils taken from roots of the two plant species, especially in the beginning of May. Conclusions: The experimental data indicates that the daily variations of the element concentrations in plants might be a result of multiple effects of various factors. The differences in the daily element variations in the couch grass and nettle growing in the same site and collected simultaneously might be due to the fact that these plants belong to different clades. The diurnal fluctuations (that also include regular changes in the element concentrations in plants) can be different for monocotyledons (couch grass) and dicotyledons (nettle). New experimental findings on short-term variations in the concentrations of macro-nutrients and trace elements can help to gain a new insight into accumulation of the elements in different plant species and also be useful in agricultural practice.
Plants have developed special mechanisms to predict the changes in the environment caused by the Earth’s rotation [1]. By now a large amount of experimental data on diurnal variations in various organic compounds is available [2, 3, 4]. However, the mechanisms of short-term (within several hours) variations in the concentrations of mineral elements in different plants and in the rhizosphere soil of the plants are still not clearly understood. Meanwhile, it is reasonable to expect that the concentrations of elements in plants can also change over a relatively short time. The variations in the plant element composition under stable conditions have a cyclic nature due to regular rhythms of different biochemical processes. Because photosynthesis is a dominant metabolic process in the primary metabolism of green cells, other plant activities, in particular, nutrient mobilization, are often synchronized with the rhythms of photosynthesis [5].
New experimental data on rhythmical fluctuations of some mineral elements have been published in the last few years. Palmer and Stangoulis studied variations in the concentrations of Mg, K, Fe, and Zn in the phloem sap of wheat Triticum aestivum L. and found statistically significant differences between concentrations of the elements in the plants collected during daytime [6]. Recently an interesting research on daily fluctuations of Li, Na, Mg, K, Ca, Fe, Co, Zn, and Se in the alga Ostreococcus tauri was reported [7]. The authors found clear daily rhythmical changes for most of the elements. Our previous field experiments with several plant species (couch grass, plantain, dandelion) showed that concentrations of different elements in the plants that grow under the same conditions can vary significantly within a short time [8, 9, 10]. However, in many cases the experimental findings concern daily variations of only a few elements such as Mg, K, and some others. Magnesium and potassium are essential for numerous biological processes in plants. Meanwhile, the concentrations of many other elements, macro-nutrients as well as trace elements, can also be expected to change over a short period of time.
Since the rhizosphere soil plays a key role in the growth and nutrition of plants [11] and because the plant and soil on the surface of the plant roots are in close proximity and constant relationship, it would appear reasonable that similar dynamic processes will also occur both in plants and in the rhizosphere soil. Furthermore, it can be expected that some external conditions, such as climatic situation on the day of sampling and acidity of soils, can affect the distribution of elements in plants and possibly in the rhizosphere soil [12]. Besides, the biological rhythms in the plants of different age can also be different [13]. Due to this, it is important to study possible effects of the sampling time on the element concentrations in plants. Such a variation in the elemental composition of plants and rhizosphere soil over time should be taken into account during sampling the environmental objects.
Today, much attention is paid to the study of the plant species that are more efficient in phytoremediation. Toxic trace elements are a major focus for selecting the plants capable of phytoextracting the metals from contaminated soils [14, 15]. Recently differences in uptake of different elements by several plants have attracted the attention of researchers [16, 17, 18]. The differences between concentrations of essential nutrients in plants are also very important for understanding the influence of macro-elements on the physiological processes in different plant species and need to be examined in the biogeochemical studies.
It is known that phenols are among main active components of organic fraction of soil [19]. Phenolic compounds are of great importance in the plant development. The understanding the relationships between these biomolecules in the rhizosphere soil and in plants can help to gain an insight into the knowledge of their contribution to the biochemical processes in soil and element nutrition of plants. In our research, Raman spectroscopy was applied to study the differences between organic composition of the soil taken from roots of different plant species and the influence of external factors on the rhizosphere. This analytical technique has already been used to identify a range of organic compounds by their unique spectral characteristics [20, 21] and can be useful for understanding biochemical processes in soil.
The main aims of the research were (1) to study the short-term variations in the concentrations of macro- and trace elements in two widely distributed plants, couch grass and nettle, and (2) to evaluate possible differences in the ability of two different plant species growing under the same conditions to accumulate various elements.
Two widely distributed plant species, couch grass Elymus repens L. and
nettle Urtica dioica L., were collected in May 2021. The study area was
located in the south of St. Petersburg city (59°53
| Time of sampling | Air temperature °C | Soil temperature °C | Air humidity % |
| 3 May, sunrise at 4:52, sunset at 20:59 | |||
| 09:00 | 8.5 | 7.0 | 8 |
| 14:00 | 4.0 | 4.0 | 73 |
| 19:00 | 3.0 | 3.0 | 99 |
| 10 May, sunrise at 4:33, sunset at 21:19 | |||
| 09:00 | 8.0 | 7.5 | 62 |
| 14:00 | 13.0 | 8.0 | 8 |
| 19:00 | 18.0 | 12.5 | 4 |
| 25 May, sunrise at 4:00, sunset at 21:53 | |||
| 09:00 | 15.0 | 10.0 | 75 |
| 14:00 | 17.0 | 15.0 | 64 |
| 19:00 | 14.5 | 13.5 | 65 |
For digestion of plant and soil samples, each sample was weighed into the
microwave digestion vessel (SK-15 high-pressure PTFE-TFM-Teflon vessels), then 9
mL of concentrated HNO
To obtain Raman spectra of light from the soil samples, an OPTEC-785TRS-2700
Raman spectrometer (spectral range –200 … + 2700 cm
The statistical analysis of the experimental results was performed with help of
Statistica for Windows 8.0 Software packages (StatSoft, Tulsa, OK, USA). The
normality of the distribution of the analytical data was verified using the
Shapiro-Wilk test. Outliers were excluded from further analysis. Mean
concentrations of elements were calculated and analysis of variances was carried
out to assess statistically significant (p
The concentrations of different elements in plants varied significantly over daytime on all dates of sampling. Fig. 1 illustrates daily dynamics of essential plant nutrients Mg, K, and Mn in roots and leaves of the couch grass and nettle collected on different dates. In most cases the differences between concentrations of the elements in the plants collected during the days at different times were statistically significant, and often the level of the p values was below 0.01. The statistically insignificant differences are listed in the Figure captions.
 Fig. 1.
Fig. 1.Daily variations of Mg, K, and Mn in couch grass and
nettle. (a) Roots (1) and leaves (2) of the plants collected on 3 May. In couch
grass, the differences between concentrations of Mg in leaves of the plants
collected at 9:00 and 19:00, and between K concentrations in leaves and Mn
concentrations in roots of the plants collected at 14:00 and 19:00 were
statistically insignificant. In nettle, no difference was found between
concentrations of Mg and Mn in roots of the plants harvested at 9:00 and 14:00,
and between K and Mn concentrations in leaves of the plants collected at 9:00 and
19:00. In all other cases the differences between concentrations of the elements
in the plants collected at different times were statistically significant
(p
Supplementary Table 1 illustrates differences between concentrations of
other elements in the plants collected on different dates during daytime. As the
data of the Supplementary Table 1 suggests, the concentrations of many
elements in roots and leaves of both plant species collected at different times
of the days are statistically significantly (p
Although couch grass and nettle were collected simultaneously from the same
site, there were statistically significant (p
Fig. 2 shows the changes in the concentrations of Mg and P in roots and leaves of couch grass and nettle taken at different dates from the same site. During May, the variations in the concentrations of the elements in the two plant species were different. In couch grass, the concentrations of Mg and P were the highest in the plants sampled on 10 May and were lower on two other dates. On the other hand, in nettle, the concentrations of the elements were higher in the plants collected on 3 and 25 May and lower in the plants harvested on 10 May.
 Fig. 2.
Fig. 2.Variations of Mg and P in couch grass and nettle collected during May. 1 – roots. 2 – leaves.
The concentrations of Mg in roots and leaves of the couch grass and nettle
collected on 10 May differed statistically significantly (p
The concentrations of other elements were also not the same on different sampling dates. For example, the concentrations of Na in roots of couch grass were lower, and in leaves were higher in the plants collected on 10 May compared with those in the plants harvested on other dates. In nettle, the Na concentrations were more or less similar regardless of the date of sampling. In leaves of couch grass and in roots of nettle collected on 10 May, the concentration of K was minimal, while in the nettle leaves it was higher than the K content in leaves of the plants collected on 3 and 25 May. In roots of couch grass, the K concentrations slightly increased from beginning to the end of May. In roots of the couch grass and nettle collected on 10 May, the concentration of Fe was higher than in roots of the plants harvested on 3 and 25 May. In leaves of the couch grass, the Fe content slightly decreased during May, and in leaves of nettle, the concentration of Fe was lower in the plants collected on 10 May. The concentration of Ba in roots of the couch grass collected on 10 May was higher compared to the Ba content in roots of the couch grass taken on other dates. In leaves of couch grass, the Ba concentration increased during May. In roots of nettle, the Ba content increased, and in leaves it slightly decreased during May.
The temporal variations in the element concentrations in plants can be caused by various factors. One of the important factors might be climatic conditions at the site on different dates of sampling. It is known that light and temperature are among the key stimuli that can affect the plant biological rhythms in plants [22]. For example, it is commonly supposed that soil temperature can influence the uptake of elements by plants. At low soil temperature nutrient uptake can be reduced as a result of an increase of soil water viscosity and low activity of root nutrient transport [23]. On the other hand, it was reported that under lower temperature the uptake of K, Ca, Mg, Na, and Mn in sweet pepper increased [24].
In Table 1 are shown the changes in the soil/air temperature and air humidity. On 3 May, the temperature of the air and soil constantly decreased during the day, and the air humidity increased. There was also a rain from middle of the day till night. On 10 May, the air and soil temperature increased from morning to evening, while air humidity decreased. On 25 May, the temperature was higher in the middle of the day. At the same time, the concentrations of elements in plants growing in the soil did not always follow the changes in the soil temperature and often varied differently in the daytime. This indicates that the short-term variations in the uptake of elements by couch grass and nettle were not directly related to the daily changes in the soil/air temperature and air humidity. This may be due to the fact that plants have evolved light and temperature sensors capable of maintaining homeostatic balance [25].
It is also well-known that soil pH can affect the solubility of elements in the
soil [26]. The relationships between pH and concentrations of elements in plants
can be both positive and negative [27]. It was shown that the main factor
influencing the changes in the pH in the rhizosphere soil is the net release of
H
Fig. 3 illustrates the daily changes in the pH of the soil taken from roots of couch grass and nettle. On all dates of sampling, the maximum values of the pH of the rhizosphere soil of both plant species were observed in the middle of day. But as was shown in Fig. 1 and in Supplementary Table 1, the daily variations in the plant element concentrations do not always follow the changes in the soil pH. Besides, the temporal changes in the concentrations of elements in couch grass and nettle growing at the same place and harvested simultaneously were often different.
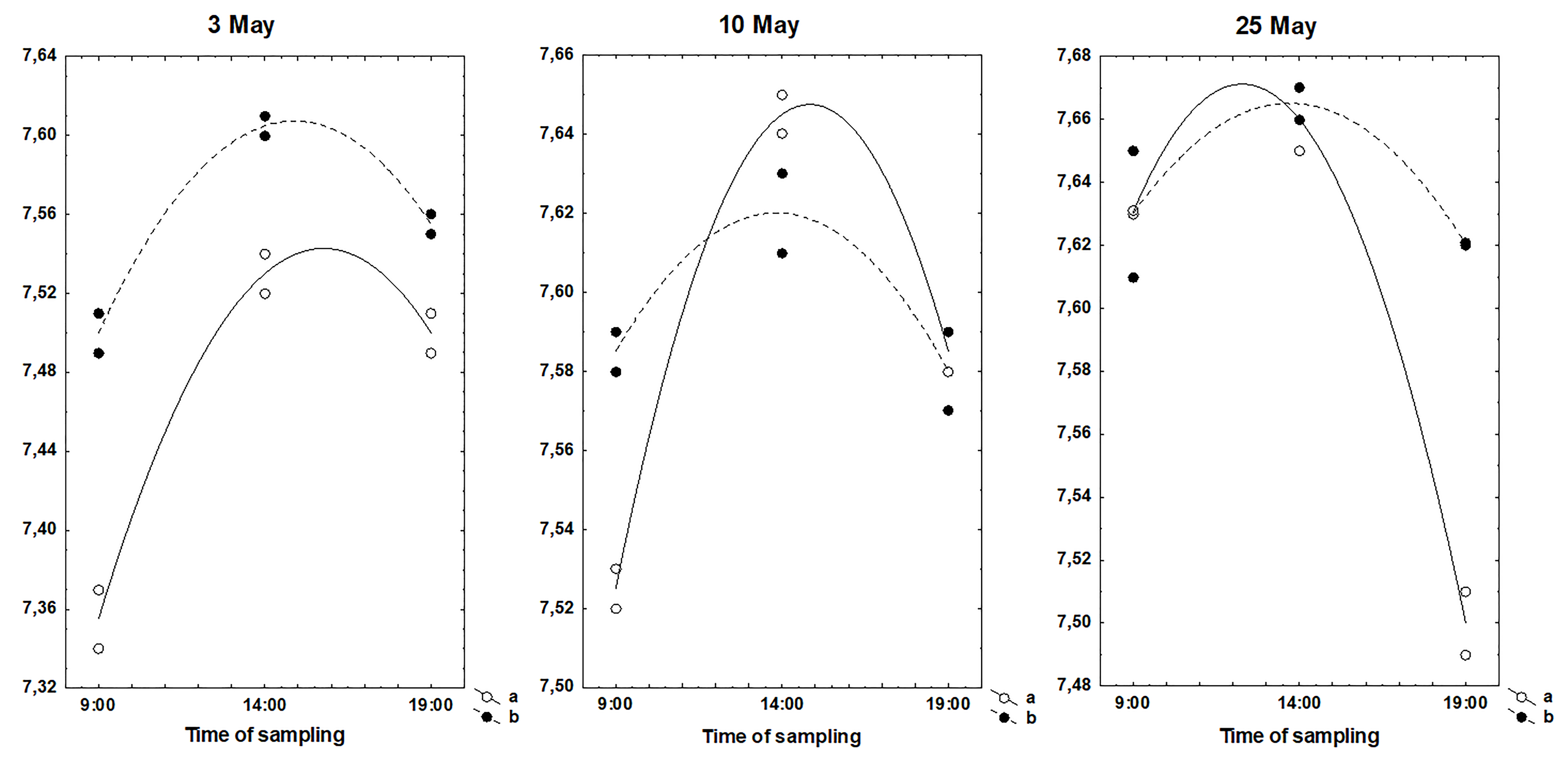 Fig. 3.
Fig. 3.Daily variations of pH of the rhizosphere soil of the plants collected during May. (a) Rhizosphere soil of couch grass. (b) Rhizosphere soil of nettle.
On the whole, it is difficult to identify any one crucial factor that would be responsible for dynamic process of element uptake. Considering that various environmental conditions during plant growth can affect the structure and anatomy of the plants, thereby affecting the uptake of elements, the observed fluctuations are result of multiple effects of various factors. It is hardly possible to explain the changes in the plant element concentrations by any one of the external factors; each of them contributes to the uptake process. Since in many cases the daily fluctuations of elements in couch grass and nettle were different, it can be assumed that these effects, at least in part, are species-specific.
Taken into account that all living organisms are under constant control of the Earth’s rotation [1, 29], it can be assumed that the circadian clock may be an important factor affecting the daily variations of the element concentrations in plants. In this case the question arises why the diurnal element variations in the couch grass and nettle growing in the same site and collected simultaneously were often different. This might be due to the fact that these plants belong to different clades, couch grass is monocotyledon and nettle is dicotyledon. It was reported that mono- and dicotyledons can differ in the ability to accumulate various macro- and trace elements [30, 31, 32]. One may speculate that the plants are also able to give different reactions on the circadian changes. As a consequence, the daily fluctuations of the element concentrations in plants can be different for monocotyledons and dicotyledons.
The ratios of nutrient concentrations can serve as an important parameter of the state of plants and the rhizosphere soil [33]. Fig. 4 illustrates the K/Na ratios in roots and leaves of couch grass and nettle and in the rhizosphere soil of the plants collected on different dates. The K/Na ratios were the lowest in the soil taken from roots of the plants, higher in roots, and the highest values of the K/Na ratios were observed in leaves. It was typical for both couch grass and nettle collected during May and was mainly due to significant differences in the K concentrations in the samples. In plants, the higher K/Na ratios resulted from both lower Na and higher (especially in leaves) K concentrations as compared to those in the rhizosphere soil. The higher K to Na ratio is desirable for the plant development [34].
 Fig. 4.
Fig. 4.Mean values of the K/Na ratios in the rhizosphere soil, roots and leaves of the plants collected during May. (a) Couch grass. (b) Nettle.
Results of correlation analysis showed that there were no statistically
significant relationships between concentrations of elements in the rhizosphere
soil and in roots of both plant species. There was a statistically significant
(p
The taxonomical status of a plant is among the most important factors responsible for uptake mechanisms. Monocotyledons and dicotyledons differ significantly at the morphological and physiological level. As a result, the plants may have different ability to accumulate various elements [35, 36, 37]. These differences may also be related to their distinct root architecture [38] leading to different strategies of uptake of elements from the rhizosphere soil and their distribution between underground and aboveground plant parts.
Cluster analysis revealed that both roots and leaves of the plants collected on different dates were well separated into different groups - couch grass and nettle. The PCA showed that main contribution to the separation was provided by K, Ca, and Mg (leaves) and Na, Ni, Ca, Mg, and Ba (roots). It is also interesting that leaves and roots of the plants collected at 9:00 were separated from leaves and roots of the couch grass and nettle harvested at 14:00 and 19:00. This is illustrated on the example of the plants collected on 3 May (Fig. 5). According to the PCA data, Co (leaves) and Ni (roots) were responsible for the separation. However, similar separation between plants collected at 9:00 and at the two other times was found only in leaves of the couch grass harvested on 10 May and in roots of the nettle harvested on 25 May. It should be mentioned that certain contribution to the separation of the plants collected on 3 May at 9:00 from the plants collected at two other times might also make a rain that took place from middle of the day till evening (precipitation levels were 0.8 mm/h at 14:00 and 0.9 mm/h at 19:00).
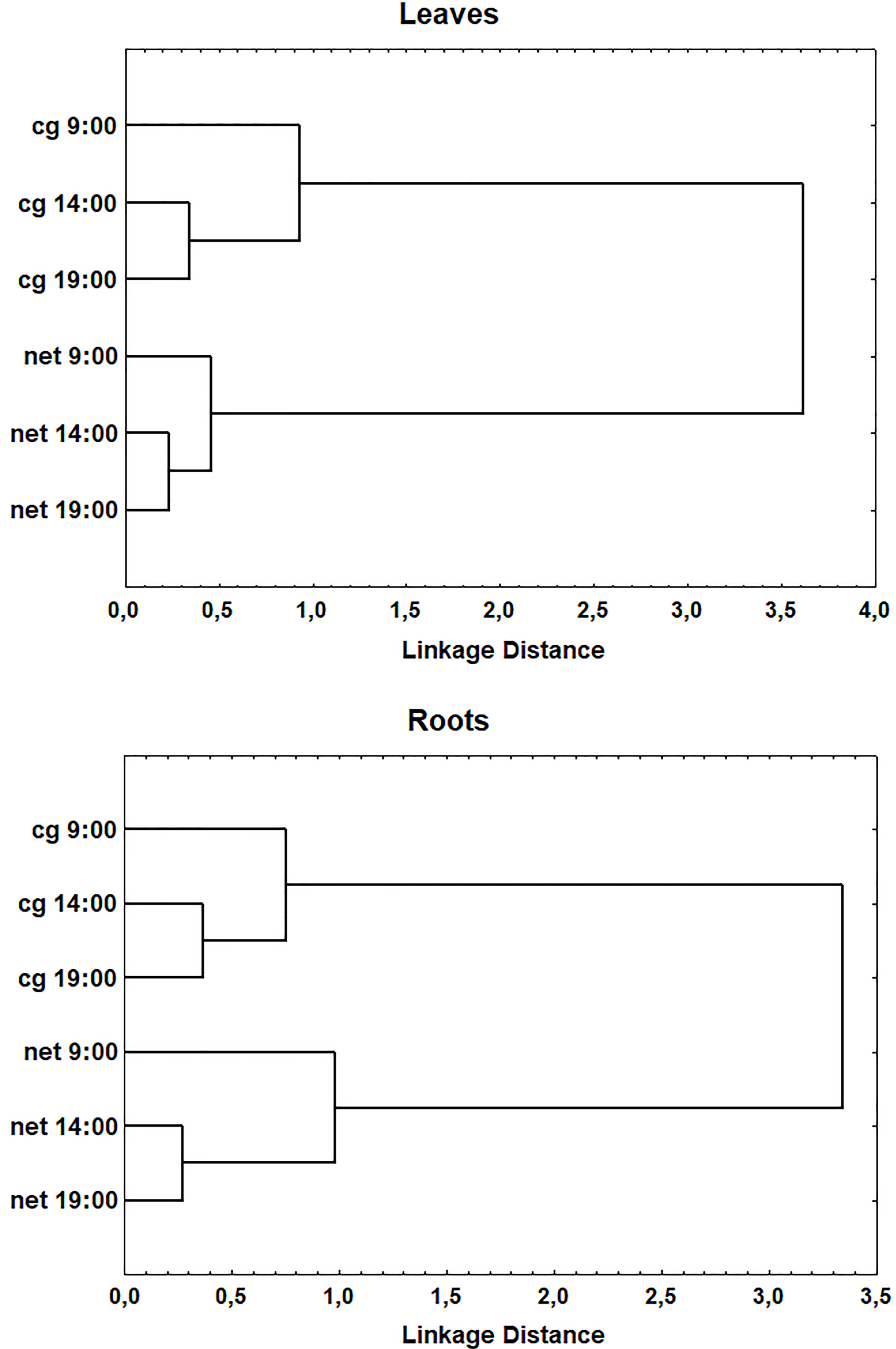 Fig. 5.
Fig. 5.Cluster analysis (Ward’s method) of the plants collected on 3 May at 9:00, 14:00, and 19:00. cg, couch grass; net, nettle.
Results of the Raman spectroscopy analysis of the soil taken from the plant roots are demonstrated in Fig. 6. Table 2 shows the compounds that were found in the soil samples.
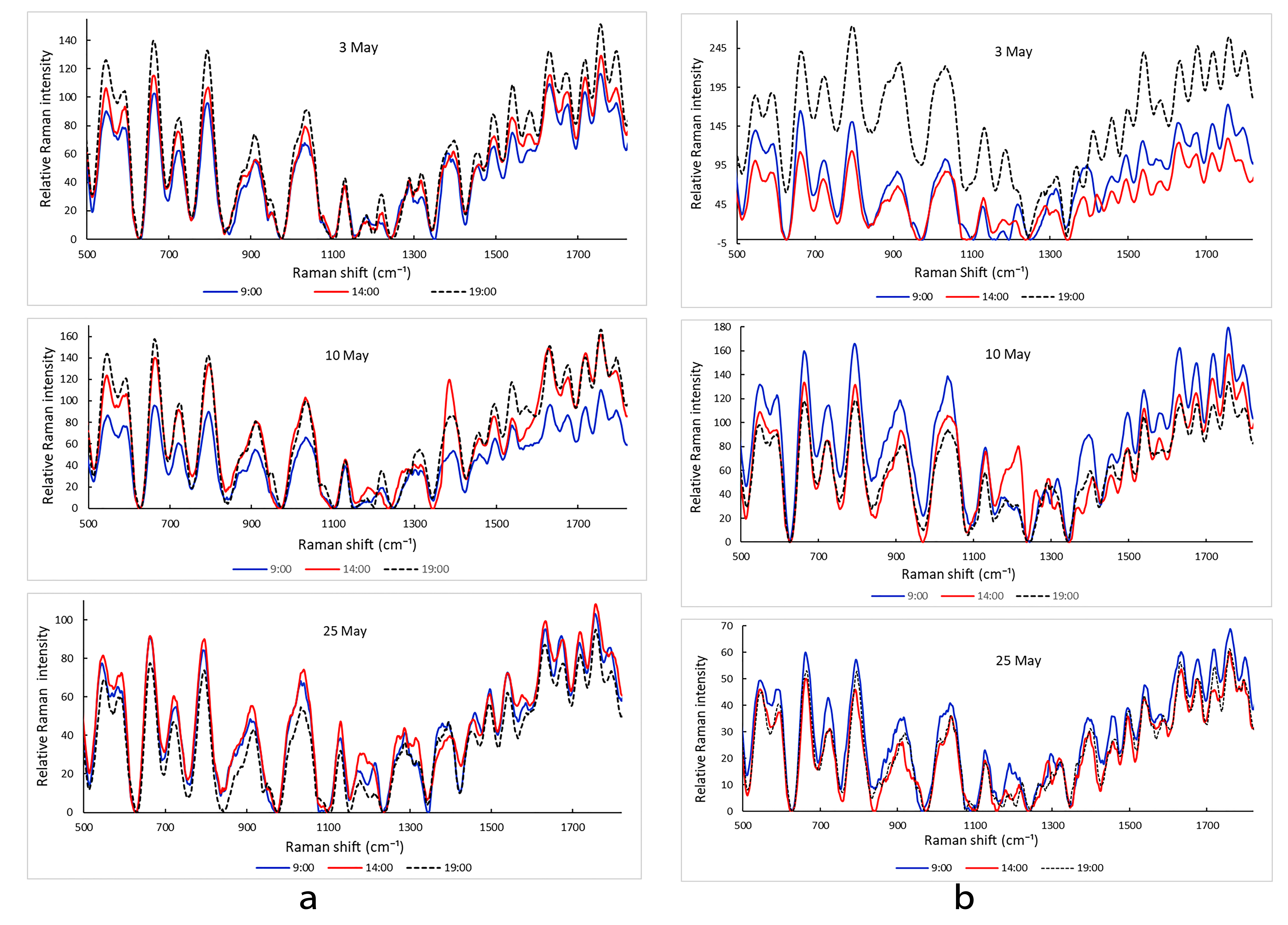 Fig. 6.
Fig. 6.Raman spectra of the rhizosphere soil. (a) Soil was taken from roots of nettle collected on 3 May, 10 May, and 25 May. (b) Soil was taken from roots of couch grass collected on 3 May, 10 May, and 25 May.
| Couch grass | Nettle | Identification |
| 592 | 592 | not identified |
| 944–950 | 948–950 | CN asym str, (CH |
| 1072–1086 | 1072–1080 | PO |
| 1178–1184 | 1178–1184 | polysaccharides (lignin, pectin, etc.) |
| 1218–1220 | 1218–1220 | PO |
| 1276–1288 | 1270–1280 | not identified |
| 1318–1322 | 1318 | ring stretching polycyclic aromatic compounds. ν(C=C) Ar, C-O phenoxyl group of phenolic compounds and benzoic acid (salicylic acid, syringic acid, protocatechuic acid, etc.) |
| 1370 | 1370 | not identified |
| 1396 | 1396 | carboxylate symmetric stretching from carboxylic acid and other fatty acids |
| 1454 | 1454 | -OCH |
| 1576–1584 | 1576–1584 | ring stretching polycyclic aromatic compounds, ν(C=C) Ar, C-O phenoxyl group of phenolic compounds and benzoic acid (salicylic acid, syringic acid, protocatechuic acid, etc.) |
| 1796 | 1796 | not identified |
On 3 May, the concentrations of organic compounds of the benzoic acid group in the rhizosphere soil of nettle slightly increased from morning to evening. On the other hand, there were rather serious differences between samples of the rhizosphere soil of couch grass collected at different times. For both plant species, the concentrations of the organic compounds in the rhizosphere soil were higher in the samples taken at 19:00.
At 9:00 on 10 May, the concentrations of the organic compounds in the soil taken from roots of nettle were lower that those in the soil collected at two other times. To the contrary, the concentrations of the compounds in the rhizosphere soil of the couch grass collected at 9:00 were higher than in the soil taken from roots of couch grass at other times.
On May 25, the concentrations of organic compounds which can be attributed to the group of benzoic acids were slightly different for the rhizosphere soil of nettle and couch grass collected at 9:00, 14:00, and 19:00.
The spectra of the rhizosphere soil of the nettle collected on 3 and 25 May were
relatively similar, but there were some differences in the area of the wave shift
of 1318 cm
Probably, the differences in the accumulation of the organic acids in the rhizosphere soil of the plants might be due to different ability of the root exudates produced by couch grass and nettle to stimulate homeostasis at the signal level. It can also be assumed that the differences between the spectra of the rhizosphere soils of the plants in certain degree might be associated with the differences between uptakes of mineral elements by these two plant species.
Our results showed that there were rather significant short-term (within few hours) variations in the concentrations of different elements in roots and leaves of couch grass and nettle as well as in the organic compounds in the rhizosphere soil of the plants. These natural temporal changes can be due to various factors and should be taken into account when chosing the time of soil and plant sampling. Although plants were collected from the same site, the temporal variations were different for couch grass and nettle. This can be explained by the fact that these plants belong to different clades – monocotyledons and dicotyledons. It can be assumed that the plants were also capable of giving different reactions on the varying environmental conditions.
IS designed the research study, performed the field experiment and multivariate statistical analysis of the experimental data. MN, IV and PP were responsible for elemental analysis of the experimental samples. AR determined main characteristics of soil material. VC and AG conducted Raman spectroscopy analysis. IS wrote the manuscript. All authors contributed to editorial changes in the manuscript, read and approved the final version of the manuscript.
Not applicable.
Not applicable.
Irina Shtangeeva acknowledges that her visit to the Oulu University was funded by Academy of Finland, grant number 333659.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
